Abstract
Clostridium difficile causes antibiotic-associated diarrhea and colitis in humans through the actions of toxin A and toxin B on the colonic mucosa. At present, broad-spectrum antibiotic drugs are used to treat this disease, and patients suffer from high relapse rates after termination of treatment. This study examined the role of both toxins in pathogenesis and the ability of orally administered avian antibodies against recombinant epitopes of toxin A and toxin B to treat C. difficile-associated disease (CDAD). DNA fragments representing the entire gene of each toxin were cloned, expressed, and affinity purified. Hens were immunized with these purified recombinant-protein fragments of toxin A and toxin B. Toxin-neutralizing antibodies fractionated from egg yolks were evaluated by a toxin neutralization assay in Syrian hamsters. The carboxy-terminal region of each toxin was most effective in generating toxin-neutralizing antibodies. With a hamster infection model, antibodies to both toxins A and B (CDAD antitoxin) were required to prevent morbidity and mortality from infection. In contrast to vancomycin, CDAD antitoxin prevented relapse and subsequent C. difficile reinfection in the hamsters. These results indicate that CDAD antitoxin may be effective in the treatment and management of CDAD in humans.
Toxigenic Clostridium difficile, a spore-forming anaerobe, is the predominant pathogen of noscomial intestinal infections (40). This organism causes about 20% of the cases of antibiotic-associated diarrhea, up to 75% of the cases of antibiotic-associated colitis, and virtually all the cases of pseudomembranous colitis (4, 22). C. difficile-associated disease (CDAD) is estimated to afflict 1% of hospitalized patients and causes substantial morbidity, increased hospital costs, and longer hospital stays (35, 43, 52). The persistence of C. difficile as a noscomial pathogen is facilitated by the ease of transmission within hospitals and nursing homes in the form of a long-lasting, heat-resistant spore. Since the 1970s, CDAD has become a major clinical problem with the increased use of broad-spectrum antibiotics, such as clindamycin, cephalosporins, and amoxicillin (3). C. difficile is unique among pathogens in that antibiotic exposure is virtually a prerequisite for infection. Nearly all antibiotics, including vancomycin (18) and even some cancer chemotherapeutics (1), can induce CDAD. Thus, antibiotic treatment is problematic for use in treating CDAD. Nonetheless, antibiotics are used, largely due to the lack of effective alternatives.
At present the two antibiotics of choice for treatment of CDAD are metronidazole for mild to moderate cases and vancomycin for moderate to severe cases. Although most patients respond to metronidazole or vancomycin, approximately 20% of patients relapse 2 to 8 weeks after the discontinuation of antibiotic therapy (14). While most of these patients respond to a second course of therapy, up to 30% of these patients will experience multiple relapses (7, 19). Several approaches have been tried to manage this difficult problem, including a pulse dose of vancomycin, slowly tapering doses of vancomycin (45), and combination therapy with vancomycin and rifampin (7) or cholestyramine (44). In attempts to normalize the colonic microbial flora, several treatments have been tried with various degrees of success: the administration of Lactobacillus GG (17) or of Saccharomyces boulardii plus metronidazole or vancomycin (28) or the rectal instillation of stool (42) or mixed broth cultures of fecal flora (48). Relapse is thought to result from either failure to eradicate the organism or reinfection from environmental or human sources (14), rather than from resistance of C. difficile to the agents used. However, C. difficile has been found to possess multiple-antibiotic resistance genes (36). Since C. difficile clinical isolates resistant to both vancomycin and metronidazole have been reported (13, 15), a major concern is that these drugs may be less effective in the future.
Recurrence of CDAD when antibiotic therapies are used may stem from the fact that they are broad spectrum and nonselective for C. difficile. These drugs are known to disrupt the normal gut flora, leading to overgrowth of toxigenic strains of C. difficile, which can predispose the patient to CDAD relapse (29). A further potential danger is that this nonselectivity of antibiotics can promote widespread drug resistance in other intestinal organisms, such as Enterococcus spp. and Staphylococcus aureus (8, 33). Vancomycin resistance in particular is of great concern because this drug is the only effective treatment for some of these opportunistic bacteria. The consequences of rampant antibiotic resistance have already been felt; methicillin-resistant S. aureus strains discovered in Japan and Michigan were found to have intermediate susceptibility to vancomycin, the only licensed antibiotic effective against methicillin-resistant S. aureus (10, 51). To combat this trend, the Centers for Disease Control and Prevention are recommending limiting the use of oral vancomycin to treat C. difficile disease (9). With these problems and limitations of today’s antibiotics, there is a clear need to develop more selective and effective alternatives to treat CDAD.
We present the strategy of developing a CDAD therapeutic that directly targets the virulence factors of the organism. Others have attempted to treat CDAD with antibodies (12, 23, 25, 26); however, there are no reports of effective immunotherapy in animals after C. difficile infection. Toxins A and B, produced by toxigenic C. difficile, are well established as the virulence factors of the disease (27). These toxins can destroy cells of the intestinal mucosa, resulting in inflammation and diarrhea. They have also been implicated in promoting C. difficile colonization (5) and neutrophil chemotaxis and activation (32, 37). We have developed avian antibodies that neutralize both toxins. By neutralization of these toxins with antibodies, the pathogenic mechanism of the organism is blocked, its ability to thrive in the gut may be diminished, and the impact on the microbial ecology could be minimized, allowing recovery of the normal flora. The medical advantages of this approach could include more-rapid recovery, fewer relapses, and relief from selective pressure for antibiotic resistance in normal gut flora. In this study we describe the effectiveness of orally delivered avian antibodies against recombinant epitopes of C. difficile toxins A and B in the hamster model of CDAD.
MATERIALS AND METHODS
Cloning and expression of recombinant toxin A and toxin B polypeptides.
