Abstract
The formation of filamentous appendages on Salmonella typhimurium has been implicated in the triggering of bacterial entry into host cells (C. C. Ginocchio, S. B. Olmsted, C. L. Wells, and J. E. Galán, Cell 76:717–724, 1994). We have examined the roles of cell contact and Salmonella pathogenicity island 1 (SPI1) in appendage formation by comparing the surface morphologies of a panel of S. typhimurium strains adherent to tissue culture inserts, to cultured epithelial cell lines, and to murine intestine. Scanning electron microscopy revealed short filamentous appendages 30 to 50 nm in diameter and up to 300 nm in length on many wild-type S. typhimurium bacteria adhering to both cultured epithelial cells and to murine Peyer’s patch follicle-associated epithelia. Wild-type S. typhimurium adhering to cell-free culture inserts lacked these filamentous appendages but sometimes exhibited very short appendages which might represent a rudimentary form of the cell contact-stimulated filamentous appendages. Invasion-deficient S. typhimurium strains carrying mutations in components of SPI1 (invA, invG, sspC, and prgH) exhibited filamentous appendages similar to those on wild-type S. typhimurium when adhering to epithelial cells, demonstrating that formation of these appendages is not itself sufficient to trigger bacterial invasion. When adhering to cell-free culture inserts, an S. typhimurium invG mutant differed from its parent strain in that it lacked even the shorter surface appendages, suggesting that SPI1 may be involved in appendage formation in the absence of epithelia. Our data on S. typhimurium strains in the presence of cells provide compelling evidence that SPI1 is not an absolute requirement for the formation of the described filamentous appendages. However, appendage formation is controlled by PhoP/PhoQ since a PhoP-constitutive mutant very rarely possessed such appendages when adhering to any of the cell types examined.
Salmonella species are an important group of enteric pathogens which penetrate the intestinal epithelial barrier to initiate disease. The specialized antigen-sampling M cells present in the follicle-associated epithelium overlying Peyer’s patches are the preferential site of Salmonella typhimurium invasion in vivo (10, 33), although the invasion of enterocytes has also been described (50, 52). S. typhimurium invasion of epithelial cells in vivo and in vitro is associated with prominent cellular changes including localized degeneration of microvilli, cytoskeletal reorganization, and membrane remodelling to produce “membrane ruffles” (14, 16, 17) and contraction of the perijunctional actinomyosin ring (30). These responses, and bacterial invasion itself, are believed to be triggered by the generation of bacterially mediated signal transduction events within the host cell (8, 18), although the precise mechanisms involved remain unclear.
The molecular and genetic bases for Salmonella adherence to and invasion of epithelial cells are distinct and complex. A large number of Salmonella genes are required for entry into cultured epithelial cells (2, 5, 11, 12, 15, 19–21, 34, 39, 45, 49). Many of these genes are located in a 40-kb “pathogenicity island” at centisome 63 on the S. typhimurium chromosome (18, 43), which includes the inv-spa and prgHIJK loci. This region has been termed Salmonella pathogenicity island 1 (SPI1) to distinguish it from the recently described second S. typhimurium pathogenicity island, SPI2 (25, 26, 44, 48). Genes within the SPI1 loci encode components of a dedicated type III protein secretion system homologous to those involved in secretion and/or surface presentation of virulence factors by a number of animal and plant pathogens including enteropathogenic and enterohemorrhagic Escherichia coli and Shigella, Yersinia, Xanthomonas, Pseudomonas, and Erwinia species (6, 7, 13, 18, 23, 24, 28, 29, 38, 47). Some of these genes also exhibit homology to genes involved in flagellar export and assembly (18, 38). Out of about 30 proteins encoded by SPI1, at least 16 constitute the type III secretion apparatus. Components of this apparatus include inner membrane protein InvA (20), cytoplasmic ATPase InvC (12), InvG, which probably forms a protein-conducting channel in the outer membrane (34), InvE (21), whose subcellular location is not known, and PrgH (45), which by homology to MxiG from Shigella flexneri (1) is likely to be associated with both membranes. The targets of the SPI1-encoded type III secretion system include the Salmonella-secreted proteins A to D (SspA to -D [SipA to -D, respectively]) which show substantial homology with the Shigella Ipa proteins and which are encoded within SPI1 (27, 35, 36). With the exception of SspA (SipA) each of the Ssp (Sip) proteins are required for bacterial entry into cultured epithelial cells (27, 35, 36). Expression of the SPI1-encoded type III secretion system and epithelial invasion by S. typhimurium have been shown to be controlled by the PhoP/PhoQ regulatory system (5, 27, 45) and other regulatory factors including SirA (32) and HilA (3, 4, 39, 45).
Ginocchio et al. (22) showed that the adherence of S. typhimurium to cultured epithelial cells is associated with the transient appearance of filamentous appendages on the surfaces of the bacteria. Noninvasive S. typhimurium strains carrying mutations in genes encoding proteins which are part of the type III secretion apparatus exhibited different patterns of expression of these surface appendages compared to the wild type. Specifically, invC and invG mutants lacked these appendages while noninvasive strains carrying mutations in invA or invE produced appendages which were longer than those on the parent strain and which did not disappear after prolonged adherence. These authors therefore concluded that the induction and the subsequent shedding or contraction of the cell contact-stimulated surface appendages depend on a functional type III secretion apparatus and play a role in the process of invasion. Consequently, it has been speculated (27, 55) that protein secretion via the type III system encoded by SPI1 might participate in the assembly of these surface appendages.
In the present study we have further examined the triggering of filamentous-appendage formation on S. typhimurium by comparing the distribution of appendages on S. typhimurium grown in the absence of cells with those on bacteria in contact with Madin-Darby canine kidney (MDCK) cells, human intestinal Caco-2 cells, or murine Peyer’s patches in vivo. We have also tested the hypothesis that the SPI1-encoded protein secretion system and the PhoP/PhoQ regulon are determinants of appendage formation by examining the distributions of the appendages on invasion-deficient S. typhimurium strains with mutations in invA, invG, prgH, and sspC (sipC) and on an S. typhimurium mutant which constitutively expresses PhoP-activated (pag) genes and in which PhoP-repressed (prg) genes are constitutively repressed (PhoPc mutant).
MATERIALS AND METHODS
Bacterial strains and culture.
The S. typhimurium strains listed in Table 1 were grown as previously described (9). Briefly, a single colony grown on Luria-Bertani (LB) agar was inoculated into 2 ml of LB broth and incubated with agitation at 37°C for 7 h. From this starter culture, 103 bacteria were inoculated into 5 ml of LB broth (in a sealed 6-ml vial) and grown as a static culture overnight (16 h) at 37°C. For the culture of mutant strains the LB broth was supplemented with 60 μg of kanamycin or 10 μg of ampicillin per ml, as appropriate.
TABLE 1.
S. typhimurium strains used in this study
Infection of cultured cells.
MDCK strain II cells (passages 116 to 123) were grown in Eagle’s minimal essential medium supplemented with 2 mM l-glutamine, 10% fetal calf serum, 1% nonessential amino acids, and 100 U of kanamycin per ml at 37°C in a humidified atmosphere of 5% CO2. Human colonic adenocarcinoma cell line Caco-2 (passages 100 to 111) was grown in Dulbecco’s modified Eagle’s medium containing 4.5 g of glucose/liter and supplemented with 2 mM l-glutamine, 10% fetal calf serum, 1% nonessential amino acids, and 60 μg of gentamicin per ml at 37°C in a humidified atmosphere of 5% CO2. When confluent, cells were passaged and seeded at high density (0.5 × 106 to 1.0 × 106/cm2) onto tissue culture inserts containing an inorganic membrane (growth area, 0.5 cm2; Anocell; Nunc, Roskilde, Denmark). On the third day after seeding for MDCK cells and the fourteenth after seeding for Caco-2 cells, the medium was replaced by a modified Krebs buffer (137 mM NaCl, 5.4 mM KCl, 1 mM MgSO4, 0.3 mM KH2PO4, 0.3 mM NaH2PO4, 2.4 mM CaCl2, 10 mM glucose, and 10 mM Tris; adjusted to pH 7.4 at 37°C with HCl). After equilibration of the inserts in this medium for 30 min at 37°C in air, the apical bathing medium was replaced with 0.5 ml of the same medium to which S. typhimurium cells had been added 60 min previously to a final total count of 108/ml. The monolayers were then maintained at 37°C in air for 60 min. To examine bacterial morphology in the absence of cell contact, tissue culture inserts without cells were incubated with MDCK cell culture medium and infected with S. typhimurium strains as described above.
