Abstract
Abstract
We provide a review of the North American ants (north of Colombia) of the ant genus Mycocepurus, including keys to the workers and females, illustrations and distribution maps. The distribution of M. tardus is extended to Nicaragua and Costa Rica. The female of M. curvispinosus is described.
Resumen
Se revisan las especies del género Mycocepurus de Norte América (al norte de Colombia). Se incluyen claves para la identificación de las obreras y las hembras, ilustraciones y mapas de distribución. Se amplia hacia el norte la distribución de M. tardus, incluyendo ahora Nicaragua y Costa Rica y se describe la hembra de M. curvispinosus.
Keywords: fungus-growing ants, tribe Attini, tropical rain forest, Neotropics
Introduction
The symbiosis between fungus-growing ants (tribe Attini, Subfamily Myrmicinae) and their fungi (primarily tribe Leucocoprini, Basidiomycotina: Agaricales: Lepiotaceae) has existed for 45 to 65 million years (Mueller et al. 1998, 2001, Currie et al. 2003). The mostly Neotropical tribe is monophyletic (Chapela et al. 1994, Meier and Schultz 1996), and consists of the “lower” attines (Mycocepurus + Apterostigma - the apterostigmoid clade) and the “higher” attines (Cyphomyrmex and the remainder of the attines, except for Myrmicocrypta - the attoid clade) (Mueller et al. 1998). The genus Myrmicocrypta appears to be paraphyletic with respect to these two clades (Schultz and Meier 1995).
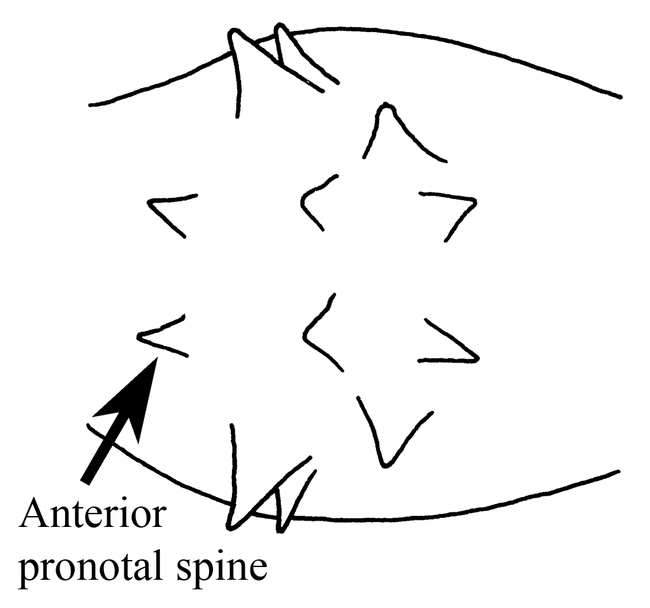
Ants of the genus Mycocepurus are among the most easily recognized among the fungus-growing ants of the tribe Attini. They are monomorphic ants, characterized by the numerous spines on most bodily surfaces, including a pair of occipital spines (Fig. 1), and usually 2 to 3 pairs on the pronotum, 5 to 6 pairs on the mesonotum and 2 pairs on the propodeum (Fig. 3), with the last pair being the most developed. The dorsum of the petiole has 2 pairs of spines (Fig. 6). There is a definite circlet or crown of spines on the promesonotum (Fig. 2). No other genus in the New World has this crown of spines on the promesonotum.
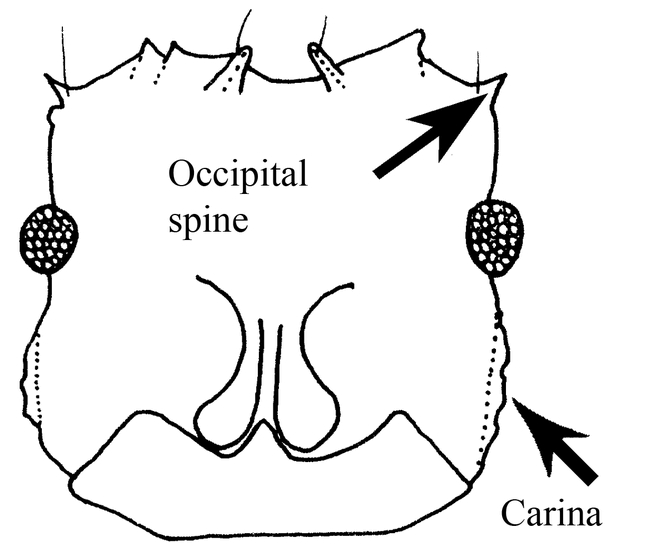
Figure. 1. Head (frontal view) of a worker of Mycocepurus curvispinosus (modified from Mackay, 1998).
