Abstract
Global importance:
Hypokalaemic polymyopathy is a genetic disease of Burmese cats that has been encountered in Australasia, Europe and South Africa.
Clinical features:
Affected cats usually present with signs of muscle weakness and muscle pain in the first year of life. Although certain clinical features, such as ventroflexion of the head and neck, are especially characteristic, some cats do not display these signs. Usually weakness is periodic or episodic, but occasionally it is incessant.
Diagnostic challenges:
In the past, diagnosis was problematic in that clinical signs and a lowered serum potassium concentration were not always observed synchronously. This necessitated serial serum potassium concentration determinations, testing of serum creatine kinase activity and exclusion of other potential causes of muscle disease in cats (including muscular dystrophies, Toxoplasma myositis, immune-mediated polymyositis, organophosphorus intoxication and envenomations). Signs in affected cats often waxed and waned, possibly in response to changes in dietary factors and stress, and some cats could apparently ‘grow out of’ the condition.
Recent advances and future prospects:
Recent molecular genetics research has identified a single nonsense mutation in the gene (WNK4) coding for lysine-deficient 4 protein kinase, an enzyme present primarily in the distal nephron. The underlying pathomechanism in affected cats is therefore likely to be a potassium wasting nephropathy, as this enzyme is involved in complex sodium/potassium exchange mechanisms in the kidney. Additional functional characterisation of the condition is warranted to define precisely how, why and when the serum potassium concentration declines. The diagnosis of Burmese hypokalaemia is now straightforward, as an inexpensive PCR test can identify affected homozygous individuals, as well as carriers. The elimination of this condition from the Burmese breed, and also from pedigree cats infused with Burmese lines, such as the Bombay, Tonkinese and Tiffanie breeds, should therefore be possible.
In 2012, periodic hypokalaemia of Burmese and Tonkinese cats was shown to be caused by a nonsense mutation (a point mutation resulting in a premature stop codon) in the gene coding for the enzyme lysine-deficient 4 protein kinase (WNK4) 1 (see Online Mendelian Inheritance in Animals entry OMIA 001759-9685). This autosomal recessive inherited condition was the first feline disease phenotype characterised using a genome-wide association study (GWAS). A first-generation Illumina single nucleotide polymorphism microarray (SNP chip), encompassing approximately 63,000 SNPs, localised the causative mutation to cat chromosome E1. Subsequent sequencing of candidate genes eventually resulted in detection of the mutation. WNK4 is one of four members of the WNK kinases, which are atypical protein kinases with pleiotropic actions.2 –5 Their genomic structure is complex, with several transcripts detected in different tissues.2 –5

Pathways and pathomechanisms
WNK4 is expressed in tissues containing secretory epithelia. High expression is detected in the kidney, while the pancreas, bile duct, brain, epididymis and skin show lower enzyme levels.2,3 It has a kinase domain that phosphorylates other kinases in the kidney, an autoinhibitory domain that regulates its own activity and an autophosphorylation site in the activation loop. 4 WNK4 reduces the activity of the sodium chloride co-transporter (NCC) by decreasing its abundance at the plasma membrane; this results in fewer sodium and chloride ions being transported into cells of the distal convoluted tubule (DCT) in the kidney. WNK4 also plays a crucial role in controlling the epithelial sodium channel (ENaC) involved in sodium reabsorption and reducing the abundance of the renal outer medullary potassium channel 1 (ROMK1) at the cell membrane.
Thus, WNK4 regulates complex pathways in the DCT that collectively regulate the activity of all major sodium and potassium transporters, 5 acting as a molecular switch between angiotensin II–aldosterone-mediated volume retention and aldosterone-mediated potassium wasting, by regulating NCC, ENaC and ROMK1. In humans, mutations in the WNK4 gene cause type II pseudohypoaldosteronism (see Online Mendelian Inheritance in Man entry OMIM 145260) and acquired forms of hypertension caused by salt sensitivity, 6 but, interestingly, not hypokalaemia. Many questions regarding the role of WNK4 remain unanswered,2 –6 and so its involvement in hypokalaemic polymyopathy is novel and intriguing. 1
Ultimately, altered function of WNK4 in Burmese cats somehow causes sufficient potassium loss into the urine to result in symptomatic hypokalaemia; this, in turn, causes muscle weakness, by shifting the resting membrane potential of muscle cells further away from the threshold for action potential generation, and polymyopathy. Functional studies of whole body potassium balance and more focused studies of the kidney are required to decipher the pathomechanisms of renal potassium loss. Hypokalaemia is manifest clinically by signs linked to muscle pain and weakness, and leakage of enzymes such as creatine kinase (CK) from the cytoplasm of muscle cells. Enigmatically, signs tend to be episodic or periodic, rather than incessant.
The discovery that a mutation in WNK4 causes periodic hypokalaemia is remarkable in that the underlying pathophysiology had not been anticipated by researchers who had been investigating this condition since its first description in the early 1980s.7,8 However, such discrepancies are by no means without precedence. This situation arises because in the first part of a GWAS, molecular genetic tools require no a priori assumptions about causal pathomechanisms to locate the general region where the genetic defect is located; the critical requirement is a crisp delineation of normal vs affected phenotypes.