Infection of mouse Peyer’s patches.
Ligated jejunal and ileal gut segments containing Peyer’s patches were created in anesthetized adult female BALB/c mice and infected as described previously (9, 10) with S. typhimurium strains which had been grown as described above, pelleted, washed twice, and resuspended in phosphate-buffered saline (PBS) at 3 × 109 bacteria/ml. The gut loops were harvested after 60 min, and the mice were culled by cervical dislocation.
Morphological studies.
Tissue culture inserts were washed extensively in PBS, fixed in 2% glutaraldehyde (in 100 mM sodium phosphate buffer, pH 7.3) at 4°C for at least 16 h, and processed for scanning electron microscopy (SEM) as described previously (31). Tissues harvested from infected gut loops were pinned flat, mucosal surface uppermost, on cork boards, rinsed thoroughly in PBS, fixed in glutaraldehyde, and processed for SEM as described above. After dehydration, critical-point drying, and gold sputter coating, the samples were examined with a Cambridge S240 scanning electron microscope. Adherent bacteria were examined for the presence of surface features. Bacteria with and without surface appendages were counted in randomly selected fields by two independent observers applying a selection criterion of six appendages per bacterium. Results were pooled from observations made on 4 to 11 cell monolayers, 2 to 4 cell-free culture supports, or 2 to 4 Peyer’s patches.
Quantification of S. typhimurium invasion and adherence in vitro.
After incubation with S. typhimurium strains for 60 min at a final total count of 2 × 107 bacteria/ml, cell monolayers on filter units were washed six times in PBS to remove nonadherent bacteria and transferred to PBS at 4°C to halt bacterial invasion. S. typhimurium adherence and invasion were then quantified by differential immunocytochemical staining as described previously (31). Briefly, the monolayers were incubated sequentially (at 4°C) with goat anti-Salmonella antibodies and fluorescein isothiocyanate (FITC)-conjugated rabbit anti-goat immunoglobulin to label extracellular bacteria. After permeabilization in methanol, the monolayers were incubated with anti-Salmonella antibodies and tetramethyl rhodamine isothiocyanate (TRITC)-conjugated rabbit anti-goat immunoglobulin at room temperature to label extracellular and intracellular bacteria. Monolayers were then examined with an epifluorescent microscope (Nikon Diaphot or Leica DM RBE). Counts of adherent (FITC-labelled) and total (TRITC-labelled) bacteria associated with the monolayers were made by two independent observers and used to calculate the number of invading bacteria per unit area. Results were expressed as numbers of invading bacteria per cell by using the measured cell densities of 106 MDCK cells per cm2 and 4 × 105 Caco-2 cells per cm2.
RESULTS
Invasion of cultured epithelia by S. typhimurium strains.
Immunocytochemical staining of S. typhimurium allowed the quantification of invading bacteria (Table 2). As previously described for MDCK or other cell lines, the S. typhimurium mutant strains examined, i.e., invA (20), invG (40), sspC (28), PhoPc (5), and prgH (5) strains, were all severely deficient for invasion of both MDCK and Caco-2 cells compared to their parent strains (Table 2).
TABLE 2.
Invasion of cell monolayers by S. typhimurium strains
| Strain | Invasiona of:
|
|
|---|---|---|
| MDCK cells | Caco-2 cells | |
| SL1344 | 81.56 ± 41.50 (6) | 53.16 ± 5.47 (4) |
| EE638 | 0.14 ± 0.09 (3) | 0.12 ± 0.07 (4) |
| SR11 | 54.40 ± 4.80 (5) | 42.48 ± 14.34 (4) |
| SB111 | 0.15 ± 0.10 (5) | 0.13 ± 0.09 (7) |
| ATCC 14028s | 48.35 ± 2.60 (6) | 65.19 ± 7.74 (8) |
| CS022 | 0.32 ± 0.00 (3) | 0.48 ± 0.08 (5) |
| IB040 | 0 (9) | 0.10 ± 0.06 (5) |
| TNP-5 | 32.40 ± 2.60 (6) | 24.60 ± 2.81 (6) |
| 83 | 0.08 ± 0.03 (5) | 0.04 ± 0.04 (6) |
Invasion was measured by differential immunocytochemical staining of adhering and invading bacteria. Results are expressed as mean numbers of bacteria per 100 epithelial cells ± standard errors of the means. Figures in parentheses are the numbers of individual monolayers examined.
Contact with epithelial cells promotes formation of short filamentous appendages by wild-type S. typhimurium.
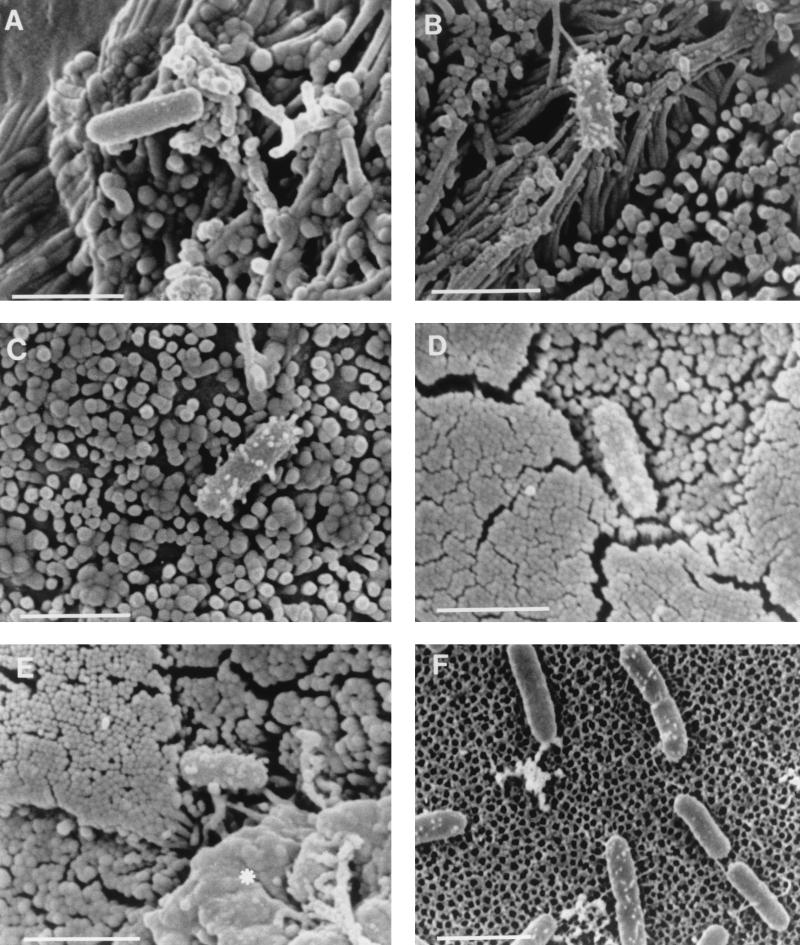
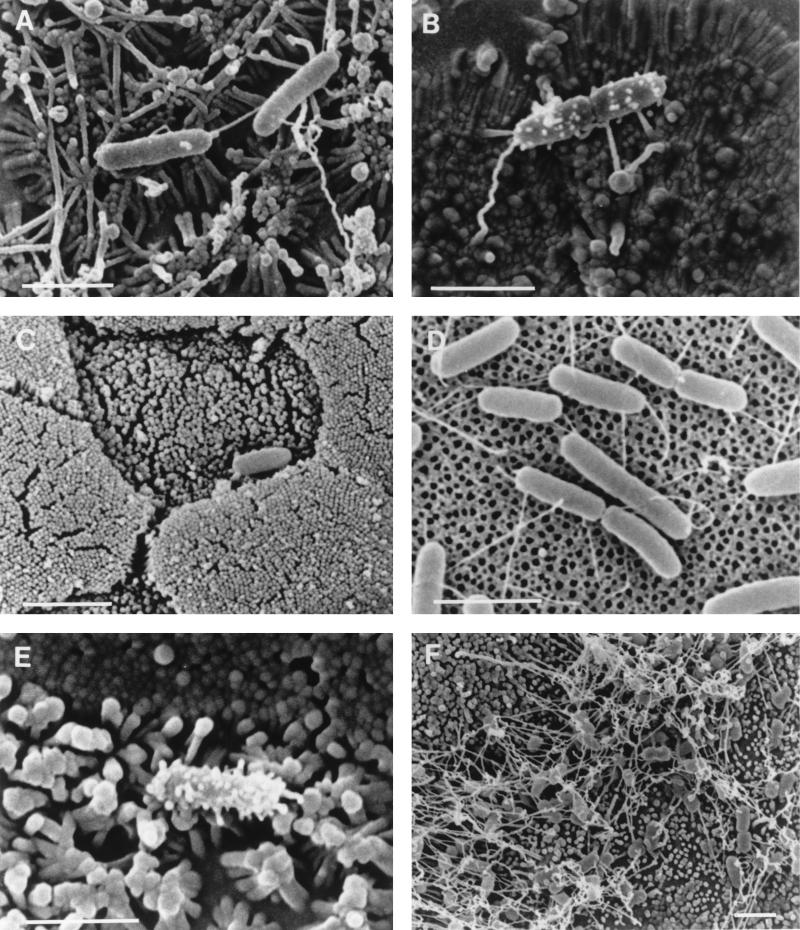