The genes of C. difficile toxins A and B have been cloned and sequenced previously (2, 41) and encode proteins of 2,710 and 2,367 amino acids (aa), respectively. In this study, segments of toxin A and toxin B genes were cloned either by screening a genomic library with specific DNA probes or by using PCR to amplify specific regions. High-molecular-weight DNA from C. difficile ATCC 43255 (American Type Culture Collection, Rockville, Md.) grown under anaerobic conditions in brain heart infusion medium was isolated as described elsewhere (54). A genomic library of size-selected PstI-digested C. difficile genomic DNA was prepared by standard molecular-biology techniques (39) and screened with an oligonucleotide probe (5′-CTATCTAGGCCTAAAGTAT-3′) specific for the sequence encoding the carboxy-terminal region of toxin A. All other regions of the toxin A gene and segments composing the entire toxin B gene were cloned by PCR with a proofreading thermostable DNA polymerase (native Pfu polymerase; Stratagene, La Jolla, Calif.). Overlapping DNA fragments representing the entire gene of each toxin were cloned into the prokaryotic expression vectors pMALc (New England Biolabs, Beverly, Mass.), pET23a-c, and pET16b (Novagen, Inc., Madison, Wis.). Engineered restriction sites in the PCR primers enabled in-frame fusions of the amplified PCR fragments with the expression vectors. All constructs containing putative recombinant inserts were confirmed by restriction analysis.
In order to express proteins in Escherichia coli, recombinant pMALc constructs were transformed into E. coli BL21 (Novagen), while recombinant pET constructs were transformed into E. coli BL21(DE3) or BL21(DE3)LysS hosts (Novagen). Protein preparations of induced small-scale (5-ml) cultures were grown in medium containing ampicillin, and recombinant clones were screened by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis (53) utilizing an affinity-purified goat anti-toxin A or goat anti-toxin B antibody (Tech Lab, Inc., Blacksburg, Va.).
Purification of toxin A and toxin B recombinant proteins.
Large-scale cultures (500 to 1,000 ml) of the recombinant clones grown in Luria-Bertani or 2× yeast extract-tryptone medium containing ampicillin were induced with isopropyl-β-d-thiogalactopyranoside (IPTG) to 1 mM and harvested by centrifugation as described elsewhere (53). The cells were lysed by freezing and thawing with lysozyme, and the supernatant containing the soluble recombinant proteins was collected by centrifugation. The recombinant toxin DNA fragments expressed in the pMAL-c vector produced proteins that contained a maltose-binding protein (MBP) fusion, allowing purification by using an amylose resin. Extracts containing the MBP fusion proteins were chromatographed in buffer (10 mM sodium phosphate, 0.5 M sodium chloride, and 10 mM β-mercaptoethanol [pH 7.2]) and eluted from the amylose resin (New England Biolabs) with 10 mM maltose (53). Proteins expressed in the pET vectors contained up to 10 amino- or carboxy-terminal polyhistidine sequences that allowed for affinity purification on a ligand-containing column. Extracts of the histidine-tagged recombinant proteins were applied to a nickel chelate column (Qiagen, Chatsworth, Calif., or Novagen) and eluted with imidazole as described by the manufacturer. The identity, integrity, and estimated purity of the recombinant proteins were determined by SDS-PAGE and Western blot analysis.
Production of antibodies against the toxin A and toxin B recombinant proteins.
Antibodies were raised against the recombinant toxin A and toxin B proteins in egg-laying White Leghorn hens. Purified recombinant proteins were each diluted in phosphate-buffered saline (PBS) (150 mM NaCl–10 mM sodium phosphate buffer [pH 7.2 to 7.4]) and mixed with either Freund’s adjuvant (GIBCO, Grand Island, N.Y.), Titer Max adjuvant (Vaxcel, Inc., Norcross, Ga.), Quil A (Accurate Chemical and Scientific Corp., Westbury, N.Y.) or Gerbu adjuvant (C-C Biotech, Poway, Calif.). Approximately 0.5 to 1.5 mg of recombinant protein in 0.5 to 1.0 ml was injected at multiple sites in the hens. The hens were immunized at least three times before eggs were harvested. Yolk antibodies (immunoglobulin Y [IgY]) were fractionated from the collected yolks either by a two-step polyethylene glycol procedure (31) or by ammonium sulfate fractionation. For positive and placebo antibody controls, hen antibodies against purified native toxin A and toxin B (Tech Lab, Inc.) and preimmune antibodies from eggs from nonimmunized hens were also prepared. The concentrations of fractionated IgY’s were estimated by measuring the absorbance at 280 nm (an optical density at 280 nm of 1.3 equals 1 mg of IgY/ml). For qualitative determinations of levels of IgY against the recombinant proteins, antibody titer values were estimated by enzyme-linked immunoassays (EIAs). Antibody titers were defined as the reciprocal of the highest dilution of antirecombinant IgY generating a signal about threefold higher than that of preimmune IgY. Recombinant proteins at 2.5 μg/ml in PBS were used to coat 96-well microtiter plates (100 μl/well), followed by the addition of the immune or preimmune IgY diluted in PBS containing 1% bovine serum albumin and 0.05% Tween 20. To determine titers of antibodies against the MBP fusion proteins, separate microtiter plates were coated with MBP to permit comparison with the reactivity of antibodies to the fusion partner. IgY reactivity was detected with alkaline phosphatase-conjugated rabbit anti-chicken IgG and p-nitrophenyl phosphate substrate. The plates were read at 410 nm on a microtiter plate reader 10 min after the addition of substrate.
In vitro and in vivo neutralization of C. difficile toxin A and toxin B.
An in vitro toxin neutralization model was developed to assess the ability of the anti-recombinant toxin A and toxin B to neutralize the activity of native C. difficile toxin A and toxin B. For the in vitro toxin neutralization assay, a 100% lethal dose of each toxin for 40- to 50-g golden Syrian hamsters (Charles River Laboratories, Wilmington, Mass.) was first determined. For oral administration, 30 μg of native toxin A was mixed with 1 ml of 0.1 M carbonate buffer, pH 9.5, to help protect the IgY from acid degradation in the stomach. Five micrograms of native toxin B was mixed in 1 ml of PBS for intraperitoneal (i.p.) injection. The toxins were incubated for 1 h at 37°C with approximately 20 mg of antirecombinant, preimmune, or anti-native toxin IgY. For the toxin A mixtures, 1 ml was orally administered with an 18-gauge feeding needle (Popper and Sons, Inc., New Hyde Park, N.Y.) to each hamster. For the toxin B mixtures, 1 ml was administered i.p. with a 27-gauge needle to each hamster. The animals were then observed for the onset of diarrhea and/or death after a period of about 24 h for the toxin A assay and 2 h for the toxin B assay.