First records of periodic hypokalaemia in the literature
The first report of hypokalaemia causing myopathic weakness was made in 1983 by Eger and colleagues from Murdoch University in Western Australia. 7 (Clive Eger was a surgeon with a special interest in neurology, having spent time with Sandy deLahunta at Cornell University, USA, after training as a specialist surgeon at the University of Ontario, Canada.) The seminal paper, in the Journal of Small Animal Practice, concerned a cat with Conn’s syndrome (hyperaldosteronism). However, in their Discussion, the authors included a succinct but lucid description of two Burmese kittens (siblings) with clinical signs virtually identical to the cat of their report (which had an aldosterone-producing adrenal tumour). 7 As all three animals were hypokalaemic, the authors correctly surmised that the characteristic clinical signs displayed by these cats were attributable to hypokalaemia.
A more comprehensive report of this entity – by Alison Blaxter and colleagues from the Feline Centre, Bristol, UK – was published in 1986 as a ‘Letter to the Editor’ of The Veterinary Record. 8 This captures the salient features of the clinical syndrome of hypokalaemia in Burmese cats. An expanded description of the same dataset is provided in a wonderful article by Tim Gruffydd-Jones, also of the Feline Centre, Bristol, written for the Burmese Cat Club. 9 At that stage, the disease in Burmese cats was thought to resemble hypokalaemic periodic paralysis, a human disease characterised by abnormal electrical excitability of the muscle cell membrane. Subsequently, Steven Dow, Rick LeCouteur and colleagues from Colorado State University, USA, described the clinical impact of hypokalaemia in the setting of chronic kidney disease (CKD).10 –14 At that time, hypokalaemia was especially common because a popular ‘prescription diet’ used to manage CKD was marginally deficient in potassium, exacerbating the tendency for cats with CKD to become hypokalaemic due to excessive potassium loss in the urine.10 –14
Although quite separate phenomenologically from the inherited disease of young Burmese cats, the Colorado papers had a huge impact by raising the profile of hypokalaemia as an entity in feline medicine. This resulted in the development of various commercial potassium supplements designed specifically for use in feline practice. Interestingly, Burmese cats with hypokalaemia have never been reported from the USA, because the breed in North America is generally distinct from the breed in Europe, South Africa and Australasia. (For this reason, other genetic diseases seen commonly in Burmese cats in Europe and Australasia – diabetes mellitus, feline orofacial pain syndrome, cutaneous asthenia – are likewise not encountered in North American Burmese lines.) 15
Burmese hypokalaemic polymyopathy was subsequently reported from a number of countries around the world; namely, New Zealand, 16 Germany 17 and the Netherlands. 18 The entity was reviewed by Boyd Jones and Tim Gruffydd-Jones in the Cornell Veterinarian, 19 and more recently by three of the current authors (FJM, MNG and VHM) and colleagues from The Cat Clinic in Brisbane, Australia, in a conference Abstract. 20 The latter provided additional pertinent insights, including the observation that Tonkinese cats can be affected. 20 Together with a call for cases in ‘Control & Therapy’ (an unrefereed journal produced quarterly by the Centre for Veterinary Education of the University of Sydney, Australia), 21 this conference Abstract afforded the current author group the opportunity of obtaining additional DNA specimens from affected patients in Australia. This, in turn, facilitated a GWAS in collaboration with feline genomics groups in the USA, UK, Ireland, Germany and New Zealand. 1
Mechanism of genetic inheritance
Chronologically, the next piece of the puzzle was solved by Ken Mason. (Though now best known as a veterinary dermatologist, researcher and medicated shampoo inventor, Mason was interested in neurology early in his career, following a Master’s degree in canine neural angiostrongyliasis at the University of Queensland, Australia). He saw his first affected Burmese in 1972, but it was 16 years before he went on to document his findings concerning a series of seven cats. 22 In his publication, Mason provided many pertinent clinical observations about these cats; and, having obtained sufficient genealogical information, determined that the condition was inherited as a highly penetrant autosomal recessive trait.
Clinical syndromes observed in association with hypokalaemia
Signalment
Most (but not all) affected cats first develop clinical signs when 2–10 months of age, the majority of them by 4–6 months.7,8,16 –20,22 –24 The average age of onset in Mason’s series of seven cats was 7 months. 22 Kittens usually display no abnormal signs while being nursed by their dams, suggesting there is sufficient potassium in the queen’s milk.
All cases to date have been observed in Burmese,7,8,16 –24 Bombay (see later) and Tonkinese cats, 20 although the potential exists for the problem to develop in breeds that have been based on Burmese outcrossings, such as the Burmilla, Australian Mist (formerly Spotted Mist) and Tiffanie (see later).