Figure 3.
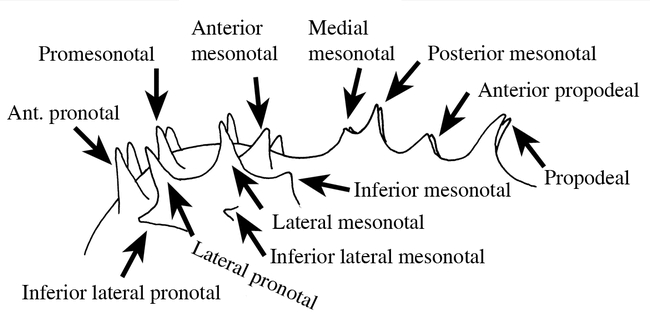
Mesosoma (side view) of a worker of Mycocepurus goeldi, showing the variety of spines.
Figure 6.
Mesosoma and petiole (side view) of the holotype worker of Mycocepurus curvispinosus (modified from Mackay, 1998).
Figure 2.
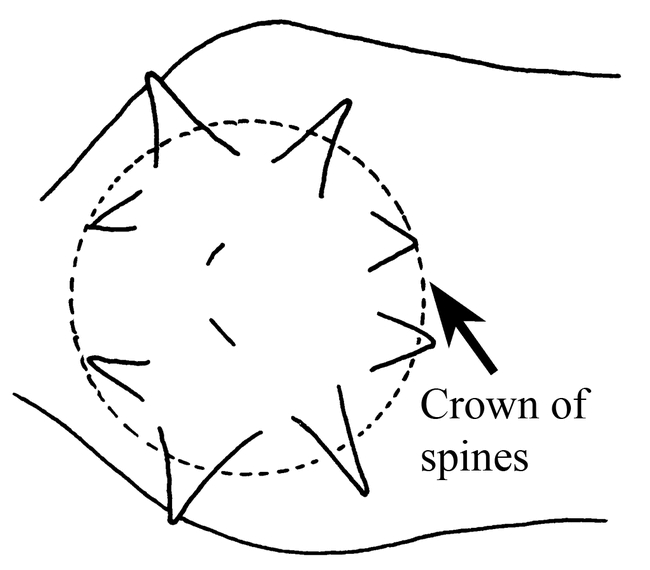
Pronotum and mesonotum (top view) of a worker of Mycocepurus smithii, showing the crown of spines on the promesonotum (modified from Mackay, 1998). All figures are oriented with the anterior end of the ant to the left.
The genus Mycocepurus may occupy a basal, divergent phylogenetic position, based on its unusual mandibular gland secretion (Blum et al. 1981), although other characters (especially characteristics of the larvae) place it with Apterostigma in a distinct lineage (Schultz and Meier 1995, Wetterer et al. 1998).
The genus Mycocepurus uses a variety of organic matter as a substrate for their fungal gardens, ranging from dry leaves and caterpillar dung (Kempf 1963) to fruit matter on the seeds of Hymenaea courbaril (Caesalpiniaceae), which facilitates germination of the plant (Oliveira et al. 1995).
The genus consists of a small group (5 species) of cryptic ants, which are found from México south to Brazil and Argentina (Kempf, 1963, Mackay, 1998). Two of the species are relatively common and widely distributed from México to Argentina and Brazil, as well as the Caribbean (M. smithii Forel), and Guyana, Brazil and Argentina (M. goeldii Forel), one is known from México to Panamá (M. curvispinosus Mackay), another from Nicaragua south to Panamá (M. tardus Weber), and the remaining species is presently known only from the type locality (M. obsoletus Emery from Santarém, Pará, Brazil). Kempf (1963) suggests that M. obsoletus may be a slightly aberrant variant of M. smithii.