An examination of cell monolayers infected with wild-type S. typhimurium SL1344 by SEM revealed that some adherent bacteria possessed numerous prominent surface features which were heterogeneous in morphology, ranging from small surface bumps to filaments 30 to 50 nm in diameter and up to 300 nm in length. The approximately 300-nm short filamentous appendages were morphologically indistinguishable from those described by Ginocchio et al. (22) (Fig. 1A to C). A quantitative analysis of the expression of these short filamentous appendages of S. typhimurium adhering to cells showed that they were present on a greater proportion of bacteria adhering to Caco-2 cells (62%) than to MDCK cells (15%) (Table 3). Bacteria with short filamentous appendages were observed both on cells displaying normal brush border morphology and on membrane ruffles. Similar filamentous appendages were observed on a proportion of S. typhimurium SL1344 cells adhering to the follicle-associated epithelium (M cells and columnar enterocytes) after infection of murine Peyer’s patches (Fig. 1D and E). S. typhimurium SL1344 cells attached to cell culture inserts treated with MDCK culture medium lacked the prominent filamentous appendages observed on some bacteria adhering to cells, but approximately 15% possessed some very short surface appendages which were indistinguishable from those seen on a proportion of cell-associated bacteria (Fig. 1F; Table 4). These very short appendages may represent a rudimentary form of the filamentous appendages seen after cell contact. A SEM analysis of other wild-type S. typhimurium strains adhering to epithelial cells or to culture inserts revealed distributions of short filamentous appendages similar to those described for S. typhimurium SL1344 (Tables 3 and 4).
FIG. 1.
Scanning electron micrographs of S. typhimurium SL1344 adhering to Caco-2 cells (A and B), MDCK cells (C), murine Peyer’s patches (D and E) and cell-free culture supports (F). A proportion of wild-type S. typhimurium cells adhering to target cells formed numerous short filamentous appendages when they adhered to either Caco-2 cells (B) or MDCK cells (C), while others lacked such appendages (A). Similar appendages were present on some S. typhimurium SL1344 cells that adhered to M cells (D and E) after infection of murine Peyer’s patches in ligated intestinal loops. The asterisk in panel E indicates an area of membrane remodelling associated with bacterial invasion. A small proportion of S. typhimurium SL1344 cells attached to cell-free culture supports possessed appendages which exhibited a similar distribution but which were shorter than many of those on cell-adhered bacteria (F). Bars, 2 μm.
TABLE 3.
Filamentous appendage expression by S. typhimurium adhering to cell monolayers
| Strain | Short filamentous appendage expressiona (%) on:
|
|
|---|---|---|
| MDCK cells | Caco-2 cells | |
| SL1344 | 15.4 (20/130) | 62.5 (158/253) |
| EE638 | 32.2 (73/226) | 98.2 (111/114) |
| SR11 | 56.4 (124/220) | 79.3 (172/219) |
| SB111b | 57.8 (70/121) | 85.0 (125/147) |
| ATCC 14028s | 65.5 (150/229) | 64.6 (170/263) |
| CS022 | 0.0 (0/153) | 1.2 (2/172) |
| IB040b | 3.3 (4/121) | 43.0 (49/114) |
| TNP-5 | 46.5 (128/275) | 69.5 (180/259) |
| 83 | 25.6 (45/176) | 73.3 (115/157) |
The expression of short filamentous appendages by S. typhimurium cells adhering to cultured epithelial cells was measured as the proportions of bacterial cells observed by SEM to express at least six such appendages. Numbers in parentheses are the numbers of bacterial cells expressing short filamentous appendages divided by the numbers of bacterial cells sampled.
The invA (SB111) and prgH (IB040) mutants exhibited heterogeneous adherence patterns, with many bacteria forming aggregates composed of bacteria which almost invariably lacked the short filamentous appendages examined here. Data for these strains refer only to those bacteria which adhered outside such aggregates.
TABLE 4.
Expression of rudimentary appendages by S. typhimurium adhering to cell culture inserts
| Strain | % of cells exhibiting rudimentary appendagesa |
|---|---|
| SL1344 | 14.6 (35/239) |
| EE638 | 13.4 (17/126) |
| SR11 | 15.1 (41/271) |
| SB111 | 24.6 (34/138) |
| ATCC 14028s | 15.3 (40/261) |
| CS022 | 1.3 (1/75) |
| IB040 | 18.4 (25/136) |
| TNP-5 | 8.3 (30/360) |
| 83 | 0.0 (0/126) |
The expression of very short (rudimentary) appendages by S. typhimurium in the absence of cells was measured as the proportion of bacterial cells observed by SEM to express at least six such appendages. Numbers in parentheses are the numbers of bacteria exhibiting rudimentary appendages divided by the numbers of bacteria sampled.
Invasion-deficient S. typhimurium strains carrying mutations in SPI1 genes form short filamentous appendages on contact with epithelia.