Physical findings
Clinical signs are sometimes very distinctive, almost pathognomonic – but not in every case! In some cats, the presentation can be cryptic, 23 and the diagnosis difficult. The key findings in affected cats reflect muscle weakness due to hyperpolarisation of the muscle cell membrane, and possibly muscle pain (myalgia). To quote Gruffydd-Jones: ‘It is an episodic problem and we see tremendous variation in the pattern’. 9
Weakness of the neck and thoracic limb girdle
The most characteristic sign of myopathic weakness in the cat is passive ventroflexion of the head and neck,7,23,24 presumably because the cervical muscles are too weak to support the head (see Figures 1a,b and 2a; also Videos 1–3 in the Supplementary material). Thus, the chin sinks with progressive cervical muscle fatigue; in extreme cases, cats adopt a ‘swan-like’ cervical posture, with the chin tucked into the sternum. Most cats with hypokalaemia display this sign to a variable extent, although some cats show no sign of passive ventroflexion.
Figure 1.
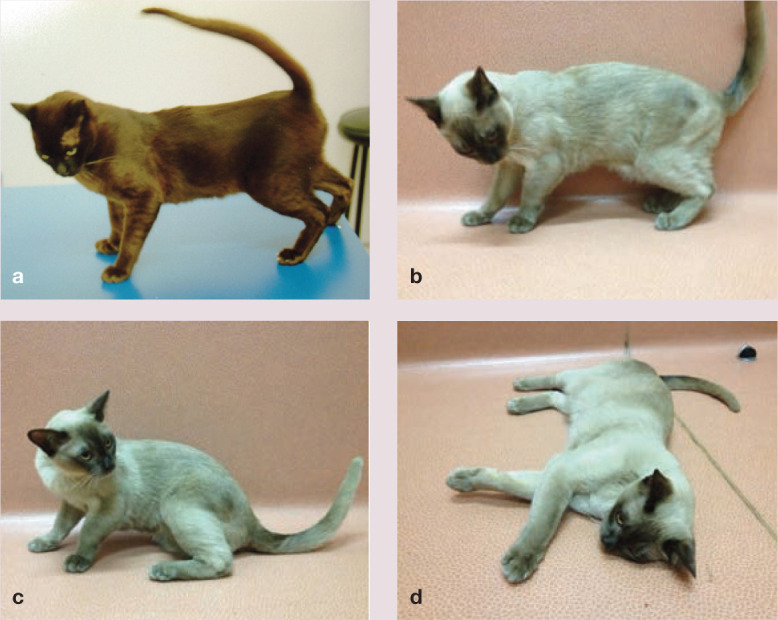
Different postures of two Burmese cats with hypokalaemic polymyopathy. Note the passive ventroflexion of the head and neck, most obvious in (a) and (b)
Figure 2.
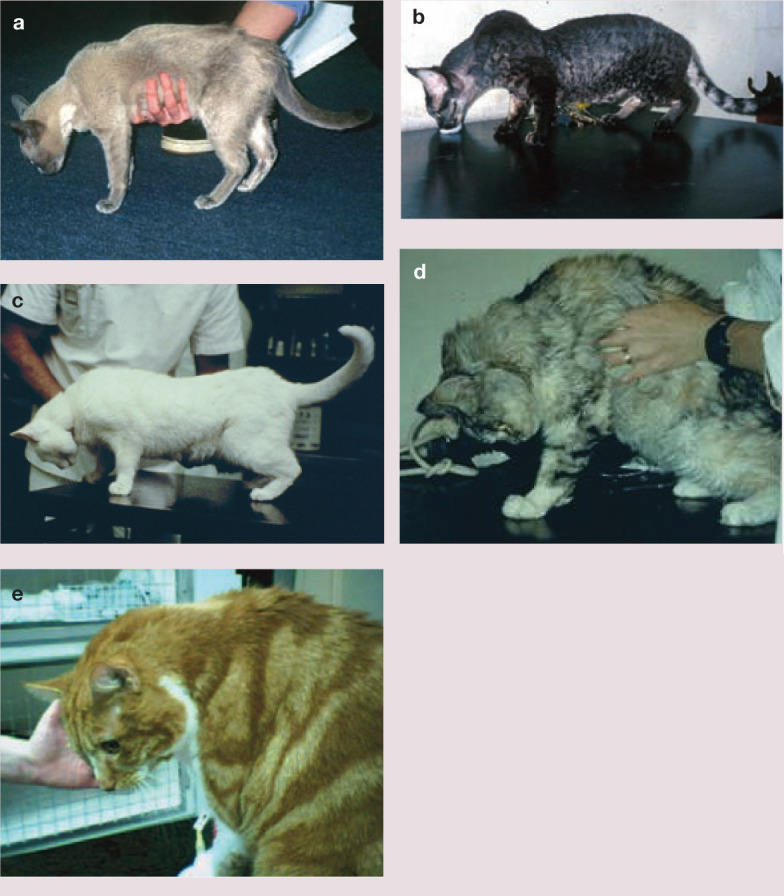
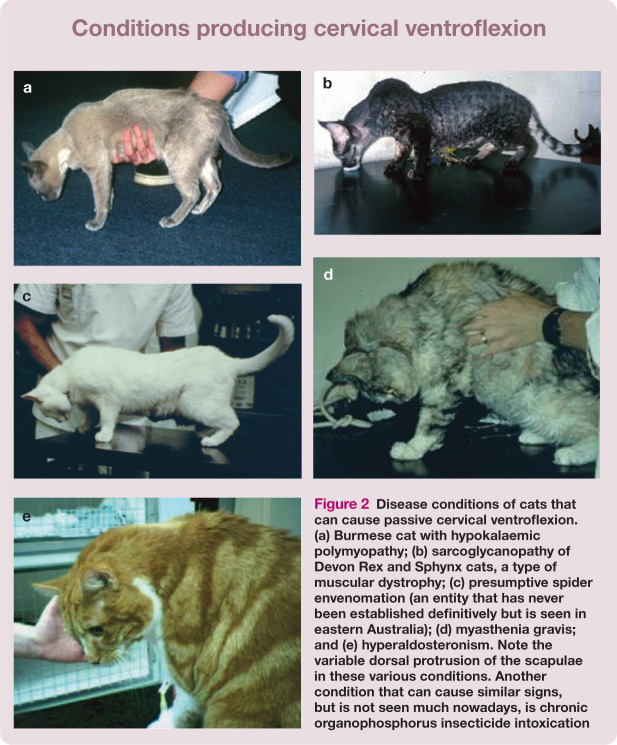
Disease conditions of cats that can cause passive cervical ventroflexion. (a) Burmese cat with hypokalaemic polymyopathy; (b) sarcoglycanopathy of Devon Rex and Sphynx cats, a type of muscular dystrophy; (c) presumptive spider envenomation (an entity that has never been established definitively but is seen in eastern Australia); (d) myasthenia gravis; and (e) hyperaldosteronism. Note the variable dorsal protrusion of the scapulae in these various conditions. Another condition that can cause similar signs, but is not seen much nowadays, is chronic organophosphorus insecticide intoxication

Note that passive cervical ventroflexion seen with myopathic weakness is quite different to the active ventroflexion of the head and neck observed in cats with the ‘seizures’ or spasms of thiamine deficiency, 25 or precipitated by vestibular manoeuvres, such as dropping the cat towards the ground. (In the past, this led to thiamine deficiency being misinterpreted as a cause for the clinical signs in affected Burmese cats. 22 ) Myopathic weakness is also distinct clinically from the neuropathic weakness observed with longstanding diabetes mellitus (manifest as a plantigrade stance, with severely affected cats ‘walking on their hocks’).