Results
The following key will separate the workers of the species of Mycocepurus:
-
1
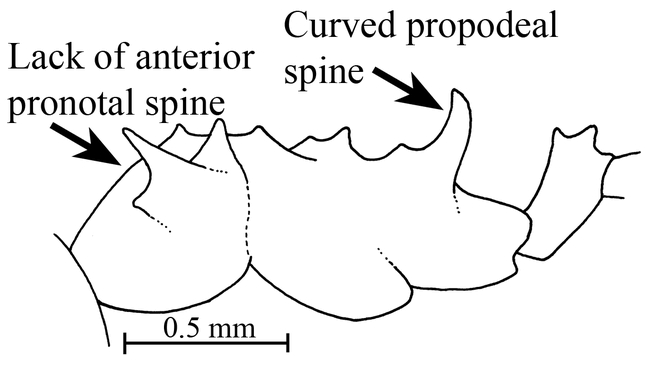
1Pronotum with pair of anterior pronotal spines (Fig. 3), which defines anterior border of crown of spines on promesonotum (Fig. 3); propodeal spines usually straight (Fig. 8)
2
-
-
-Pronotum lacking pair of anterior pronotal spines (Figs. 5 and 6); propodeal spines strongly curved upwards (Fig. 6)
curvispinosus Mackay
-
2(1)
2(1)Pair of promesonotal spines present in center of crown (Fig. 7)
3
-
-
-Promesonotal spines lacking in center of crown, although raised area or low crest may be present (Figs. 2 & 8)
4
-
3(2)
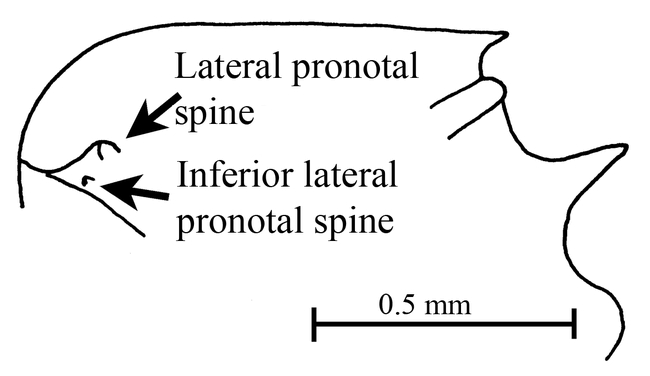
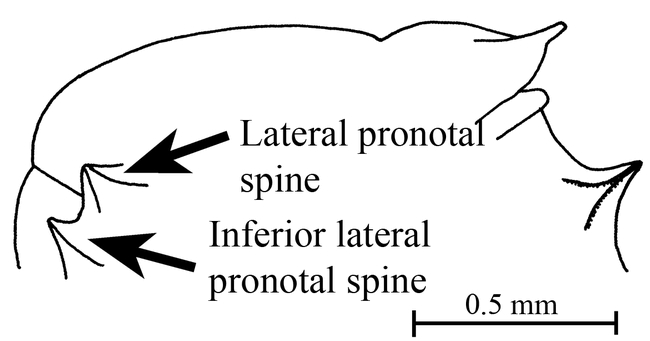
3(2)Inferior lateral pronotal spine poorly developed or absent (Fig. 9); Central America
tardus Weber
-
-
-Inferior lateral pronotal spine well developed (Fig. 3); Guyana, Brazil and Argentina
goeldii Forel
-
4(2)
4(2)Lateral pronotal spines low and blunt (Fig. 8); known only from the type locality in Brazil (Pará: Santarém)
obsoletus Emery
-
-
-Lateral pronotal spines high and sharp (Fig. 7); widely distributed (México: Jalisco, Nayarit, Tabasco south throughout Central America to Argentina and Brazil)
smithii Forel
Figure 8.
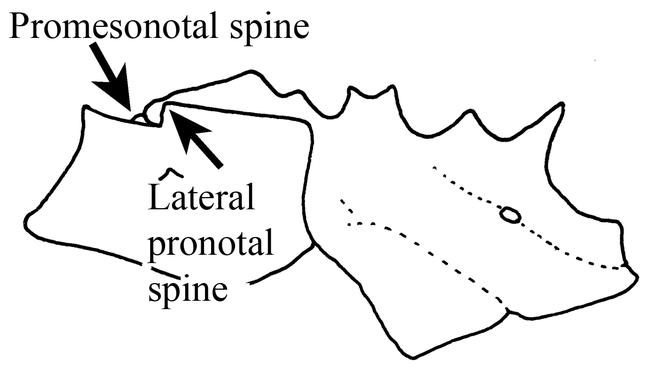
Mesosoma (side view) of the holotype worker of Mycocepurus obsoletus (modified from Emery, 1913).
Figure 5.
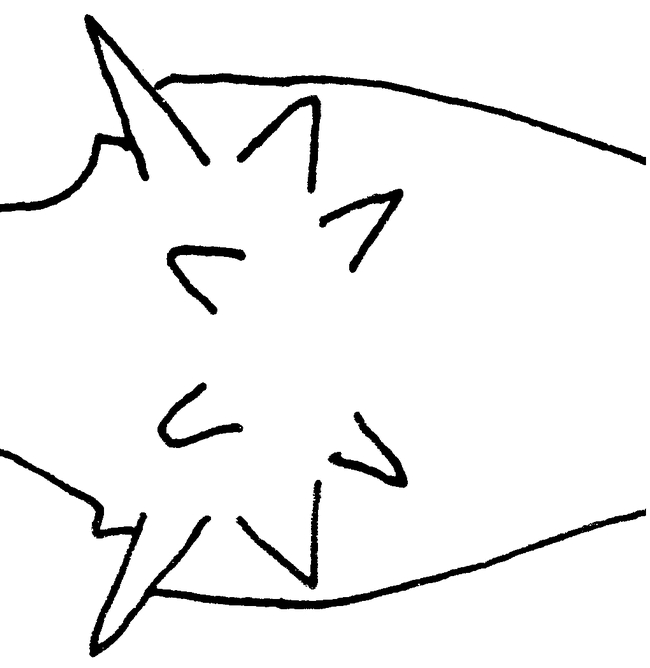
Promesonotum (top view) of a worker of Mycocepurus curvispinosus (modified from Mackay, 1998).