Neutralizing antibodies identified by the in vivo toxin neutralization assay were also tested in the golden Syrian hamster model of C. difficile disease (11, 16). Briefly, 80- to 90-g golden Syrian hamsters (Charles River) were challenged orally with 104 toxigenic C. difficile organisms 18 to 24 h after predisposition to the disease with clindamycin phosphate (1 mg i.p./100 g of body weight). In these experiments, several different C. difficile strains were used (ATCC 43596, ATCC 43255, or VPI 7698). In therapeutic studies, hamsters were given oral doses ranging from 20 to 80 mg of immune and control IgY preparations for several days, starting from 4 to 8 h postchallenge. In prophylactic treatment studies, hamsters were treated with 40 to 80 mg of IgY 24 h before C. difficile infection, and then treatment was resumed about 1 h after challenge. Hamsters were dosed orally, by using an 18-gauge feeding needle, with 1 to 2 ml of IgY resuspended in 0.1 M carbonate buffer (pH 9.5). During these studies, hamsters were given food and water ad libitum and usually housed two to four animals per cage. During and after treatment, death and/or the presence of diarrhea in the animals was monitored. Chi-square analysis was used to determine statistical significance.
RESULTS
Production of recombinant toxin A and toxin B proteins.
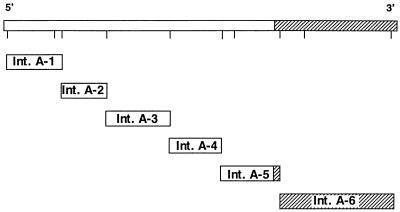
The locations of the cloned gene fragments of toxin A and toxin B are shown in Fig. 1 and 2. Six DNA fragments for toxin A (intervals [Int.] A-1 to A-6) and seven DNA fragments for toxin B (Int. B-1 to B-7) were cloned. Each was expressed in the pET or pMAL bacterial vector expression system, and the products were purified. Verification of each recombinant insert was confirmed by dideoxy-DNA sequencing (Sequenase system; Amersham Corporation, Arlington Heights, Ill.) of either the 3′ end or the entire insert. All the toxin A and toxin B recombinant epitopes diagrammed in Fig. 1 and 2 produced high levels of recombinant protein upon induction with IPTG (more than 1 mg of purified intact recombinant protein/liter of culture). After a one-step affinity purification, the purity of the full-length recombinant-protein product ranged from 50 to 90%, as estimated by SDS-PAGE. Many of the recombinant clones containing toxin DNA fragments smaller or larger than those shown were found to produce levels of induced protein that were too low to purify in amounts suitable for immunization.
FIG. 1.
C. difficile toxin A gene and locations of expression clones. The amino acid interval expressed in each clone is as follows: for Int. A-1, aa 30 to 100; for Int. A-2, aa 300 to 660; for Int. A-3, aa 660 to 1100; for Int. A-4, aa 1100 to 1610; for Int. A-5, aa 1450 to 1870; and for Int. A-6, aa 1870 to 2680. The shaded region at the 3′ end represents the repetitive domain of the toxin A gene.
FIG. 2.
C. difficile toxin B gene and locations of expression clones. Int. B-4 and Int. B-(1+2) each comprise a pool of two or three smaller fragments. The amino acid interval expressed in each clone is as follows: for Int. B-4, aa 10 to 330 and aa 260 to 520; for Int. B-(1+2), aa 510 to 1110, aa 820 to 1110, and aa 1110 to 1530; for Int. B-5, aa 1530 to 1750; and for Int. B-3, aa 1750 to 2360. The shaded region at the 3′ end represents the repetitive domain of the toxin B gene.
Western blot analysis using chicken or goat antibodies against native toxin A or native toxin B shows that each of the respective purified recombinant proteins was recognized. There was little cross-reactivity of the toxin A recombinants or the toxin B recombinants with the other anti-native toxin. At least 90% of total immunoreactivity was found to be directed to the carboxy-terminal end of each toxin, where repetitive domains are located (2, 41). The recombinant protein encoded by the Int. A-6 region of the toxin A gene and the protein encoded by the Int. B-3 region of the toxin B gene thus contained the immunodominant epitopes of the toxins.
Levels of avian antibodies against the toxin A and toxin B recombinant proteins.
High-titer antibodies were generated by hyperimmunizing hens against the toxin A and toxin B recombinant proteins. Antibodies to some recombinant segments, such as Int. B-(1+2) or Int. B-4, were generated against a mixture of several recombinant proteins. IgY from eggs collected about 7 days after the last boost was fractionated, resuspended in PBS, and assayed by EIA. Titers of antibodies against all the recombinant proteins by the qualitative EIA ranged from 12,500 to >93,750. All the adjuvants tested were able to elicit good antibody responses in the hens.
Identification of toxin A and toxin B recombinant proteins that induce toxin-neutralizing antibodies.
Results from the in vitro toxin A neutralization assay demonstrated that only antibodies against Int. A-6 recombinant protein could completely prevent death from an otherwise lethal dose of native toxin A in the hamsters (data not shown). In addition, these antibodies significantly prevented the onset of diarrhea in six of seven treated hamsters. As expected, the positive-control antibodies generated in chickens against native toxin A completely protected all hamsters from diarrhea and death from toxin A. In contrast, preimmune IgY and antibodies against Int. A-1, Int. A-2, Int. A-3, Int. A-4, and Int. A-5 recombinant proteins had no effect on the onset of diarrhea and death in the hamsters due to toxin A. In the in vitro toxin B neutralization assay, only antibodies to Int. B-3 recombinant protein prevented death in all the hamsters (data not shown). The positive-control antibodies against native toxin B also protected the hamsters from death. Antibodies to the other recombinant proteins encoded by the Int. B-(1+2), Int. B-4, and Int. B-5 regions, as well as preimmune IgY, were ineffective, and all the hamsters died within 2 h. Because of the route of administration and the rapid time course of death in the in vitro toxin B neutralization assay, diarrhea was not seen in these hamsters.
The toxin-neutralizing epitopes encoded by the Int. A-6 and Int. B-3 regions were designated recombinant toxin A (rTox A) and recombinant toxin B (rTox B). The amino acid positions in relation to the native-toxin amino acid sequence are 1870 to 2680 for Int. A-6 and 1750 to 2360 for Int. B-3. These epitopes both reside at the carboxy termini of the native toxins and are encoded by segments of 94 kDa for rToxA and 74 kDa for rToxB. Both recombinant fragments were subcloned into a pET23a derivative, and the purified expressed products were used to immunize hens in order to generate additional antitoxin.