There are additional manifestations of cervical muscle weakness. Cats cannot properly fixate their head during locomotion, so head ‘bobbing’ or ‘nodding’ is prominent. To see their environment during periods of weakness, cats can adopt a ‘meerkat-like’ posture, which some people refer to as ‘periscoping’. They also have another characteristic myopathic posture whereby they place their head to one side, resting it on outstretched thoracic limbs.9,19,24
Similar signs can be seen in other disease states in which muscle weakness is prominent. Examples include the sarcoglycanopathy of Devon Rex and Sphynx cats (Figure 2b); 26 some presumptive spider envenomations (David Church, Liz Dill-Macky and RM, unpublished observations [Figure 2c]); the immune-mediated junctionopathy myasthenia gravis (Figure 2d); 27 immune-mediated polymyositis; protozoan polymyopathy (toxoplasmosis with muscle involvement); and chronic organophosphorus intoxication (rarely seen nowadays). 28 Curiously, these signs of myopathic weakness are generally absent in cases of tick paralysis due to Ixodes holocyclus and snake envenomation.29,30 Of course, similar signs are seen in other disorders that cause hypokalaemia, such as hyperaldosteronism (Conn’s syndrome [Figure 2e]) 31 and the potassium wasting nephropathy commonly observed in many cats with end-stage kidney disease.
Appendicular weakness
Appendicular weakness is variable in cats with Burmese hypokalaemia.7 –9,20 In some patients, mainly the forelimbs are affected, while in others the weakness affects mainly the hindlimbs; in others still, it is a combination of all four limbs. When the hindlimbs are affected, the most prominent feature may be an inability to ‘jump up’, which might not be appreciated by owners unless a careful history is taken. More severely affected cats have hindlimb paresis that may be misinterpreted as ataxia. Their gait has been described as ‘swaying’ or ‘crouching’ (see Videos 1 and 2 in the Supplementary material).
Cats with myopathic weakness need to recruit more motor units than normal cats for any given level of activity. For this reason, they can demonstrate tremor, especially when muscles fatigue. This tremor is usually more prominent in the thoracic limbs. Muscles controlling the digits can sometimes become weak, leading to protrusion of the claws. Some cats demonstrate signs consistent with myalgia, such as a shifting lameness, stiff or stilted gait and pain on deep palpation of muscle groups.7 –9,16 –23 Video 3, in the Supplementary material, kindly loaned by Clare Rusbridge, shows a Burmese cat with comparatively mild signs that displayed a progressively stilted gait, head bobbing and dorsal protrusion of the scapulae. Interestingly, the cat hid its weakness by stopping walking and rolling apparently nonchalantly on its back, when in reality it was resting after its appendicular muscles became fatigued.