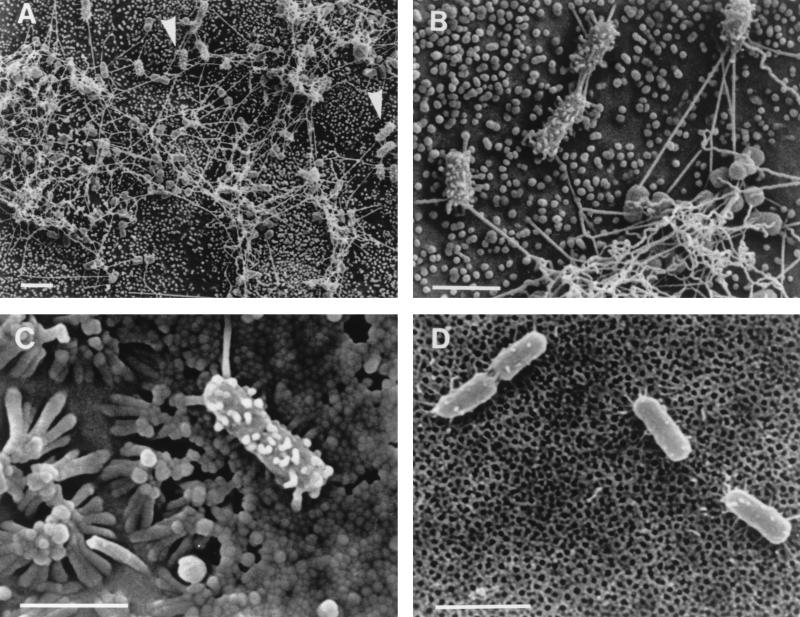
An examination of Caco-2 and MDCK monolayers infected with the S. typhimurium invA mutant SB111 revealed a heterogeneous pattern of adherence (Fig. 2A) which was quite different from those of its parent, SR11, and other wild-type strains. The majority of adherent S. typhimurium SB111 cells were in dense aggregates linked by long (3- to 5-μm) filaments which were sparsely distributed on the surfaces of bacteria. Almost all bacteria in these aggregates (approximately 99%) lacked the short filamentous appendages described above and by Ginocchio et al. (22) (Fig. 2B). Adherent S. typhimurium SB111 cells which were independent of the aggregates generally (85% for Caco-2 cells; 58% for MDCK cells; Table 3) had a dense covering of short filamentous appendages (Fig. 2B) which were similar to those on cell-adhered wild-type bacteria. As observed with S. typhimurium SL1344 and other wild-type strains including SR11, a significant proportion (25%) of SB111 cells expressed very short surface appendages in the absence of cells; these appendages were less prominent and sparser than those on bacteria adhering to cells (Fig. 2D; Table 4).
FIG. 2.
Scanning electron micrographs of invA mutant S. typhimurium SB111 adhering to MDCK cells (A and B), Caco-2 cells (C), and cell-free culture supports (D). A heterogeneous adherence pattern is observed after infection of MDCK cell monolayers with S. typhimurium SB111 (A). The majority of bacteria formed aggregates linked by long filaments and lacked the shorter filamentous appendages observed on wild-type bacteria. Isolated bacteria (arrowheads in panel A) were largely proficient in the formation of these short filamentous appendages. The marked difference in surface morphology between the two bacterial subtypes is clear at higher magnification (B). Similar patterns of adherence and appendage expression were observed when S. typhimurium SB111 adhered to Caco-2 cells, with individual bacteria expressing short filamentous appendages (C). The appendages elaborated by S. typhimurium SB111 were generally thicker than those observed on wild-type strains (compare panels B and C with Fig. 1B and C). S. typhimurium SB111 adhering to cell-free culture supports produced much smaller and sparser appendages (D). Bars, 2 μm.
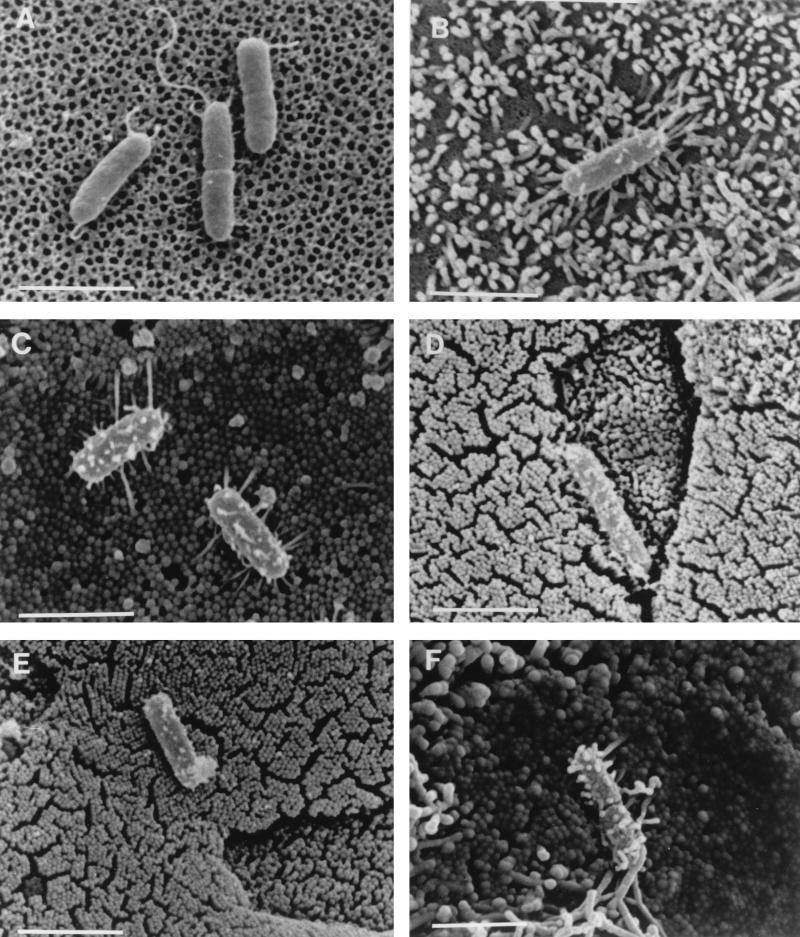
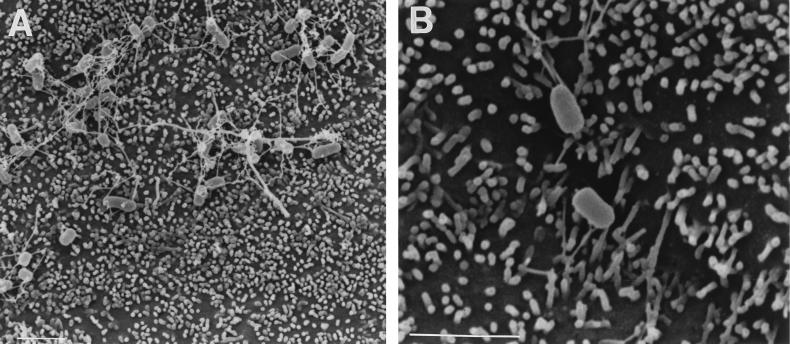
To investigate the suggested involvement of InvG in the formation of short filamentous appendages (22), we examined the S. typhimurium invG mutant 83, which is derived from strain TNP-5 (Table 1). Although this mutant differed from wild-type S. typhimurium, its parent, and invA mutant SB111 in that it lacked even very short appendages in the absence of cells (Fig. 3A; Table 4), in the presence of cells (Fig. 3B and C) it resembled the wild-type strains and TNP-5. A quantitative analysis revealed that approximately 26% of S. typhimurium 83 cells expressed short filamentous appendages on MDCK cells and that this proportion increased to 73% on Caco-2 cells (Table 3). Filamentous appendage formation by S. typhimurium 83 on MDCK cells was variable; the proportion of bacteria with appendages ranged from 8 to 53% in individual experiments. The pattern of appendage formation was less variable on Caco-2 cells, where the proportion of bacteria exhibiting filamentous appendages ranged from 70 to 82%. This mutant did not display the heterogeneous pattern of adherence associated with invA mutant SB111 on either cell line. Similar surface appendages were observed on invG mutant strain 83 after infection of murine Peyer’s patches (Fig. 3D and E), where short filamentous appendages were present on approximately 18% (24 of 130 cells) of the bacteria adhering to M cells and approximately 6% (6 of 99 cells) of those adhering to enterocytes. Because our finding that an invG mutant exhibits no defect in the formation of the described cell contact-induced filamentous appendages does not agree with data on a different invG mutant (22), we also examined the latter strain, SB161, under our experimental conditions. When adhering to MDCK cells, SB161 exhibited a heterogeneous adherence pattern similar to that observed with the invA mutant SB111 but not to those observed with the invG mutant originally examined (strain 83) and with wild-type bacteria (Fig. 4A). The majority of bacteria adhered in aggregates which were linked by longer filaments; the bacteria within these aggregates lacked the described surface appendages (Fig. 4A). In addition almost all of the bacteria which appeared to adhere independently of these aggregates lacked the short filamentous appendages focused on in this study (Fig. 4B). Thus, strain SB161 exhibits phenotypic differences from the wild type (SR11) that extend to its pattern of adherence as well as to the production of contact-dependent appendages.