Testing of toxin-neutralizing antibodies in the hamster infection model.
We found that antibodies to both rToxA (anti-rToxA) and rToxB (anti-rToxB) were necessary to achieve full efficacy in the hamster model of disease (Fig. 3). These antibodies against the toxin-neutralizing epitopes were tested individually or as a mixture to determine if either or both are required for efficacy against C. difficile in the hamster model. Antibody treatment was initiated 8 h after C. difficile challenge (approximately 104 organisms/hamster) and continued for 4 days three times a day (t.i.d.) in three groups of 19 animals. One group of hamsters was given 80 mg of placebo (preimmune IgY), a second group was given 40 mg of anti-rTox A and 40 mg of anti-rTox B, and a third group was given 40 mg of anti-rTox A and 40 mg of placebo. As shown in Fig. 3, the mixture of anti-rTox A and anti-rTox B was 100% effective in preventing mortality. In contrast, all the placebo-treated hamsters died by day 3, whereas anti-rTox A with placebo prevented mortality in 70% of the hamsters. On necropsy, the treated hamsters showed no gross signs of toxic megacecum. Histological sections of the cecum revealed virtually no inflammation, edema, hemorrhage, or erosion of the mucosa and lamina propria, all characteristic of CDAD in the hamster. While anti-rTox A could significantly protect the hamsters from mortality, morbidity, such as weight loss and diarrhea, from C. difficile disease was not prevented by treatment with anti-rTox A alone but could be completely prevented by antibodies to both recombinant toxins (Table 1). The placebo treatment failed to protect the hamsters. The IgY generated against native toxin A and toxin B also supported this finding; only a mixture of antibodies against both toxins completely protected all the hamsters (data not shown). Moreover, in a separate experiment, 40 mg of anti-rToxB alone failed to protect any hamsters from morbidity and mortality from C. difficile (data not shown). Approximately equal amounts of anti-rTox A and anti-rTox B (40 mg each), designated CDAD antitoxin, were found to be an effective therapeutic mixture.
FIG. 3.
Cumulative mortalities caused by clindamycin-induced CDAD in hamsters treated t.i.d. with placebo (preimmune IgY) (⧫), anti-rTox A (▪), or a mixture of anti-rTox A and anti-rTox B (▴).
TABLE 1.
Anti-rTox A and anti-rTox B antibodies reduce CDAD morbidity
| Treatment groupa | % Diarrhea | % Weight lossb |
|---|---|---|
| Placebo | 100 | NA |
| Anti-rTox A | 90c | 16d |
| Anti-rTox A and anti-rTox B | 0d | 1c |
There were 10 hamsters in each treatment group.
Compared to starting weight. NA, not applicable.
Not significant.
P < 0.001.
Prophylactic treatment studies demonstrated that only anti-rTox A is required for efficacy. As shown in Table 2, anti-rTox A treatments initiated 24 h prior to inoculation with C. difficile significantly protected the hamsters from mortality. Hamsters treated with 40 mg (t.i.d.) of anti-rTox A for 7 to 12 days were completely protected from morbidity and mortality from CDAD. As described in the preceding section, the need for anti-rTox B for full therapeutic (postchallenge) efficacy is also illustrated by the failure of anti-rTox A alone to provide significant protection when administered after C. difficile challenge (Table 2).
TABLE 2.
Results of prophylactic and therapeutic treatment studies with anti-rTox A
| Time of 1st dose (h)a | Dose (mg) and frequency (no. of days)b | No. of hamsters | Reduction of mortality (%) |
|---|---|---|---|
| −24 | 40, t.i.d. (12) | 7 | 100 |
| −24 | 40, t.i.d. (7) | 7 | 100 |
| −24 | 40, t.i.d. (7) | 7 | 100 |
| −24 | 40, t.i.d. (7) | 7 | 100 |
| −24 | 40, t.i.d. (7) | 7 | 100 |
| −24 | 80, q.d. (4) | 10 | 60 |
| +3 | 40, t.i.d. (4) | 12 | 50 |
| +4 | 80, q.d. (3) | 9 | 56 |
| +16–24 | 80, t.i.d. (2) | 20 | 45 |
−, before challenge; +, after challenge.
A regimen of 40 mg t.i.d. for 7 days was used in four independent experiments.
Treating hamsters therapeutically with different dosing regimens beginning 2 to 8 h after challenge with C. difficile indicated that a dose of 80 mg of CDAD antitoxin once a day (q.d.) for 3 days is effective in treating CDAD in the hamster model. Doses at 40 mg or less q.d. did not significantly protect the hamster. In these experiments, hamsters treated with placebo all predictably died from CDAD within 48 h after challenge. CDAD antitoxin was further tested in the hamster model to determine a dose which can prevent mortality and morbidity (diarrhea) in 50% of the animals (50% effective dose [ED50]). Hamsters were treated 6 h after C. difficile challenge with 20, 40, 60, and 80 mg (by weight) of CDAD antitoxin and were observed for the onset of diarrhea or death. The dose-response profiles 4 days after termination of treatment with respect to prevention of mortality are shown in Fig. 4. From the results, the daily ED50 calculated by linear regression to prevent morbidity in the hamster was 32.6 mg. The ED50 to prevent morbidity was determined to be 54.7 mg (data not shown). As expected, a slightly higher dose is required to prevent morbidity than to prevent mortality in the hamsters. CDAD antitoxin was also found to be completely effective in the hamster model against different toxigenic C. difficile strains and different challenge doses (data not shown).
FIG. 4.
Effect of CDAD antitoxin dose on death in hamsters after C. difficile challenge. Seven hamsters were tested at each CDAD antitoxin dose. Hamsters were treated q.d. for 3 days. The equation of the line that approximates the best fit of the data points is shown.