If not recognised sufficiently early, cats can develop severe incapacitating weakness. They either cannot walk at all, or walk a short distance and then collapse, resting their head on outstretched thoracic limbs, as described earlier. If cats suffering an attack are subjected to excessive handling, stress or fear, pupillary dilatation and claw protrusion are said to be prominent. 22 In extreme cases, patients can apparently develop seizures and suffer cardiopulmonary arrest, requiring intubation, ventilation and external cardiac massage. 22
Clinical course
The clinical course of this condition is highly variable from case to case. The problem is generally episodic or periodic, but there is no consistent pattern. Mason noted that owners could often appreciate that their cats were ‘strange’ for a few hours preceding an attack of weakness (up to a day in some instances). 22 Some cats were completely normal between attacks of weakness, and bouts of weakness were quite infrequent. In other instances, signs were more severe and episodes more frequent, with cats probably never being normal between episodes. 22
Potential triggers
Some clinicians think stress is an important trigger. The importance of stress of one form or another on manifestations of different feline disease entities has been emphasised by Tony Buffington and colleagues, 32 although the concept remains controversial.
There are clues that diet and husbandry play a part in disease expression.9,11,22 Gruffydd-Jones, Jones, Mason and Menrath often observed improvements when kittens were placed in pet homes, or when affected patients were placed in hospital.9,19,22,23 Although improvements were attributed to a reduction in stress, such circumstantial evidence is also consistent with a change in diet ameliorating the hypokalaemia. The cat in Video 3 was approximately 5 years old when it first presented soon after a change to a high carbohydrate diet (C. Rusbridge, personal communication). Interestingly, Mason considered that feeding fish meals precipitated weakness in four of his seven cases, while feeding a fresh meat diet minimised signs in one cat. 22
Routine laboratory studies, electrodiagnostic testing and muscle histology
The cornerstone of securing a diagnosis of Burmese hypokalaemia was, until recently, based on demonstrating a serum potassium concentration ([K+]) below a threshold of 3.0 mmol/l (or 2.5 mmol/l according to some authorities).7 –14 Ideally, hypokalaemia should be demonstrable at the same time as the patient displays characteristic signs. In some cats, this was straightforward. However, in other individuals the serum [K+] would normalise by the time cats were presented for investigation, necessitating serial [K+] determinations to be made if the index of suspicion was sufficiently high. 9
Once the serum [K+] reaches a sufficiently low level, muscle cells are injured and leak cytoplasmic enzymes. These include CK (which is skeletal and cardiac muscle specific), and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (which are not muscle specific, but have longer half-lives than CK). This typically results in high to very high serum CK activity, with concurrent but more modest elevations in AST and ALT activity. Muscle leakage enzymes remain elevated after an episode of weakness even though the serum [K+] has normalised.9 –14,16 –21 Thus, consideration of [K+], CK and ALT activities in tandem often proved helpful in providing laboratory support for a diagnosis of hypokalaemic polymyopathy, even when the [K+] was initially normal.
The availability of molecular genetic testing, and ready access to inexpensive PCR-based diagnostic assays for this condition, has made many of the aforementioned considerations less pertinent. 1 However, adrenal ultrasonography and/or determination of serum aldosterone and possibly renin concentrations may be worthwhile in an older cat with hypokalaemia, to exclude a diagnosis of Conn’s syndrome. 31
The use of electromyography, nerve conduction studies, repetitive nerve stimulation, jitter analysis and muscle biopsy to investigate cats with weakness may seem logical.24,28 In practice, such studies are largely redundant when the most likely diagnosis is hypokalaemic polymyopathy. Myopathic potentials have not been detected in those cases of hypokalaemia that have undergone electrodiagnostic testing. Muscle biopsies subjected to special staining protocols, including transmission electron microscopy, have not revealed any changes; indeed, muscle is structurally normal.9,19,23

Treatment of hypokalaemia
Most cats with symptomatic hypokalaemia are still capable of eating and drinking. Therefore, oral replacement therapy is generally the best way to increase the serum [K+] and correct the likely total body potassium deficits.8 –12,19,23,24 The North American literature favours the use of potassium gluconate in a variety of different formulations (tablets, liquids, palatable pastes).10 –14 As the WNK4 mutation has never been reported in cats in North America, the patient cohort in US studies are likely cats with CKD and a potassium wasting nephropathy and/or a diet marginally deficient in potassium.11,13,14
In the authors’ experience, the easiest and most effective way to manage Burmese cats with hypokalaemia is using enteric-coated sustained-release potassium chloride (KCl) tablets (600 mg; Span-K; Rhone-Poulenc Rorer) (Figure 3). Most cats require half a tablet with food twice daily (usually given just before each meal). Breaking the enteric coat seems to cause no problems so long as tablets are given immediately before a meal. This provides a total daily supplement of 8 mmol KCl using an easily administered inexpensive formulation, and is much more practical than giving two large potassium gluconate tablets twice daily. For cats that are difficult to pill, 300 mg of analytic grade potassium chloride can be added relatively easily to an 85 g tin of commercial canned cat food, to be given twice daily. Other authors recommend palatable potassium gluconate formulations, such as Tumil-K (Virbac), which is provided in a meaty base.