FIG. 3.
Scanning electron micrographs of the S. typhimurium strains carrying mutations in invG (strain 83; A to E) or in sspC (strain EE638; F). When adhering to cell-free culture supports (A), S. typhimurium 83 lacked any of the short filamentous appendages observed on a proportion of cells of wild-type and invA mutant S. typhimurium strains (see, e.g., Fig. 1F and 2D), although longer filamentous appendages were occasionally observed. When adhering to MDCK cells (B) or Caco-2 cells (C), a variable proportion of S. typhimurium 83 cells formed numerous short filamentous appendages indistinguishable from those seen on wild-type bacteria. Similar surface features were also present on some S. typhimurium 83 cells adhering to M cells (D) and enterocytes (E) after infection of murine Peyer’s patches in ligated intestinal loops. S. typhimurium EE638 also produced short filamentous appendages when adhering to MDCK or Caco-2 cells (F); these appendages generally more closely resembled those observed on S. typhimurium invA mutant SB111. Bars, 2 μm.
FIG. 4.
Scanning electron micrographs of invG mutant S. typhimurium strain SB161 adhering to MDCK cells. SB161 exhibited a heterogeneous adherence pattern after infection of MDCK cell monolayers (A). Adherent bacteria mainly formed aggregates linked by long filaments and lacked short filamentous appendages (A). Most bacteria which appeared to adhere independently of the aggregates also lacked short filamentous appendages (B). Bars, 2 μm.
To directly investigate the possible involvement of secreted proteins SspA, SspC, and SspD (SipA, SipC, and SipD) in appendage formation, we examined noninvasive S. typhimurium sspC (sipC) mutant EE638 (27), which was constructed from SL1344 by a transposon insertion in sspC and which does not secrete any of these proteins. An analysis of this mutant after infection of cells revealed that approximately 32% of the bacteria on MDCK cells and 98% on Caco-2 cells exhibited filamentous appendages of the type described above (Fig. 3F; Table 3). In the absence of epithelial cells S. typhimurium EE638 appeared similar to the wild type and to invA mutant SB111, with a minor proportion of EE638 cells (approximately 13%) possessing a few rudimentary appendages (Table 4).
A PhoP-constitutive S. typhimurium strain is defective in the formation of short filamentous appendages.
S. typhimurium CS022 (PhoPc), a regulatory mutant of wild-type strain 14028s which constitutively represses PhoP-regulated (prg) genes and activates PhoP-activated (pag) genes (42) and which is defective in the secretion of at least 10 proteins including SspA, SspB, SspC, and SspD (SipA to -D) (45), was defective in the formation of filamentous appendages. On Caco-2 cells, which were typically more potent than MDCK cells in stimulating appendage formation on S. typhimurium, 99% (170 of 172 cells) of S. typhimurium CS022 cells lacked filamentous appendages (Fig. 5A; Table 3), although the appendages observed on the two bacterial cells which expressed them (Fig. 5B) were indistinguishable from those on wild-type bacteria, including its parent 14028s. Our examination of 153 bacteria on MDCK cells (Table 3) and 106 bacteria on mouse Peyer’s patches (Fig. 5C) infected with S. typhimurium CS022 failed to reveal any filamentous appendages. In the absence of epithelial cells all but one of the 75 bacteria observed lacked even the very short (“rudimentary”) appendages seen on a proportion of all wild-type strains and most other mutant strains examined (Fig. 5D; Table 4).
FIG. 5.
Scanning electron micrographs of PhoPc S. typhimurium strain CS022 (A to D) and prgH mutant strain IB040 (E and F). Almost all of the S. typhimurium CS022 cells observed on Caco-2 cells lacked the short filamentous appendages observed on other strains (A), although two which exhibited such features were found (B). S. typhimurium CS022 cells observed on MDCK cells (data not shown) and on murine Peyer’s patch epithelia (C) invariably lacked these surface features, as did those adhering to cell-free culture supports (D), although longer filamentous appendages were occasionally observed. S. typhimurium IB040 cells frequently formed numerous short filamentous appendages when they adhered to Caco-2 cells (E), while such features were rarely observed on strain IB040 bacteria adhering to MDCK cells (F). Most of the cell-adhered bacteria of strain IB040 were present in dense aggregates linked by long filaments (F), which resembled the adherence pattern of invA mutant strain SB111 (compare Fig. 4F with Fig. 2A). Bars, 2 μm.
To determine whether the lack of appendages on PhoPc mutant CS022 might be due to repression of the prgHIJK locus, which is involved in the secretion of at least five proteins including SspA, SspB, SspC, and SspD (SipA to -D) (27, 45), we examined polar S. typhimurium prgH mutant IB040 (also derived from wild-type strain 14028s). IB040 exhibited a heterogeneous pattern of cell binding unlike that of all wild-type strains examined and similar to that of invA mutant SB111, with aggregates of bacteria lacking the type of appendages focused on in this study (Fig. 5F). Of those bacteria which were independent of the aggregates, approximately 3% on MDCK cells and 43% on Caco-2 cells expressed prominent surface appendages (Fig. 5E; Table 3), indicating that the lack of appendage formation on CS022 is not due to the repression of prgHIJK. Although IB040 appeared to display a reduced ability to form filamentous appendages when adhering to MDCK cells, this may not be significant since a similar defect was not apparent after infection of Caco-2 cells and since this strain mainly adhered in aggregates; the analysis of appendage formation was limited to those bacteria appearing to adhere independently of the aggregates. Rudimentary surface features were also present on a proportion of prgH mutant bacteria in the absence of epithelial cells; these features were comparable to those on all wild-type bacteria and strains carrying mutations in invA and sspC (Table 4).
DISCUSSION
S. typhimurium invades epithelial cells by a mechanism which involves the induction of localized membrane ruffles at the site of bacterial cell contact (14, 16, 17). Induction of epithelial cell membrane ruffling requires the secretion of several bacterial proteins, including the invasion proteins SspB, SspC, and SspD, by a type III protein secretion system encoded by SPI1 (18, 27). The molecular mechanism by which the secreted proteins induce epithelial cell membrane ruffling is not known. Since membrane ruffles are induced only at the site of close bacterial cell contact, secreted proteins may either act as diffusible factors in high local concentrations or be located on the surface of the bacterial cell. The tendency of these proteins to form large insoluble aggregates (27) raises the possibility that they may assemble into supermolecular bacterial surface structures during the invasion process.
In accordance with the notion of an invasion-associated surface structure, Ginocchio et al. have observed that contact of S. typhimurium with the surfaces of polarized MDCK epithelial cells induces the transient formation of short, densely arrayed, filamentous appendages on the bacterial surface (22). These authors found that formation of the appendages correlated with the functional expression of the SPI1-encoded type III secretion system (22). Since secretion mutants are significantly impaired in invasiveness, the cell contact-induced formation of surface appendages was linked to bacterial cell invasion and the appendages were tentatively termed “invasomes” (55).