Figure 7.
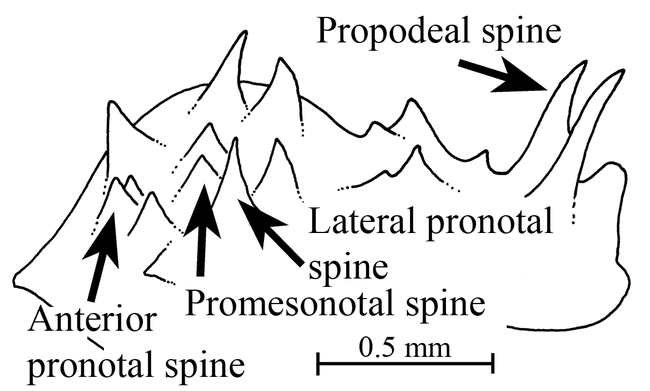
Mesosoma of a worker of Mycocepurus smithii as seen obliquely from the side.
Figure 9.
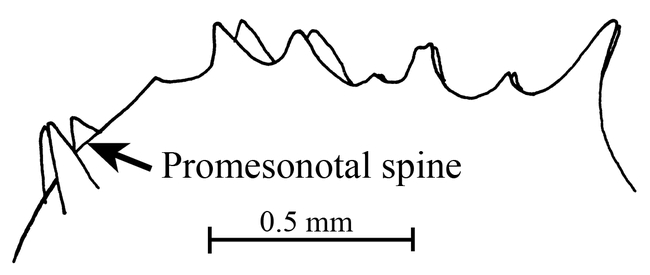
Mesosoma (side view) of a worker of Mycocepurus tardus.
The following key may separate the females of the species of Mycocepurus (the females of M. tardus and M. obsoletus are unknown, and the characters are extrapolated from the workers):
-
1
1México, Central America, and northern South America
2
-
-
-Brazil and Argentina
4
-
2(1)
2(1)Propodeal spines slightly upturned (Fig. 10); lateral pronotal spine large and thick (Fig. 10); inferior lateral tooth tiny
curvispinosusMackay
-
-
-Propodeal spines straight or slightly bent downward (Figs. 11, 12)
3
-
3(2)
3(2)Common and widely distributed; propodeal spines slightly bend downward (Fig. 11); lateral pronotal spine poorly developed (Fig. 11); inferior lateral pronotal spine tiny
smithii Forel
-
-
-Rarely collected, but widely distributed; propodeal spines probably straight (female unknown)
tardus Weber
-
4(1)
4(1)Inferior lateral tooth well developed, about same size as lateral pronotal tooth (Fig. 12)
goeldii Forel
-
-
-Inferior lateral pronotal tooth poorly developed, obsolete or at least much smaller than lateral pronotal tooth (Fig. 11)
5
-
5(4)
5(4)Common and widely distributed (México south to Argentina and Brazil; Caribbean); lateral pronotal spine moderately well developed (Fig. 11)
smithii (Forel)
-
-
-Known only from the state of Pará, Brazil; lateral pronotal spine probably poorly developed or even absent
obsoletus Emery
Figure 10.
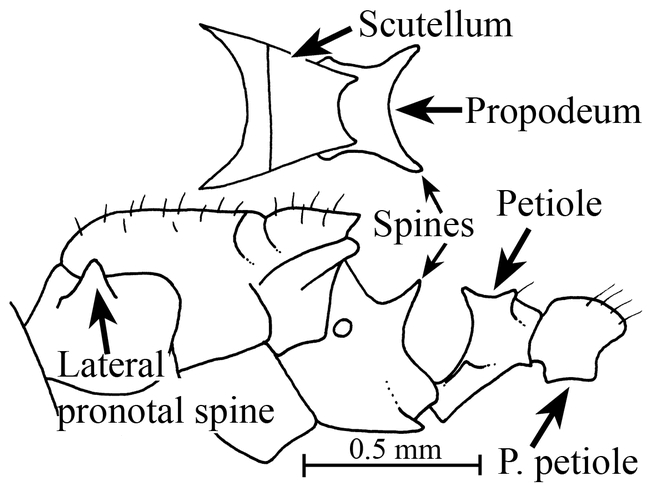
Mesosoma, petiole and postpetiole (side view) of a female of Mycocepurus curvispinosus (Río Frijolito, Panamá, CWEM). The inset shows the scutellum and propodeum as seen from above.