Hamsters successfully treated with CDAD antitoxin show no relapse of infection for weeks and months after discontinuation of therapy. Hamsters successfully treated therapeutically or prophylactically with anti-rTox A alone also did not relapse. This outcome is in sharp contrast to that of treatment with vancomycin and other drugs used to treat CDAD in the hamster model, which show essentially 100% relapse and death within days after the termination of treatment. As shown in Fig. 5, a 5 day oral treatment with vancomycin (Vancocin HCl; Eli Lilly and Company, Indianapolis, Ind.) at three different doses indicated that doses of 1.0 or 5.0 mg/kg could initially protect the hamsters from mortality compared to the untreated controls, but animals began to relapse and die from 2 to 7 days after the termination of treatment. In contrast, treatment with CDAD antitoxin provided long-lasting protection in the hamsters. The lack of relapse in hamsters treated with CDAD antitoxin has been reproduced in numerous experiments. More than 200 successfully treated hamsters have not relapsed after discontinuation of therapy when observed for weeks to months (data not shown).
FIG. 5.
Cumulative mortalities caused by clindamycin-induced CDAD in hamsters that were either untreated (⧫), treated q.d. with 0.2 (□), 1.0 (▵), or 5.0 (○) of oral vancomycin/kg, or treated with CDAD antitoxin at a 40-mg dose t.i.d. (×). Nineteen animals were used in the CDAD antitoxin treatment group, and six animals were used in each of the untreated and vancomycin-treated groups.
Surprisingly, hamsters successfully treated with CDAD antitoxin were resistant to a subsequent rechallenge with clindamycin and C. difficile. This resistance to rechallenge in these hamsters, even after numerous rechallenge attempts, has been shown in many experiments. One example is shown in Fig. 6. In this experiment, hamsters successfully treated with CDAD antitoxin were reexposed to clindamycin, followed by rechallenges with three different strains (ATCC 43596, VPI 7698, and ATCC 43255) of C. difficile (second, third, and fourth challenges, respectively) over the course of several months. The hamsters in this study were treated with one regimen of CDAD antitoxin at the beginning of the study. At each rechallenge time, a set of naive hamsters was also predisposed with clindamycin and challenged with the same strain of C. difficile to ensure that the bacteria could effectively kill those animals. As shown in Fig. 6, the CDAD antitoxin-treated hamsters completely resisted infection after four C. difficile challenges. While some hamsters died in other rechallenge experiments, a significant number were refractory to subsequent infections.
FIG. 6.
Effect of CDAD antitoxin on mortality after C. difficile rechallenge in the hamster model. Hamsters were induced with clindamycin, challenged with C. difficile ATCC 43596 (first challenge [1st]), then treated q.d. for 3 days with 80 mg of CDAD antitoxin (□) (see Materials and Methods). These treated hamsters were then rechallenged at the time points indicated with clindamycin and C. difficile ATCC 43596 (2nd), VPI 7698 (3rd), and ATCC 43255 (4th). Hamsters treated with placebo (preimmune IgY, given at 80 mg q.d. for 3 days) at the first C. difficile challenge (○) and untreated hamsters at the second (▴), third (×), and fourth (•) C. difficile challenges served as controls. Six to nine hamsters were used in each challenge experiment.
DISCUSSION
The goal of this study was to evaluate the feasibility of producing a passive immunotherapeutic for CDAD. This therapeutic should be safe, fast-acting, and effective and should protect against relapse. We have shown in the hamster model of CDAD that avian antibodies against recombinant epitopes of toxin A and toxin B are as effective as vancomycin. The CDAD antitoxin acts quickly. Animals were rescued when treatment was initiated as late as 16 h prior to their anticipated deaths. Thus far, we have observed no adverse effects of therapy. Animals do not relapse after treatment with CDAD antitoxin, in contrast to vancomycin therapy. Moreover, we unexpectedly found that treated hamsters are refractory to reinfection with C. difficile.
These results validate our approach of targeting both toxins to treat this disease. Prior to this study, the importance of toxin B as an antibody target for CDAD therapy was unclear. A present paradigm states that toxin A, an enterotoxin, binds to the intestinal mucosa, causing the initial requisite tissue injury. This insult then permits toxin B, a potent cytotoxin, to bind to its yet-unknown cell receptor and cause more severe cellular damage. Since toxin B may only work synergistically with toxin A, effective neutralization of toxin A alone may be sufficient for full efficacy. This sequence of toxin A and toxin B pathogenesis has been questioned, since C. difficile variants that produce toxin B but lack a functional toxin A are still highly pathogenic in hamsters (6). Furthermore, it has been reported that toxin B by itself is about 10 times more potent than toxin A in damaging human colonic epithelial cells in vitro (34).
Our results show that a mixture of anti-rTox A and anti-rTox B is required for complete efficacy in the C. difficile hamster model of CDAD. While anti-rTox A treatment after C. difficile challenge at the doses tested could significantly protect the hamsters from death, it did not prevent diarrhea and weight loss in the majority of the hamsters. Unexpectedly, the anti-rTox A-treated hamsters exhibited profuse diarrhea, characteristic of an enterotoxin rather than a cytotoxin. Only when anti-rTox A was combined with anti-rTox B were the hamsters completely protected from CDAD. The importance of toxin B antibodies in CDAD may have been overlooked previously because it is most relevant only during therapeutic administration. We found that prophylactically dosing hamsters with anti-rTox A alone was sufficient to protect them from mortality and detectable morbidity from C. difficile. It is possible that anti-rTox A, administered before C. difficile challenge, can efficiently prevent toxin A from damaging the mucosal barrier, thereby obviating the need for anti-rTox B. In contrast, when anti-rTox A alone is administered after challenge, these antibodies are unable to prevent a low level of mucosal disruption by toxin A, thereby allowing toxin B to act. In the presence of anti-rTox B, toxin B is not available at levels necessary to cause significant damage. As expected, if toxin B antibodies alone are administered therapeutically, the full toxic effects of toxin A, and possibly those of low levels of unbound toxin B, are manifested, resulting in an overall lack of protection in the hamsters. Toxin B may be important in clinical scenarios where the patient’s mucosa is compromised by drugs, other pathogens, or trauma.
Analysis of antibodies against the recombinant toxin A and toxin B epitopes in toxin neutralization studies indicated that the carboxy-terminal regions of each toxin were most effective in generating toxin-neutralizing polyclonal antibodies in hens. These regions each possess a series of repetitive amino acid domains (49). This region of toxin A is functionally important and contains the putative intestinal cell-binding domain (24). Monoclonal antibodies directed against the C terminus of toxin A have been reported to neutralize the enterotoxicity of toxin A in vitro (12). While the carboxy terminus of toxin B has sequence homology with that of toxin A and other carbohydrate-binding domains (50), direct evidence of its function has not been reported. Recent studies have demonstrated that the toxins share the same enzymatic activity; both are UDP-glucosyl-transferases that bind to Rho proteins, causing F-actin disorganization of the cell cytoskeleton (20, 21). Differences between the toxins may only lie in their affinities or the cell specificities of their binding domains. Our study indicates that the C-terminal region of toxin B contains neutralizing epitopes and, as predicted, probably functions as the cell-binding domain.