In this study we confirm that S. typhimurium, when adhering to epithelial cells, expresses a heterogeneous surface morphology including short filamentous appendages which closely resemble the appendages described by Ginocchio et al. (22). While some bacteria had no detectable appendages or a few long (over 3-μm) filaments, a variable proportion exhibited a dense array of short filamentous appendages up to 300 nm in length and 30 to 50 nm in diameter. On other adherent bacteria less prominent appendages of similar diameter, which may represent rudimentary forms of the short filamentous appendages, were apparent. We also show for the first time that similar appendages are expressed by S. typhimurium adhering to murine Peyer’s patch M cells and enterocytes in vivo. Although the resolution of the appendages by the conventional SEM techniques used in the present study is slightly inferior to that achieved by Ginocchio et al. (22), surface appendages were readily apparent and could be recognized at relatively low magnifications.
The observations made by Ginocchio et al. (22) implied the possibility that proteins which are secreted by the SPI1-encoded type III secretion system might be structural components of the cell contact-induced surface appendages. In order to test this hypothesis, and because Ssp proteins are known to form aggregates (27), we analyzed a mutant (EE638) impaired in the expression of secreted proteins SspC, SspD, and SspA (27). Since this mutant expressed surface appendages indistinguishable from those on the wild type, these three proteins are clearly not essential structural components of the observed appendages. Thus, the identity of these surface structures remains unclear. The possibility that they represent fimbriae cannot be excluded since they appear similar in morphology to appendages previously identified as type 1 fimbriae on E. coli viewed by SEM (53, 54).
An analysis of other strains which are defective in epithelial invasion due to mutations in SPI1 components (invA, invG, and prgH) revealed that each produced short surface appendages similar to those on wild-type bacteria in contact with polarized epithelia. These data demonstrate that expression of the short filamentous surface appendages does not correlate either with S. typhimurium invasiveness or SPI1-encoded protein secretion. S. typhimurium 83, which exhibits dramatically reduced invasiveness in vitro (10; this study) due to a mutation in invG encoding a type III secretion system component (40), produces appendages like those of wild-type strains when in contact with epithelial cells both in vivo and in vitro. Thus, InvG is not essential to form cell contact-induced short filamentous appendages, and, since InvG appears to be an integral part of the SPI1-encoded type III secretion apparatus (18, 34, 46), our data indicate that the entire system is not required for the formation of these appendages. In contrast to invG mutant 83, a different invG mutant, SB161, lacked short filamentous appendages after the infection of cultured epithelial cells, by either the protocol employed by Ginocchio et al. (22) or that used in the present study. The reason for this difference between different invG mutants has not been determined, although it may be related to differences in the mutations carried by the two strains. Strain 83 has a transposon insertion 163 bp into the 1,691-bp sequence, while SB161 has an internal deletion forming a nonpolar mutation (34). It remains possible that SB161 has an additional defect besides that in SPI1-encoded protein secretion. The recent finding that some S. typhimurium strains carrying transposon insertions in SPI2 genes exhibit reduced SPI1-mediated protein secretion and invasiveness (26) indicates that truncated proteins of one type III secretion system can interfere with the operation of those of another. Thus, it is possible to speculate that SB161, which would be expected to produce a moderately truncated InvG protein (34), may have a defect in a distinct type III secretion system. This possibility remains to be examined. Interestingly, invG mutant strain SB161 and other strains with mutations of the SPI1-encoded type III secretion system (i.e., invA and prgH mutants) exhibited a heterogeneous pattern of adherence which differed strikingly from the adherence pattern of wild-type S. typhimurium. Each of these mutants formed aggregates linked by long filaments. Almost all bacterial cells within these aggregates lacked the short filamentous appendages. For invA and prgH mutants some bacterial cells adhered independent of the aggregates, and these single bacteria exhibited a typical pattern of appendage formation indistinguishable from those of wild-type strains. It is unclear how the type III secretion system mutations can influence the bacterial adherence pattern. Nevertheless, the fact that singly adhering invA and prgH mutant bacteria expressed surface appendages similar to those of wild-type bacteria demonstrates again that the SPI1-encoded type III secretion system is not required for the formation of these appendages.
Our demonstration that the protein secretion system encoded by SPI1 is not required for formation of the described cell contact-dependent filamentous appendages suggests that an alternative protein secretion system is involved in this phenomenon. The type III protein secretion system encoded by SPI2 (25, 26, 44, 48) might be considered as a candidate for this role since, by analogy with other type III systems, it is predicted to be triggered by cell contact. However, three S. typhimurium strains (P8G12, P7G2, and P2D6) which exhibit dramatically reduced virulence due to mutations in the putative regulator and two components of the SPI2-encoded type III secretion apparatus (25, 48) formed short filamentous appendages similar to those seen on other strains when adhering to Caco-2 cells (unpublished observations). It thus appears unlikely that protein secretion by SPI2 is the principal cause of the appendages formed in these experiments, although we cannot rule out the possible contribution of this type III secretion system in the assembly of surface structures under these or other conditions. The apparent repression of appendage formation exhibited by S. typhimurium CS022 supports the conclusion that SPI2-mediated protein secretion is not required since SPI2 genes have recently been shown to be induced in macrophages (51) where the PhoP/PhoQ regulatory system would be expected to be activated. Macrophage induction of SPI2 genes was shown to be controlled by the regulatory system encoded in SPI2 (SsrA/SsrB) and not by PhoP/PhoQ (51).
We found that the proportion of bacteria possessing short filamentous appendages varied according to the different cell types used and was typically greater on Caco-2 cells than on MDCK cells. The basis of this observation is not known, but it is conceivable that different surface components and/or physicochemical properties of the cell surfaces may be responsible for these differences. Interestingly, and in contrast to observations by Ginocchio et al. (22), we also observed surface appendages on some bacteria independent of contact with epithelial cells. However, the appendages observed in the absence of cells were all very short and might represent rudimentary forms of the appendages observed after cell contact. Expression of the rudimentary appendages might be induced by the tissue culture medium, which was absent from the preparations examined by Ginocchio et al. (22). It thus appears possible that the expression of the S. typhimurium surface appendages is stimulated to different extents by various stimuli.
The only mutant (CS022) which was severely impaired in all phenotypes analyzed in this study carries a constitutively active form (PhoPc) of the global Salmonella virulence gene regulator PhoP (41, 42). Thus, appendage formation can be added to the list of S. typhimurium characteristics that are controlled by the two-component PhoP/PhoQ regulatory system. The prgH operon, which encodes part of the SPI1-encoded type III secretion system, is known to be repressed in the PhoPc strain (5, 45). However, the effect of PhoPc on appendage formation appears to be independent of the repression of prgH, since a prgH mutant differed from the PhoPc strain in that it was capable of expressing surface appendages under certain conditions. Since the PhoPc mutant is more severely impaired in the secretion of extracellular proteins than the prgH mutant (45), it is possible that one or more of the secreted proteins which are absent from the supernatant of the PhoPc mutant but which are secreted by the prgH mutant might be involved in appendage formation.
In summary, we have confirmed previous results by Ginocchio et al. (22), who showed that contact with epithelial cells promotes the formation of short filamentous appendages on the surface of S. typhimurium. We have extended these results by demonstrating that appendage formation also occurs in vivo and that different epithelial cell types vary in their abilities to induce appendage formation. Similar short filamentous appendages were observed on each of a panel of invasion-deficient S. typhimurium strains carrying mutations in invA, invG, prgH, and sspC (sipC) when in contact with Caco-2 and MDCK cells, demonstrating that these appendages themselves are not sufficient to trigger invasion. Thus, in contrast to Ginocchio et al. (22) we were unable to demonstrate a correlation between the formation of the described appendages and either SPI1-mediated protein secretion or bacterial invasion. For this reason we are reluctant to use the term invasome, which was recently coined to describe these appendages (55). Formation of these unusual surface appendages may nevertheless be related to S. typhimurium virulence since their expression appears to be controlled by the global virulence gene regulatory system, PhoP/PhoQ. The present data cannot exclude the possibility that SPI1-mediated protein secretion and invasion involves the formation of alternate surface appendages, which were not identified in these experiments. It is noteworthy that the analogous type III secretion system of enteropathogenic E. coli has been shown to be involved in the formation of transient appendages on the bacterial surface which appear to play a direct role in the translocation of secreted proteins into host cells (37). It is clear that the enteropathogenic E. coli appendages are morphologically distinct from those on Salmonella described by ourselves and others (22). The possibility that Salmonella produces equivalent surface appendages remains an open question.