Figure 11.
Mesosoma (side view) of a female of Mycocepurus smithii (Parque Nacional Soberanía, Panamá, CWEM).
Figure 12.
Mesosoma (side view) of a female of Mycocepurus goeldii (Rio Claro, Brazil, CWEM).
Mycocepurus curvispinosus Mackay Figs. 1, 5, 6, 10; Map 1
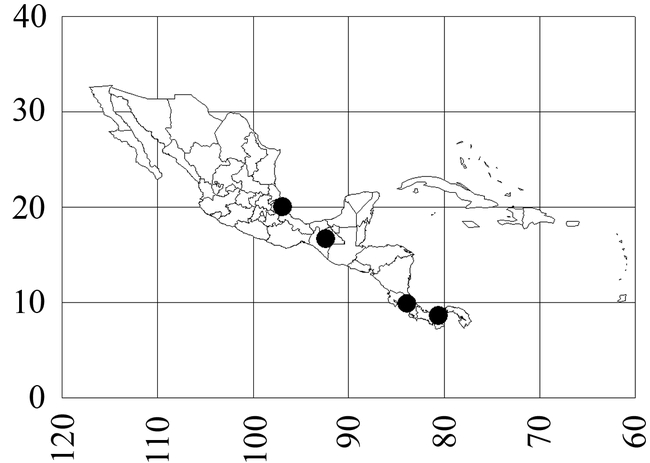
Map 1. Mycocepurus curvispinosus.
Mycocepurus curvispinosus Mackay, 1998:423–424 + Figs. 5, 6 & 9, Costa Rica, Lomas Barbudal, worker
Discussion
The worker of this species is easily recognized, as it lacks the anterior pronotal spines (Fig. 6). Additionally, the propodeal spines are strongly curved upwards (Fig. 6). No other species has this combination of characters.
The female can be recognized by the large lateral pronotal spines, and the slightly upturned propodeal teeth (Fig. 10). It is relatively small (total length slightly more than 2.5 mm), smaller than the females of M. smithii. The first two characteristics easily separate this species from M. smithii and would probably separate it from the unknown females of M. tardus.
Description of female (mm): Head length (HL, anterior edge of median lobe of clypeus to midpoint of posterior margin) 0.71, head width (HW, maximum, at posterior edge of eye) 0.67, scape length (SL, excluding basal condyle) 0.58, eye length (maximum) 0.17, Weber's length (anterior border of pronotum to posterior angle of metapleuron) 1.07, cephalic index (HL/HW X 100) 95, scape index (SL/HL X 100) 81.
Mandible apparently with 5 teeth; frontal lobes expanded, covering insertions of antennae; sides of head nearly parallel, slightly narrowed in region of eyes, which extend past sides of head; posterior margin of head concave, occipital spines poorly developed; scape barely reaches posterior lateral corner; lateral pronotal spine well-developed, with wide base, inferior lateral pronotal spine poorly developed; scutellum angulate posteriorly, overhanging metanotum; propodeal spines well-developed, slightly upturned, with broad base; subpeduncular process poorly developed, peduncle elongated, apex of petiole with two sets of spines.
Anterior margin of clypeus with several, erect hairs, dorsum of head with several, erect hairs, hairs on scape decumbent, dorsum of mesosoma with erect hairs, petiole nearly lacking erect hairs, postpetiole and gaster with abundant, erect hairs, hairs on tibiae mostly suberect.
Entire ant very roughly sculptured, except for mandibles, which are striate and moderately shining, much of rough sculpture, especially on dorsum of gaster, arranged in poorly defined striate.
Ferrugineous red.
The single female specimen is deposited in the collection of William and Emma Mackay (CWEM).
Distribution
México (Veracruz: Reserva Ecológica La Mancha, Chiapas: 24 k SW Cintalapa), Costa Rica (Heredia: Lomas Barbudal); Panamá (Colón, Río Frijolito in El Parque Nacional Soberanía, female).
Habitat
Found in a variety of communities, ranging from slashed and burned areas, sub-deciduous forests to tropical rain forests.
Biology
Most specimens have been collected in pitfall traps, or litter samples. This species apparently lives in nests of M. smithii in México (La Mancha, Veracruz). The workers are slow and timid and forage together with those of M. smithii. A single, dealate female was collected in Panamá on 18-v-1995.