Figure 3.

The potassium chloride formulation the authors recommend for treating hypokalaemic Burmese cats. Most cats require half a tablet given immediately before each meal. This formulation is inexpensive and readily available
It can be difficult to re-establish normal serum [K+] using oral supplementation alone in some cats. In this minority of problematic patients, two of the authors (RM and CS) have found that spironolactone (1–2 mg/kg q12–24h) can be a helpful adjunct.
The authors’ impression is that when given correct potassium supplementation, and occasionally spironolactone, affected cats become completely normal. Others suggest that potassium supplementation does not prevent the signs altogether, but reduces the frequency of ‘attacks’ and the extent of the weakness.9,22
Further work
As Burmese hypokalaemia is likely attributable to a very specific potassium wasting nephropathy, it should be possible to determine the physiological basis for the aforementioned, but somewhat enigmatic, clinical observations. Such work is being planned by two of the authors (BG and LAL) who have established a small colony of affected and carrier cats (see box below). Monitoring urinary [K+], blood pressure and the fractional excretion of potassium in affected cats may provide insights into pathomechanisms underlying changes in the serum [K+].9,11,14,23
No fractional excretion studies of urinary electrolytes have been published in peer-reviewed journals, and the interaction between serum [K+], dietary composition (water, sodium and potassium content) and other physiological factors (stress, dehydration, acid/base status, volume depletion, etc) is likely to be complex.11,14,23 Indeed, it has not been determined whether cats that are heterozygous for the WNK4 mutation are mildly affected, or not affected at all. Interestingly, in an unrefereed article, Gruffydd-Jones states ‘one (affected cat) had slightly higher levels (of potassium) than would normally be expected (in urine)’. 9 Clearly, more work is needed; for example, to determine potassium concentrations in the urine of affected cats while they are fed various diets (canned vs extruded vs fresh meat) under different environmental conditions. 11
Molecular genetic testing
Since the groundbreaking paper elucidating the WNK4 mutation, 1 testing for hypokalaemia has been greatly simplified. The test involves a straightforward PCR, using whole blood (anticoagulated with EDTA) or cheek swabs, available commercially through the Veterinary Genetics Laboratory at UC Davis (USA) and Langford Veterinary Services at the University of Bristol (UK). This service is available to veterinarians, and also to owners and cat breeders interested in determining the genotype of their cats. Results are generally available a few days after sample submission.
In suspect cases, the test can establish an unequivocal diagnosis of inherited hypokalaemia (due to WNK4 dysfunction), even in patients in which the serum [K+] is within the reference interval, forgoing the requirement for sequential serum [K+] and CK determinations. Furthermore, and importantly, owners and breeders can determine if phenotypically normal Burmese cats, and breeds that have infused Burmese bloodlines, are heterozygous for the defective gene (ie, are carriers). As collecting cheek swabs is quick, easy and non-invasive, specimens can be collected from cats prior to mating and from kittens prior to sale.
How this genetic disease can be eliminated from the Burmese breed
Genetic testing in any cat breed depends on reliable identification of individual cats. For this purpose, microchip technology is superior to alternatives such as tattooing and photography. Once individuals can be reliably identified, it is only a matter of submitting samples from each cat for genetic testing, most conveniently by cheek swabs 34 or fresh whole blood anticoagulated in EDTA. Ideally, this should be done by a veterinarian, to ensure that the integrity of the process is preserved. (For cats to be placed on the International Cat Care ‘Hypokalaemia Register’ [www.icatcare.org/advice/breeders] the mouth swab or blood sample must be taken by a veterinarian who confirms the cat’s identity using its microchip number. The microchip number must be written on both the submission form and sample.) DNA profiling can also confirm the identity of cats and correlation with their genetic tests. 35
The fastest way to eliminate the defective gene is by insisting affected cats and carriers are not used for future matings. The major problem with this approach is that otherwise desirable individuals are excluded from contributing to subsequent generations.36,37 The Burmese breed has already undergone substantial genetic bottlenecks and selections associated with breed formation and preservation of breed standards.36,37 Exclusion of many individuals (perhaps 22% of the population; see Table 1) from the breeding stock could be extremely dangerous, due to the rapid and significant reduction in genetic variation, and hence the possibility of new diseases being selected.
Table 1.