ACKNOWLEDGMENTS
We thank C. L. Francis, J. E. Galán, J. Stephen, and D. W. Holden for gifts of bacterial strains; N. L. Simmons for supplying MDCK cells; and A. Leard and M. Geggie for assistance with cell culture.
This work was supported by a Wellcome Trust Veterinary Research Fellowship (041573/Z/94/Z) awarded to M.A.C., a Medical Research Council grant (G9405434) to B.H.H., a Royal Society research grant to M.A.J. (17996), and a National Institute of Health grant AI34079 to S.I.M. K.A.R. was supported by a studentship from the Biotechnology and Biological Sciences Research Council, and C.J.H. was supported by a personal grant from the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie, Germany. The School of Medical Sciences Cell Imaging Facility, University of Bristol, is supported by a Medical Research Council Infrastructure Award (G4500006).
REFERENCES
- 1.Allaoui A, Sansonetti P J, Menard R, Barzu S, Mounier J, Phalipon A, Parsot C. MxiG, a membrane protein required for secretion of Shigella spp. Ipa invasins: involvement in entry into epithelial cells and in intercellular dissemination. Mol Microbiol. 1995;17:461–470. doi: 10.1111/j.1365-2958.1995.mmi_17030461.x. [DOI] [PubMed] [Google Scholar]
- 2.Altmeyer R M, McNern J K, Bossio J C, Rosenshine I, Finlay B B, Galán J E. Cloning and molecular characterisation of a gene involved in Salmonella adherence and invasion of cultured epithelial cells. Mol Microbiol. 1993;7:89–98. doi: 10.1111/j.1365-2958.1993.tb01100.x. [DOI] [PubMed] [Google Scholar]
- 3.Bajaj V, Hwang C, Lee C A. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol. 1995;18:715–727. doi: 10.1111/j.1365-2958.1995.mmi_18040715.x. [DOI] [PubMed] [Google Scholar]
- 4.Bajaj V, Lucas R L, Hwang C, Lee C A. Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol Microbiol. 1996;22:703–714. doi: 10.1046/j.1365-2958.1996.d01-1718.x. [DOI] [PubMed] [Google Scholar]
- 5.Behlau I, Miller S I. A PhoP-repressed gene promotes Salmonella typhimurium invasion of epithelial cells. J Bacteriol. 1993;175:4475–4484. doi: 10.1128/jb.175.14.4475-4484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman T, Erickson K, Galyov E, Persson C, Wolf-Watz H. The lcrB (yscN/U) gene cluster of Yersinia pseudotuberculosis is involved in Yop secretion and shows high homology to the spa gene clusters of Shigella flexneri and Salmonella typhimurium. J Bacteriol. 1994;176:2619–2626. doi: 10.1128/jb.176.9.2619-2626.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogdanove A J, Wei Z M, Zhao L, Beer S V. Erwinia amylovara secretes harpin via a type III pathway and contains a homolog of yopN of Yersinia spp. J Bacteriol. 1996;178:1720–1730. doi: 10.1128/jb.178.6.1720-1730.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L-M, Hobbie S, Galán J E. Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science. 1996;274:2115–2118. doi: 10.1126/science.274.5295.2115. [DOI] [PubMed] [Google Scholar]
- 9.Clark M A, Jepson M A, Simmons N L, Hirst B H. Preferential interaction of Salmonella typhimurium with mouse Peyer’s patch M cells. Res Microbiol. 1994;145:543–553. doi: 10.1016/0923-2508(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 10.Clark M A, Reed K A, Lodge J, Stephen J, Hirst B H, Jepson M A. Invasion of murine intestinal M cells by Salmonella typhimurium inv mutants severely deficient for invasion of cultured cells. Infect Immun. 1996;64:4363–4368. doi: 10.1128/iai.64.10.4363-4368.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collazo C M, Zierler M K, Galán J E. Functional analysis of the Salmonella typhimurium invasion genes invI and invJ and identification of a target of the protein secretion apparatus encoded in the inv locus. Mol Microbiol. 1995;15:25–38. doi: 10.1111/j.1365-2958.1995.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 12.Eichelberg K, Ginocchio C G, Galán J E. Molecular and functional characterization of the Salmonella typhimurium invasion genes invB and invC: homology of InvC to the F0F1 ATPase family of proteins. J Bacteriol. 1994;176:4501–4510. doi: 10.1128/jb.176.15.4501-4510.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenselau S, Balbo I, Bonas U. Determinants of pathogenicity in Xanthomonas campestris pv. vesicatora are related to proteins involved in secretion in bacterial pathogens of animals. Mol Plant-Microbe Interact. 1992;5:390–396. doi: 10.1094/mpmi-5-390. [DOI] [PubMed] [Google Scholar]
- 14.Finlay B B, Falkow S. Salmonella interactions with polarised human intestinal Caco-2 epithelial cells. J Infect Dis. 1990;162:1096–1106. doi: 10.1093/infdis/162.5.1096. [DOI] [PubMed] [Google Scholar]
- 15.Finlay B B, Starnbach M N, Francis C L, Stocker B A D, Chatfield S, Dougan G, Falkow S. Identification and characterization of TnphoA mutants of Salmonella that are unable to pass through a polarised MDCK epithelial cell monolayer. Mol Microbiol. 1988;2:757–766. doi: 10.1111/j.1365-2958.1988.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 16.Finlay B B, Ruschkowski S, Dedhar S. Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. J Cell Sci. 1991;99:283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- 17.Francis C L, Ryan T A, Jones B D, Smith S J, Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993;364:639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- 18.Galán J E. Molecular genetic bases of Salmonella entry into host cells. Mol Microbiol. 1996;20:263–271. doi: 10.1111/j.1365-2958.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 19.Galán J E, Curtiss R., III Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci USA. 1989;86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galán J E, Ginocchio C, Costeas P. Molecular and functional characterization of the Salmonella invasion gene invA: homology to members of a new protein family. J Bacteriol. 1992;174:4338–4349. doi: 10.1128/jb.174.13.4338-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginocchio C, Pace J, Galán J E. Identification and molecular characterization of a Salmonella typhimurium gene involved in triggering the internalization of Salmonellae into cultured epithelial cells. Proc Natl Acad Sci USA. 1992;89:5976–5980. doi: 10.1073/pnas.89.13.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ginocchio C C, Olmsted S B, Wells C L, Galán J E. Contact with epithelial cells induces the formation of surface appendages on Salmonella typhimurium. Cell. 1994;76:717–724. doi: 10.1016/0092-8674(94)90510-x. [DOI] [PubMed] [Google Scholar]
- 23.Gough C L, Genin S, Zischek C, Boucher C A. hrp genes of Pseudomonas solanacearum are homologous to pathogenicity determinants of animal pathogenic bacteria and are conserved among plant pathogenic bacteria. Mol Plant-Microbe Interact. 1992;5:384–389. doi: 10.1094/mpmi-5-384. [DOI] [PubMed] [Google Scholar]
- 24.Groisman E A, Ochman H. Cognate gene clusters govern invasion of host epithelial cells by Salmonella typhimurium and Shigella flexneri. EMBO J. 1993;12:3779–3787. doi: 10.1002/j.1460-2075.1993.tb06056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hensel M, Shea J E, Gleeson C, Jones M D, Dalton E, Holden D W. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- 26.Hensel M, Shea J E, Raupach B, Monack D, Falkow S, Gleeson C, Kubo T, Holden D W. Functional analysis of ssaJ and the ssaK/U operon, 13 genes encoding components of the type III secretion apparatus of Salmonella pathogenicity island 2. Mol Microbiol. 1997;24:155–167. doi: 10.1046/j.1365-2958.1997.3271699.x. [DOI] [PubMed] [Google Scholar]