Mycocepurus smithii (Forel) Figs. 2, 7,11; Map 2
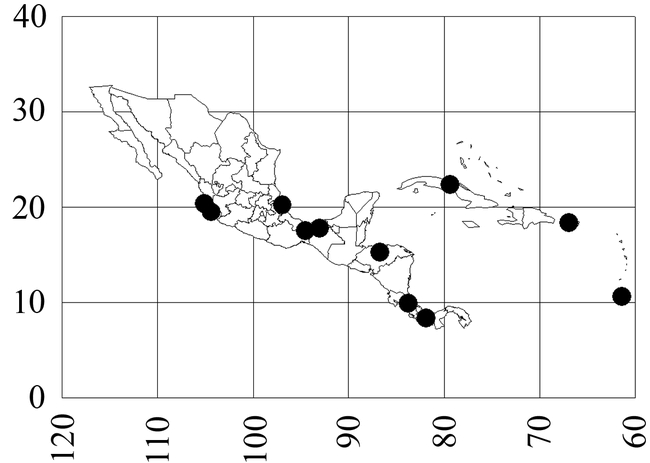
Map 2. Mycocepurus smithii.
Atta (Mycocepurus) smithii Forel, 1893:370, Antilles, worker
Trachymyrmex attaxenus Eidmann, 1936:85, worker, female (Kempf, 1963:425)
Mycocepurus bolivianus Weber, 1938:155 (Kempf, 1963:425)
Atta (Mycocepurus) smithii var. borinquenensis Wheeler, 1907:718 (Kempf, 1963:425)
Mycocepurus smithii var. eucarnitae Forel, 1913:235 (Kempf, 1963:425)
Mycocepurus manni Weber, 1938:156 (Kempf, 1963:425)
Mycocepurus reconditus Borgmeier, 1937:246 (Kempf, 1963:425)
Atta (Mycocepurus) smithii var. tolteca Wheeler, 1907:718 (Kempf, 1963:425)
Mycocepurus smithii var. trinidadensis Weber, 1937:378 (Kempf, 1963:425)
Discussion
This is one of the two species, in which the workers lack the well-developed promesonotal spines in the center of the crown (Fig. 2). Generally there is a very low, sharp crest in this region. In most cases, it can be separated from the other species that lacks these spines, the Brazilian M. obsoletus, on the basis of the distributions. Otherwise, it can be separated by the lateral pronotal spines, which are long and sharp (Fig. 7), not short and blunt as in M. obsoletus (Fig. 8). Specimens that have bumps in the center of the crown of spines, could be confused with M. tardus, but can be usually separated as M. tardus has definite promesonotal spines (Fig. 9).
The females have small lateral pronotal spines, and tiny inferior lateral pronotal spines (Fig. 11). The females are slightly larger than those of M. curvispinosus (∼ 3 mm total length). Additionally the propodeal spines are bent slightly downward (Fig. 11). These characters would easily separate it from females of M. curvispinosus, but may not work to separate it from the unknown females of M. tardus, which occur in the same areas.
The name of this species is often misspelled as M. smithi.
Distribution
México (Jalisco: 39 k N Colima; Nayarit: Tepic, 54 k S Rosamorada; Tabasco: 10 k N Cárdenas; Veracruz: Reserva Ecológica La Mancha, 26 k S Puente Tampico, Arroyo Seco Acayucán), Honduras, Costa Rica, Panamá (Parque Nacional Soberanía near Gamboa), Colombia (Valle: Buga, Sevilla), Cuba, Puerto Rico (Río Grande at El Verde Field Station), Perú, Trinidad, Guianas, Bolivia (Río Bení), Brazil (Pará, Bahia, Mato Grosso, Goias, Minas Gerais, Rio de Janeiro, São Paulo: Ilha dos Buzios, Espírito Santo), Argentina (Formosa).
Habitat
Ranging from open, disturbed areas, burned rain forest, sub-deciduous tropical forests, to tropical rain forests, and moist gullies.
Biology
Kempf (1963) reviewed the biology of this species. It nests only in the soil (Torres 1989), often with the entrance surrounded by a turret. Specimens in México are often found nesting under stones. The nest cavity is very small, only a few cm in diameter, and is connected with several fine, threadlike, tunnels. The nest may be as deep as a meter. There are often several nests in a single area, which suggests a single large nest with multiple entrances. The colonies are polygynous.
The small, sluggish workers carry dry leaves, caterpillar dung (Kempf, 1963) and bat dung (Levins et al. 1973) back to the nest, where they culture their fungus. Populations vary greatly over time, and they may be abundant in some areas (Torres 1989, Majer, 1997).