The successful production of recombinant toxin epitopes was key in enabling us to prepare specific reagents to dissect the importance of antibodies against each toxin fragment when developing an effective CDAD therapeutic. To generate toxin antigens at levels required for large-scale immunization, the optimal approach is to use recombinant proteins rather than using culture filtrates or purified native toxins. Culture filtrates normally contain only small amounts of toxins, with impurities that can themselves generate unwanted antibodies. Culture filtrates as a source for toxin antigens also contain variable and uncontrollable levels of each toxin. Both culture filtrates and purified native toxins are potentially dangerous and require formalin inactivation to eliminate the hazard to personnel and injected animals. This in turn, may also destroy key conformational epitopes of the toxins. Proper conformation of the toxin epitopes is also uncertain in preparing them recombinantly. Another possible drawback of the recombinant approach is the inability to express adequate amounts of recombinant protein for immunization. Indeed, only low expression levels were seen in most of the recombinant fragments that encoded proteins greater than 100 kDa. Only recombinant toxin fragments that encoded proteins in the 50- to 100-kDa range expressed amounts of protein useful for immunization. Production of nontoxic recombinant toxins allows for the efficient production of each toxin epitope without cross-contamination, while eliminating the hazard of intact toxins. In addition, the recombinant approach facilitates single-step purification using expression systems that encode affinity tags. This resulted in the production of substantially pure recombinant-toxin epitopes.
Polyclonal IgY’s from hens were chosen as the neutralizing agents because prior work has demonstrated their potent ability to neutralize other biological poisons, including other clostridial toxins and snake venoms (46, 47). The generation of high-titer IgY in the hens by multiple immunizations and the ability to purify large quantities of material reproducibly and economically from egg yolk were also important factors in selecting avian antibodies to treat CDAD. All the recombinant-toxin protein fragments we prepared elicited high polyclonal-antibody levels in the hens. Polyclonal antibodies also have several advantages relative to monoclonal antibodies in that (i) they often have much higher affinities for their targets, resulting in superior potency; (ii) they can react to multiple toxin epitopes, so the emergence of drug resistance by point mutations is unlikely; and (iii) they can recognize multiple toxin isotypes, which is especially relevant since polymorphisms of the toxin A and toxin B genes of C. difficile have been reported (38).
CDAD antitoxin was effective in the hamster model of C. difficile. This model has clear advantages over in vitro or ileal-loop assays used in toxin neutralization studies. The hamster model offers clear end points and is reproducible, and the disease manifestations, such as diarrhea and colitis, are similar to those found in humans. In our experience, virtually all the placebo-treated hamsters died from CDAD within 24 to 48 h after C. difficile challenge. Results in the hamster model may also be more relevant clinically because the disease is initiated by the C. difficile infection, not simply by toxin administration, as with in vitro assays. Any significant efficacy in this model is important due to the very rapid progression and severity of CDAD in hamsters. Furthermore, the effect of antibody treatment on disease relapse can be monitored in this model. Overall, this model shows promise for predictions of drug performance in human CDAD.
Hamsters treated with CDAD antitoxin showed no relapse of infection weeks and months after discontinuation of therapy. This is in contrast to the findings of a previous study (26), where bovine antibodies against a culture filtrate were unable to protect the hamsters beyond the treatment regimen. This inability to achieve long-term efficacy is also seen in the hamster model when antibiotics, such as vancomycin, are used. Both the prophylactic and the therapeutic administration of anti-rToxA and anti-rTox B completely protected the hamsters against CDAD relapse. Since prior antibiotic therapy causes CDAD, the selective targeting of the toxigenic C. difficile by CDAD antitoxin may be the key to explaining these results. The toxins may be involved in colonization or survival of C. difficile within the intestines. Targeting the toxins may more effectively eradicate the organism and minimize the disruption of the microflora compared to antibiotics. This enables the normal colonic microflora in the gut to effectively compete with and prevent colonization by C. difficile. Also, in contrast to antibiotics, CDAD antitoxin may not promote the formation of spores, which is thought to be involved in reinfection after treatment (30). Surprisingly, in addition to lack of relapse, most of the successfully treated hamsters were refractory to CDAD even after rechallenge. Why the CDAD antitoxin effectively prevents CDAD in the hamster in this manner is unknown. Only very low levels of serum IgG to the toxins were found in these hamsters (data not shown), so the presence of a protective humoral response to afford long-term protection may be unlikely. In fecal samples, low levels of mucosal IgA to the toxins have also been detected. We are investigating whether this is a local IgA or cellular immune response. Clinical studies are in progress to determine the efficacy of CDAD antitoxin in the treatment and prevention of CDAD in humans. It would be anticipated that the human clinical trial would evaluate CDAD antitoxin appropriately formulated to improve its gastrointestinal survival.
ACKNOWLEDGMENTS
We thank D. L. Hottmann, C. M. Clemens, and L. M. Byrne for their valuable technical assistance. We also thank R. W. Schatz, D. C. Stafford, R. Godiska, and S. B. Carroll for manuscript review and Lynn Affetto for help in preparing the manuscript.