- 27.Hueck C J, Hantman M J, Bajaj V, Johnston C, Lee C A, Miller S I. Salmonella typhimurium secreted invasion determinants are homologous to Shigella Ipa proteins. Mol Microbiol. 1995;18:479–490. doi: 10.1111/j.1365-2958.1995.mmi_18030479.x. [DOI] [PubMed] [Google Scholar]
- 28.Jarvis K G, Kaper J B. Secretion of extracellular proteins by enterohemorrhagic Escherichia coli via a putative type III secretion system. Infect Immun. 1996;64:4826–4829. doi: 10.1128/iai.64.11.4826-4829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarvis K G, Giron J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jepson M A, Collares-Buzato C B, Clark M A, Hirst B H, Simmons N L. Rapid disruption of epithelial barrier function by Salmonella typhimurium is associated with structural modification of intercellular junctions. Infect Immun. 1995;63:356–359. doi: 10.1128/iai.63.1.356-359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jepson M A, Lang T F, Reed K A, Simmons N L. Evidence for a rapid, direct effect on epithelial monolayer integrity and transepithelial transport in response to Salmonella invasion. Pflueg Arch Eur J Physiol. 1996;432:225–233. doi: 10.1007/s004240050128. [DOI] [PubMed] [Google Scholar]
- 32.Johnston C, Pegues D A, Hueck C J, Lee C A, Miller S I. Transcriptional activation of Salmonella typhimurium invasion genes by a member of the phosphorylated response-regulator superfamily. Mol Microbiol. 1996;22:715–727. doi: 10.1046/j.1365-2958.1996.d01-1719.x. [DOI] [PubMed] [Google Scholar]
- 33.Jones B D, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialised epithelial M cells. J Exp Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaniga K, Bossio J C, Galán J E. The Salmonella typhimurium invasion genes invF and invG encode homologues of the AraC and PulD family of proteins. Mol Microbiol. 1994;13:555–568. doi: 10.1111/j.1365-2958.1994.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 35.Kaniga K, Trollinger D, Galán J E. Identification of two targets of the type III protein secretion system encoded by the inv and spa loci of Salmonella typhimurium that have homology to the Shigella IpaD and IpaA proteins. J Bacteriol. 1995;177:7078–7085. doi: 10.1128/jb.177.24.7078-7085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaniga K, Tucker S, Trollinger D, Galán J E. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J Bacteriol. 1995;177:3965–3971. doi: 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knutton, S., I. Rosenshine, M. J. Pallen, I. Nisan, B. C. Neves, C. Bain, C. Wolff, G. Dougan, and G. Frankel. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J., in press. [DOI] [PMC free article] [PubMed]
- 38.Lee C A. Type III secretion systems: machines to deliver bacterial proteins into eukaryotic cells? Trends Microbiol. 1997;5:148–156. doi: 10.1016/S0966-842X(97)01029-9. [DOI] [PubMed] [Google Scholar]
- 39.Lee C A, Jones B D, Falkow S. Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proc Natl Acad Sci USA. 1992;89:1847–1851. doi: 10.1073/pnas.89.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lodge J, Douce G R, Amin I I, Bolton A J, Martin G D, Chatfield S, Dougan G, Brown N L, Stephen J. Biological and genetic characterization of TnphoA mutants of Salmonella typhimurium TML in the context of gastroenteritis. Infect Immun. 1995;63:762–769. doi: 10.1128/iai.63.3.762-769.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller S I, Mekalanos J J. Constitutive expression of the PhoP regulon attenuates Salmonella virulence and survival within macrophages. J Bacteriol. 1990;172:2485–2490. doi: 10.1128/jb.172.5.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller S I, Kukral A M, Mekalanos J J. A two-component regulatory system (phoP and phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci USA. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mills D M, Bajaj V, Lee C A. A 40 kilobase chromosomal fragment encoding Salmonella typhimurium invasion genes is absent from the corresponding region of the Escherichia coli K-12 chromosome. Mol Microbiol. 1995;15:749–759. doi: 10.1111/j.1365-2958.1995.tb02382.x. [DOI] [PubMed] [Google Scholar]
- 44.Ochman H, Soncini F C, Soloman F, Groisman E A. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc Natl Acad Sci USA. 1996;93:7800–7804. doi: 10.1073/pnas.93.15.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pegues D A, Hartman M J, Behlau I, Miller S I. PhoP/PhoQ transcriptional repression in Salmonella typhimurium invasion genes: evidence for a role in protein secretion. Mol Microbiol. 1995;17:169–181. doi: 10.1111/j.1365-2958.1995.mmi_17010169.x. [DOI] [PubMed] [Google Scholar]
- 46.Penheiter K L, Mathur N, Giles D, Fahlen T, Jones B D. Non-invasive Salmonella typhimurium mutants are avirulent because of an inability to enter and destroy M cells of ileal Peyer’s patches. Mol Microbiol. 1997;24:697–709. doi: 10.1046/j.1365-2958.1997.3741745.x. [DOI] [PubMed] [Google Scholar]
- 47.Rosqvist R, Ruschkowski S, Foubister V, Finlay B B. Functional conservation of the secretion and translocation machinery for virulence proteins of yersiniae, salmonellae and shigellae. EMBO J. 1995;14:4187–4195. doi: 10.1002/j.1460-2075.1995.tb00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shea J E, Hensel M, Gleeson C, Holden D W. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:2593–2597. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stone B J, Garcia C M, Badger J L, Hassett T, Smith R I F, Miller V L. Identification of novel loci affecting entry of Salmonella enteritidis into eukaryotic cells. J Bacteriol. 1992;174:3945–3952. doi: 10.1128/jb.174.12.3945-3952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967;50:109–136. [PMC free article] [PubMed] [Google Scholar]
- 51.Valdivia R H, Falkow S. Fluorescence-based isolation of bacterial genes expressed within host cells. Science. 1997;277:2007–2011. doi: 10.1126/science.277.5334.2007. [DOI] [PubMed] [Google Scholar]
- 52.Wallis T S, Starkey W G, Stephen J, Haddon S J, Osborne M P, Candy D C A. The nature and role of mucosal damage in relation to Salmonella typhimurium-induced fluid secretion in the rabbit ileum. J Med Microbiol. 1986;22:39–49. doi: 10.1099/00222615-22-1-39. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto T, Yokota T. In vitro adherence study using formalin-treated human small intestine: fimbria-mediated adherence of enterotoxigenic Escherichia coli and Salmonella typhi oral-route vaccine strain possessing E. coli colonization factor antigen to villi and lymphoid follicle epithelium. FEMS Microbiol Lett. 1988;55:335–340. [Google Scholar]
- 54.Yamamoto T, Fujita K, Yokota T. Adherence characteristics to human small intestinal mucosa of Escherichia coli isolated from patients with diarrhea or urinary tract infections. J Infect Dis. 1990;162:896–908. doi: 10.1093/infdis/162.4.896. [DOI] [PubMed] [Google Scholar]
- 55.Zierler M K, Galán J E. Contact with cultured epithelial cells stimulates secretion of Salmonella typhimurium invasion protein InvJ. Infect Immun. 1995;63:4024–4028. doi: 10.1128/iai.63.10.4024-4028.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]