This species may be associated with the leaf-cutting ant Atta sexdens, and is often found in the same habitat as M. goeldii, with which it has mixed mating swarms, although hybridization apparently does not occur. This species apparently tolerates a number of other species in its nest, including M. curvispinosus, Solenopsis sp. (thief ants) and Acropyga sp. It forages together with M. curvispinosus.
Mycocepurus tardus Weber Fig. 9; Map 3
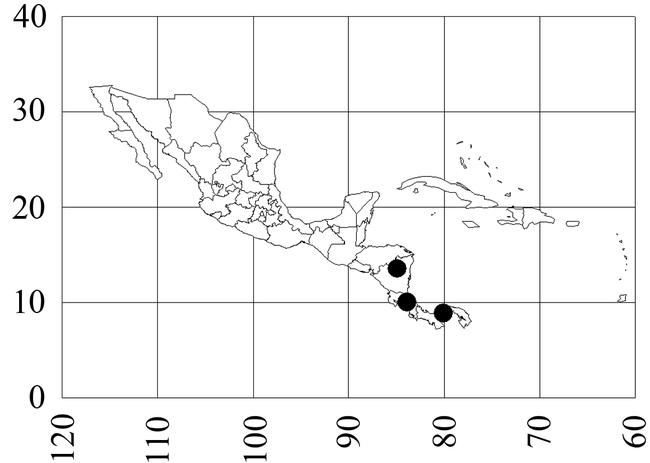
Map 3. Mycocepurus tardus.
Mycocepurus tardus Weber, 1940:416, Panama, worker
Discussion
The worker can be recognized by the pair of promesonotal spines located in the middle of the circlet of teeth on the promesonotum (Fig. 9). It can be separated from the other two species (M. curvispinosus and M. goeldii) which also have these teeth, based on several different characters. It differs from the Mexican and Central American M. curvispinosus, as the anterior pronotal spines (Fig. 3) are well developed (lacking in M. curvispinosus, Fig. 6), and propodeal spines are straight (Fig. 9), and directed somewhat vertically. It can be easily separated from the Brazilian and Argentinean M. goeldii on the basis of the distributions, and in that the inferior lateral pronotal tooth is poorly developed (well-developed in M. goeldii).
Distribution
Nicaragua (Zelaya: Bluefields), Costa Rica (Puntarenas: Cerro Helado), south to Panamá (Panamá: Barro Colorado Island).
Habitat
Lowland rain forest.
Biology
Unknown.
Figure 4.
Promesonotum (top view) of a worker of Mycocepurus goeldii (modified from Mackay, 1998).
Acknowledgments
We thank Robert Anderson for the gift of several of the specimens. Emma Mackay and three anonymous reviewers critically reviewed the manuscript.
Footnotes
Editor's Note
Paper copies of this article will be deposited in the following libraries. Senckenberg Library, Frankfurt Germany; National Museum of Natural History, Paris, France; Field Museum of Natural History, Chicago, Illinois USA; the University of Wisconsin, Madison, USA; the University of Arizona, Tucson, Arizona USA; Smithsonian Institution Libraries, Washington D.C. U.S.A.; The Linnean Society, London, England.
References
- Blum M, Brand J, Amante E. o-aminoacetophenone: identification in a primitive fungus-growing ant (Mycocepurus goeldii) Experentia. 1981;37:816–817. [Google Scholar]
- Borgmeier T. 1937 Formigas novas ou pouco conhecidas da América do sul e Central, principalmente do Brazil (Hym. Formicidae). Archivos do Instituto de Biologia Vegetal, Rio de Janeiro. 3:217–255.+ 5 plates. [Google Scholar]
- Chapela I, Rehner S, Schulz T, Mueller U. Evolutionary history of the symbiosis between fungus-growing ants and their fungi. Science. 1994;266:1691–1694. doi: 10.1126/science.266.5191.1691.0193-4511(1994)266<1691:EHOTSB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Currie CR, Wong B, Stuart A, Schultz T, Rehner S, Mueller U, Sung G, Spatafora J, Straus N. Ancient tripartite Coevolution in the attine ant-microbe symbosis. Science. 2003;299:386–388. doi: 10.1126/science.1078155.0193-4511(2003)299<0386:ATCITA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Eidmann H. Ökologisch-faunistische Studien an südBrazilianischen Ameisen. Arbeiten über Physiologische und angewandte Entomologie aus Berlin-Dahlem. 1936;3:81–114.0365-5326(1936)003<0081:KSASA>2.0.CO;2 [Google Scholar]
- Emery C. Études sur les Myrmicinae V. Les genres des Attini; descriptions de nouvelles formes de Mycocepurus et de Myrmicocrypta. Annales de la Société Entomologique de Belgique. 1913;57:250–262.0774-5915(1913)057<0250:TSLMVL>2.0.CO;2 [Google Scholar]