REFERENCES
- 1.Anand A, Glatt A E. Clostridium difficile infection associated with antineoplastic chemotherapy: a review. Clin Infect Dis. 1993;17:109–113. doi: 10.1093/clinids/17.1.109. [DOI] [PubMed] [Google Scholar]
- 2.Barroso L A, Wang S Z, Phelps C J, Johnson J L, Wilkins T D. Nucleotide sequence of Clostridium difficile toxin B. Nucleic Acids Res. 1990;8:4004. doi: 10.1093/nar/18.13.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartlett J G. Antimicrobial agents implicated in Clostridium difficile toxin-associated diarrhea or colitis. Johns Hopkins Med J. 1981;149:6–9. [PubMed] [Google Scholar]
- 4.Bartlett J G. Clostridium difficile: history of its role as an enteric pathogen and the current state of knowledge about the organism. Clin Infect Dis. 1994;18:S265–S272. doi: 10.1093/clinids/18.supplement_4.s265. [DOI] [PubMed] [Google Scholar]
- 5.Borriello S P, Welch A R, Barclay F E, Davies H A. Mucosal association by Clostridium difficile in the hamster gastrointestinal tract. J Med Microbiol. 1988;25:191–196. doi: 10.1099/00222615-25-3-191. [DOI] [PubMed] [Google Scholar]
- 6.Borriello S P, Wren B W, Hyde S, Seddon S V, Sibbons P, Krishna M M, Tabaqchali S, Manek S, Price A B. Molecular, immunological, and biological characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile. Infect Immun. 1992;60:4192–4199. doi: 10.1128/iai.60.10.4192-4199.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buggy B P, Fekety R, Silva J. Therapy of relapsing Clostridium difficile-associated diarrhea with the combination of vancomycin and rifampin. J Clin Gastroenterol. 1987;9:155–159. doi: 10.1097/00004836-198704000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Nosocomial enterococci resistant to vancomycin—United States, 1989. Morbid Mortal Weekly Rep. 1993;42:597–599. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Recommendations for preventing the spread of vancomycin resistance: recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC) Morbid Mortal Weekly Rep. 1995;44:1–13. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morbid Mortal Weekly Rep. 1997;46:471–476. [PubMed] [Google Scholar]
- 11.Chang T, Bartlett J G, Gorbach S L, Onderdonk A B. Clindamycin-induced enterocolitis in hamsters as a model of pseudomembranous colitis in patients. Infect Immun. 1978;20:526–529. doi: 10.1128/iai.20.2.526-529.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corthier G, Muller M C, Wilkins T D, Lyerly D, L’Haridon R. Protection against experimental pseudomembranous colitis in gnotobiotic mice by use of monoclonal antibodies against Clostridium difficile toxin A. Infect Immun. 1991;59:1192–1995. doi: 10.1128/iai.59.3.1192-1195.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dworczynski A, Sokol B, Meisel-Mikolajczyk F. Antibiotic resistance of Clostridium difficile isolates. Cytobios. 1991;65:149–153. [PubMed] [Google Scholar]
- 14.Fekety R. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. Am J Gastroenterol. 1997;92:739–750. [PubMed] [Google Scholar]
- 15.Fekety R, Shah A B. Diagnosis and treatment of Clostridium difficile colitis. JAMA. 1993;269:71–75. [PubMed] [Google Scholar]
- 16.Fekety R, Silva J, Toshniwal R, Allo M, Armstrong J, Browne R, Ebright J, Rifkin G. Antibiotic-associated colitis: effects of antibiotics on Clostridium difficile and the disease in hamsters. Rev Infect Dis. 1979;1:386–397. doi: 10.1093/clinids/1.2.386. [DOI] [PubMed] [Google Scholar]
- 17.Gorbach S L. Successful treatment of relapsing Clostridium difficile colitis with Lactobacillius GG. Lancet. 1987;ii:1519. doi: 10.1016/s0140-6736(87)92646-8. [DOI] [PubMed] [Google Scholar]
- 18.Hecht J R. Clostridium difficile colitis secondary to intravenous vancomycin. Dig Dis Sci. 1989;34:148–149. doi: 10.1007/BF01536172. [DOI] [PubMed] [Google Scholar]
- 19.Jobe B, Grasely A, Deveney K. Clostridium difficile colitis: an increasing hospital acquired illness. Am J Surg. 1995;169:480–483. doi: 10.1016/S0002-9610(99)80199-8. [DOI] [PubMed] [Google Scholar]
- 20.Just I, Selzer J, Wilm M, von Eichel-Streiber C, Mann M, Aktories K. Glucosylation of rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 21.Just I, Wilm M, Selzer J, Rex G, von Eichel-Streiber C, Mann M, Aktories K. The enterotoxin from Clostridium difficile (toxin A) monoglucosylates the rho proteins. J Biol Chem. 1995;270:13932–13936. doi: 10.1074/jbc.270.23.13932. [DOI] [PubMed] [Google Scholar]
- 22.Kelly C P, Pothoulakis C, LaMont J T. Clostridium difficile colitis. N Engl J Med. 1994;330:257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 23.Kim P H, Iaconis J P, Rolfe R D. Immunization of adult hamsters against Clostridium difficile-associated ileocecitis and transfer of protection to infant hamsters. Infect Immun. 1987;55:2984–2992. doi: 10.1128/iai.55.12.2984-2992.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kriven H C, Clark G F, Smith D F, Wilkins T D. Cell surface binding site for Clostridium difficile enterotoxin: evidence for a glycoconjugate containing the sequence Galα1-3Galβ1-4GlcNAc. Infect Immun. 1986;53:573–581. doi: 10.1128/iai.53.3.573-581.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Libby J M, Jortner B S, Wilkins T D. Effects of the two toxins of Clostridium difficile antibiotic-associated cecitis in hamsters. Infect Immun. 1982;36:822–829. doi: 10.1128/iai.36.2.822-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyerly D M, Bostwick E F, Binion S B, Wilkins T D. Passive immunization of hamsters against disease caused by Clostridium difficile by use of bovine immunoglobulin G concentrate. Infect Immun. 1991;59:2215–2218. doi: 10.1128/iai.59.6.2215-2218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyerly D M, Krivan H C, Wilkins T D. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988;1:1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McFarland L V, Surawicz C M, Greenberg R N, Fekety R, Elmer G W, Moyer R A, Melcher J A, Bowen K E, Cox J L, Noorani Z, Harrington G, Rubin M, Greenwald D. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;217:1913–1918. [PubMed] [Google Scholar]