Combined results of WNK4 genotyping at Langford Veterinary Services (Bristol, UK) and the Veterinary Genetics Laboratory (Davis, California, USA) from 2012–2014
| Breed | Normal | Carrier (heterozygous) | Affected (homozygous) | Total | WNK4 allele frequency |
|---|---|---|---|---|---|
| Asian | 20 (77%) | 6 (23%) | 0 (0%) | 26 | 0.1154 |
| Australian Mist | 31 (100%) | 0 (0%) | 0 (0%) | 31 | 0 |
| Bombay* | 89 (72%) | 26 (21%) | 9 (7%) | 124 | 0.1774 |
| Burmese | 1654 (79%) | 411 (20%) | 34 (1.6%) | 2099 | 0.1141 |
| Burmilla | 116 (87%) | 17 (13%) | 0 (0%) | 133 | 0.0639 |
| Tiffanie | 14 (67%) | 7 (33%) | 0 (0%) | 21 | 0.1667 |
| Tonkinese | 18 (95%) | 0 (0%) | 1 (5%) | 19 | 0.0526 |
| Total | 1942 (79%) | 467 (19%) | 44 (2%) | 2453 | 0.1131 |
A single Bombay cattery in New Zealand had a high prevalence of carriers and affected cats
The alternative approach recommended by most authorities is to neuter affected homozygous individuals (perhaps 1–2% of the population), but permit the use of heterozygous carriers of good quality on the proviso they are mated with genotypically normal cats, and that the subsequent generation is screened for the WNK4 mutation using PCR. In essence, the suggestion is that, in the short term, it should be possible to eliminate the disorder without eliminating the mutation. Complete elimination of the mutation is, however, the ultimate long-term aim.
WNK4 genotyping results for samples submitted to Langford Veterinary Services and the Veterinary Genetics Laboratory at UC Davis from 2012 to September 2014 have been pooled and are provided in Table 1. Most of the cats tested were domiciled in the UK, Europe or Australasia. Of Burmese cats tested, 411/2099 (20%) were carriers, while 34/2099 (1.6%) were homozygous-affected (ie, had two copies of the WNK4 mutation), a distribution consistent with Hardy–Weinberg equilibrium (P = 0.33). Interestingly, in four breeds derived from the Burmese that were also tested, 26/124 (21%) Bombays, 17/133 (13%) Burmillas and 7/21 (33%) Tiffanies were carriers, while 9/124 (7%) Bombays and 1/19 (5%) Tonkinese were homozygous-affected (ie, had inherited hypokalaemia), consistent with the historical infusion of genetic material from the Burmese breed. Only 21 Australian Mist cats were tested; no affected or carrier cats were detected.
Key Points
The discovery of a WNK4 mutation as the cause for hypokalaemia in Burmese and related breeds will probably result in the eradication of the condition due to this mutation over the next few generations.
Ironically, this will make unravelling the physiological basis for the [K+] derangement a moot point, although probably of great interest to renal physiologists and cell biologists interested in WNK4 and related kinases.
A colony of carrier and affected cats has been established at the University of Missouri, USA, to preserve the mutation in the research laboratory for basic functional studies of the WNK4 mutation, while it is eliminated from the owned cat population.
It is exciting to to have found a causal mutation, developed a discriminatory PCR test and laid the foundation for elimination of this genetic problem in such a short span of time. Such is the power of a modern, internationally collaborative
Acknowledgments
The authors wish to thank Clare Rusbridge and Frank Nicholas for their meticulous reading of the manuscript. Clare Rusbridge also kindly provided Video 3, which appears as Supplementary material online. Joanna White, David Church, Vanessa Barrs and Andrea Harvey were involved in the management of some of the cases used in the photographs in Figure 2. Finally, thanks are due to all the clinicians at the Cat Clinic, Brisbane, Australia, for insights and observations that were useful in preparing this review. Richard Malik is supported by the Valentine Charlton Bequest of the Centre of Veterinary Education of the University of Sydney Australia.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article.
The authors have no conflict of interest to declare.