- Forel A. Formicides de l'Antille St. Vincent. Récoltées par Mons. H. H. Smith. Transactions of the Entomological Society of London. 1893;1893:333–418. [Google Scholar]
- Forel A. Fourmis d'Argentine du Brésil, du Guatemala & de Cuba reçues de M. M. Bruch, Prof. v. Ihering, Mlle Báez, M. Peper et M. Rovereto. Bulletin de la Société Vaudoise des Sciences Naturelles. 1913;49:203–250.0037-9603(1913)049<0203:FDDBDG>2.0.CO;2 [Google Scholar]
- Kempf W. A review of the ant genus Mycocepurus Forel, 1893 (Hymenoptera: Formicidae) Studia Entomologica. 1963;6:417–432. [Google Scholar]
- Levins R, Pressick M, Heatwole H. Coexistence patterns in insular ants. American Scientist. 1973;61:463–472.0003-0996(1973)061<0463:CPIIA>2.0.CO;2 [Google Scholar]
- Mackay W. Dos especies nuevas de hormigas de la tribu Attini de Costa Rica y México: Mycetosoritis vinsoni y Mycocepurus curvispinosus (Hymenoptera: Formicidae) Revista de Biología Tropical. 1998;46:421–426.0034-7744(1998)046<0421:DENDHD>2.0.CO;2 [Google Scholar]
- Major J, Delabie J, McKenzie N. Ant litter fauna of forest, forest edges and adjacent grassland in the Atlantic rain forest region of Bahia, Brazil. Insectes Sociaux. 1997;44:255–266.0020-1812(1997)044<0255:ALFOFF>2.0.CO;2 [Google Scholar]
- Meier R, Schultz T. Pilzzucht und Blattschneiden bei Ameisen-Präadaptionen und evolutive Trends. Sitzungsberichte der Gesellschaft Naturforschender Freunde zu Berlin. 1996;35:57–76. [Google Scholar]
- Mueller UG, Rehner S, Schultz T. The evolution of agriculture in ants. Science. 1998;281:2034–2038. doi: 10.1126/science.281.5385.2034.0193-4511(1998)281<2034:TEOAIA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mueller UG, Schultz T, Currie C, Adams R, Malloch D. The origin of the attine ant-fungus mutualism. The Quarterly Review of Biology. 2001;76:169–197. doi: 10.1086/393867.0033-5770(2001)076<0169:TOOTAA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Oliveira P, Galetti M, Pedroni F, Morellato L. Seed cleaning by Mycocepurus goeldii ants (Attini) facilitates germination in Hymenaea courbaril (Caesalpiniaceae) Biotropica. 1995;27:518–522.0006-3606(1995)027<0518:SCBMGA>2.0.CO;2 [Google Scholar]
- Schultz TR, Meier R. A phylogenetic analysis of the fungus-growing ants (Hymenoptera: Formicidae: Attini) based on morphological characters of the larvae. Systematic Entomology. 1995;20:337–370.0307-6970(1995)020<0337:APAOTF>2.0.CO;2 [Google Scholar]
- Torres J. The status of the fungus-grower ants (Hymenoptera: Formicidae) in Puerto Rico and adjacent islands. Journal of Agriculture of the University of Puerto Rico. 1989;73:401–403.0041-994X(1989)073<0401:TSOTFA>2.0.CO;2 [Google Scholar]
- Weber N. 1937 The biology of the fungus growing ants. Part. l. New forms. Revista de Entomologia. 7:378–409.+ 11 Figs. [Google Scholar]
- Weber N. 1938 The biology of the fungus-growing ants. Part IV. Additional new forms; Part V. The Attini of Bolivia. Revista de Entomologia. 9:154–206.+ 21 Figs. [Google Scholar]
- Weber N. 1940 The biology of the fungus-growing ants. Part VI. Key to Cyphomyrmex, new Attini and a new guest ant. Revista de Entomologia. 11:406–427.+ 16 Figs. [Google Scholar]
- Wetterer JK, Schultz T, Meier R. Phylogeny of fungus-growing ants (Tribe Attini) based on mtDNA sequence and morphology. Molecular Phylogenetics and Evolution. 1998;9:42–47. doi: 10.1006/mpev.1997.0466.1055-7903(1998)009<0042:POFATA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wheeler W. 1907 The fungus-growing ants of North America. Bulletin of the American Museum of Natural History. 23:669–807.+ 5 plates and 31 Figs. [Google Scholar]