- 29.Nord C D, Edlund C. Impact of antimicrobial agents on the intestinal microflora. J Chemother. 1990;2:218–237. doi: 10.1080/1120009x.1990.11739021. [DOI] [PubMed] [Google Scholar]
- 30.Onderdonk A B, Cisneros R L, Bartlett J G. Clostridium difficile in gnotobiotic mice. Infect Immun. 1980;28:277–282. doi: 10.1128/iai.28.1.277-282.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polson A, von Wechmar B M, Fazakerley G. Antibodies to proteins from yolk of immunized hens. Immunol Commun. 1980;9:495–514. doi: 10.3109/08820138009066011. [DOI] [PubMed] [Google Scholar]
- 32.Pothoulakis C, Sullivan R, Melnick D A, Triadafilopoulos G, Gadenne A S, Meshulam T, LaMont J T. Clostridium difficile toxin A stimulates intracellular calcium release and chemotactic response in human granulocytes. J Clin Invest. 1988;81:1741–1745. doi: 10.1172/JCI113514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rafferty M E, McCormick M I, Bopp L H, Baltch A L, George M, Smith R P, Rheal C, Ritz W, Schoonmaker D. Vancomycin-resistant enterococci in stool specimens submitted for Clostridium difficile cytotoxin assay. Infect Control Hosp Epidemiol. 1997;18:342–344. doi: 10.1086/647623. [DOI] [PubMed] [Google Scholar]
- 34.Riegler M, Sedivy R, Pothoulakis C, Hamilton G, Zacherl J, Bischof G, Cosentini E, Feil W, Schiessel R, LaMont J T, Wenzl E. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J Clin Invest. 1995;95:2004–2011. doi: 10.1172/JCI117885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riley T V. Antibiotic-associated diarrhoea: a costly problem. PharmacoEconomics. 1996;10:1–3. doi: 10.2165/00019053-199610010-00001. [DOI] [PubMed] [Google Scholar]
- 36.Roberts M C, McFarland L V, Mullany P, Mulligan M E. Characterization of the genetic basis of antibiotic resistance in Clostridium difficile. J Antimicrob Chemother. 1994;33:419–429. doi: 10.1093/jac/33.3.419. [DOI] [PubMed] [Google Scholar]
- 37.Rocha M F, Maia M E T, Bezerra L R P S, Lyerly D M, Guerrant R L, Ribeiro R A, Lima A A M. Clostridium difficile toxin A induces the release of neutrophil chemotactic factors from rat peritoneal macrophages: role of interleukin-1β, tumor necrosis factor alpha, and leukotrienes. Infect Immun. 1997;65:2740–2746. doi: 10.1128/iai.65.7.2740-2746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rupnik M, Braun V, Soehn F, Janc M, Hofstetter M, Laufenberg-Feldmann R, von Eichel-Streiber C. Characterization of polymorphisms in the toxin A and B genes of Clostridium difficile. FEMS Microbiol Lett. 1997;148:197–202. doi: 10.1111/j.1574-6968.1997.tb10288.x. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritisch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 40.Samore M H. Epidemiology of nosocomial Clostridium difficile infection. Compr Ther. 1993;19:151–156. [PubMed] [Google Scholar]
- 41.Sauerborn M, Eichel-Streiber C. Nucleotide sequence of Clostridium difficile toxin A. Nucleic Acids Res. 1990;18:1629–1630. doi: 10.1093/nar/18.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwan A, Sjholins S, Trottestam U, Aronsson B. Relapsing Clostridium difficile enterocolitis cured by rectal infusion of normal feces. Scand J Infect Dis. 1984;16:211–215. doi: 10.3109/00365548409087145. [DOI] [PubMed] [Google Scholar]
- 43.Silva J. Update on pseudomembranous colitis. West J Med. 1988;151:644–648. [PMC free article] [PubMed] [Google Scholar]
- 44.Tedesco F J. Treatment of recurrent antibiotic-associated pseudomembranous colitis. Am J Gastroenterol. 1982;77:220–221. [PubMed] [Google Scholar]
- 45.Tedesco F J, Gorden D, Fortson W C. Approach to patients with multiple relapses of antibiotic-associated pseudomembranous colitis. Am J Gastroenterol. 1985;80:867–868. [PubMed] [Google Scholar]
- 46.Thalley B S, Carroll S B. Rattlesnake and scorpion antivenoms from the eggs of yolks of immunized hens. Bio/Technology. 1990;8:934–938. doi: 10.1038/nbt1090-934. [DOI] [PubMed] [Google Scholar]
- 47.Thalley B S, van Boldrik M B, Carroll S B, Tepp W, Das Gupta B R, Stafford D C. Development of an avian antitoxin to type A botulinum neurotoxin. In: Das Gupta B R, editor. Botulinum and tetanus neurotoxins. New York, N.Y: Plenum Press; 1993. pp. 467–472. [Google Scholar]
- 48.Tvede M, Rask-Madsen J. Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet. 1989;i:1156–1160. doi: 10.1016/s0140-6736(89)92749-9. [DOI] [PubMed] [Google Scholar]
- 49.von Eichel-Streiber C, Laufenberg-Feldmann R, Sartingen S, Schulz J, Sauerborn M. Comparative sequence analysis of the Clostridium difficile toxins A and B. Mol Gen Genet. 1992;233:260–268. doi: 10.1007/BF00587587. [DOI] [PubMed] [Google Scholar]
- 50.von Eichel-Streiber C, Sauerborn M, Kuramitsu H K. Evidence for a modular structure of the homologous repetitive C-terminal carbohydrate-binding sites of Clostridium difficile toxins and Streptococcus mutans glucosyltransferases. J Bacteriol. 1992;174:6707–6710. doi: 10.1128/jb.174.20.6707-6710.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watanabe T, Oshashi K, Matsui K, Kubota T. Comparative studies of the bactericidal, morphological and post-antibiotic effects of arbekacin and vancomycin against methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1997;39:471–476. doi: 10.1093/jac/39.4.471. [DOI] [PubMed] [Google Scholar]
- 52.Wilcox M H, Cunniffe J G, Trundle C, Redpath C. Financial burden of hospital-acquired Clostridium difficile infection. J Hosp Infect. 1996;34:23–30. doi: 10.1016/s0195-6701(96)90122-x. [DOI] [PubMed] [Google Scholar]
- 53.Williams J A, Langeland J A, Thalley B S, Skeath J B, Carroll S B. Expression of foreign proteins in E. coli using plasmid vectors and purification of specific polyclonal antibodies. In: Glover D M, Hames B D, editors. DNA cloning: a practical approach. 2nd ed. 2. Expression systems. Oxford, United Kingdom: Oxford University Press; 1995. pp. 15–58. [Google Scholar]
- 54.Wren B W, Tabaqchali S. Restriction endonuclease DNA analysis of Clostridium difficile. J Clin Microbiol. 1987;25:2402–2404. doi: 10.1128/jcm.25.12.2402-2404.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]