References
- 1. Gandolfi B, Gruffydd-Jones TJ, Malik R, et al. First WNK4-hypokalemia animal model identified by genome-wide association in Burmese cats. PLoS ONE 2012; 7: e53173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Veríssimo F, Jordan P. WNK kinases, a novel protein kinase subfamily in multi-cellular organisms. Oncogene 2001; 20: 5562–5569. [DOI] [PubMed] [Google Scholar]
- 3. Kahle KT, Gimenez I, Hassan H, et al. WNK regulates apical and basolateral CL-flux in extrarenal epithelia. Proc Natl Acad Sci 2004; 101: 2064–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCormick JA, Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev 2011; 91: 177–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoorn EJ, Nelson JH, McCormick JA, et al. The WNK kinase network regulating sodium, potassium, and blood pressure. J Am Soc Nephrol 2011; 22: 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wilson FH, Disse-Nicodème S, Choate KA, et al. Human hypertension caused by mutations in WNK kinases. Science 2001; 293: 1107–1112. [DOI] [PubMed] [Google Scholar]
- 7. Eger CE, Robinson WF, Huxtable CRR. Primary aldosteronism (Conn’s syndrome) in a cat; a case report and review of comparative aspects. J Small Anim Pract 1983; 24: 293–307. [Google Scholar]
- 8. Blaxter A, Lievesley P, Gruffydd-Jones T, et al. Periodic muscle weakness in Burmese kittens. Vet Rec 1986; 118: 619–620. [DOI] [PubMed] [Google Scholar]
- 9. Gruffydd-Jones TJ. Presentation on hypokalaemia. Presented at the Burmese Cat Club Annual General Meeting, 11 May 1996. Published in ‘The Burmese Cat Club News’ Summer 1996, pp 17–20. http://www.bjelkes.dk/hypokalaemi.pdf (1996, accessed October 8, 2014). [Google Scholar]
- 10. Dow SW, LeCouteur RA, Fettman MJ, et al. Potassium-depletion in cats – hypokalemic polymyopathy. J Am Vet Med Assoc 1987; 191: 1563–1568. [PubMed] [Google Scholar]
- 11. Dow SW, Fettman MJ, LeCouteur RA, et al. Potassium-depletion in cats – renal and dietary influences. J Am Vet Med Assoc 1987; 191: 1569–1575. [PubMed] [Google Scholar]
- 12. Dow SW, Fettman MJ, Curtis CR, et al. Hypokalemia in cats: 186 cases (1984–1987). J Am Vet Med Assoc 1989; 194: 1604–1608. [PubMed] [Google Scholar]
- 13. Fettman MJ. Feline kaliopenic polymyopathy/nephropathy syndrome. Vet Clin North Am Small Anim Pract 1989; 19: 415–432. [DOI] [PubMed] [Google Scholar]
- 14. Schaer M. Disorders of potassium metabolism. Vet Clin North Am Small Anim Pract 1982; 12: 399–409. [DOI] [PubMed] [Google Scholar]
- 15. Kurushima JD, Lipinski MJ, Gandolfi B, et al. Variation of cats under domestication: genetic assignment of domestic cats to breeds and worldwide random-bred populations. Anim Genet 2013; 44: 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones BR, Swinney GW, Alley MR. Hypokalaemic myopathy in Burmese kittens. N Z Vet J 1988; 36: 150–151. [DOI] [PubMed] [Google Scholar]
- 17. Stolze M, Lund C, Kresken JG, et al. Periodic hypokalemic polymyopathy in the Burmese cat. Kleintierpraxis 2001; 46: 517–518. [Google Scholar]
- 18. Lantinga E, Kooistra HS, van Nes JJ. Periodic muscle weakness and cervical ventroflexion caused by hypokalemia in a Burmese cat [article in Dutch]. Tijdschr Diergeneeskd 1998; 123: 435–437. [PubMed] [Google Scholar]
- 19. Jones BR, Gruffydd-Jones TJ. Hypokalemia in the cat. Cornell Vet 1990; 80: 13–16. [PubMed] [Google Scholar]
- 20. Musca F, Malik R, Menrath V, et al. Hypokalaemic polymyopathy of Burmese cats – retrospective analysis of cases, new clinical observations and a call for cases for genomic studies. Scientific proceedings of the Annual Meeting of the Australian College of Veterinary Scientists, Small Animal Medicine Chapter; 2010, p 6. [Google Scholar]
- 21. Musca F, Malik R. Hypokalaemia in Burmese and related cats: a call for cases. Control & Therapy Series of the Centre for Veterinary Education, The University of Sydney, Australia, 2010 [Google Scholar]
- 22. Mason KV. A hereditary disease in Burmese cats manifested as an episodic weakness with head nodding and neck ventroflexion. J Am Anim Hosp Assoc 1988; 24: 147–151. (Erratum: Mason KV. Hereditary potassium depletion in Burmese cats. J Am Anim Hosp Assoc 24: 481.) [Google Scholar]
- 23. Jones B. Hypokalemic myopathy in cats. In: Bonagura JD, Twedt DC. (eds). Kirk’s current veterinary therapy. Saunders, Elsevier. pp 1136–1138. [Google Scholar]
- 24. Gaschen F, Jaggy A, Jones B. Congenital diseases of feline muscle and neuromuscular junction. J Feline Med Surg 2004; 6: 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Everett GM. Observations on the behaviour and neurophysiology of acute thiamine deficient cats. Am J Physiol 1944; 141: 439–448. [Google Scholar]
- 26. Martin PT, Shelton GD, Dickinson PJ, et al. Muscular dystrophy associated with alpha-dystroglycan deficiency in Sphynx and Devon Rex cats. Neuromuscul Disord 2008; 18: 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shelton GD, Ho M, Kass PH. Risk factors for acquired myasthenia gravis in cats: 105 cases (1986–1998). J Am Vet Med Assoc 2000; 216: 55–57. [DOI] [PubMed] [Google Scholar]
- 28. Schunk KL. Feline polymyopathy. Proceedings of the 2nd Annual Forum of the American College of Veterinary Internal Medicine, 1984; pp 197–200. [Google Scholar]
- 29. Schull DN, Litster AL, Atwell RB. Tick toxicity in cats caused by Ixodes species in Australia: a review of published literature. J Feline Med Surg 2007; 9: 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moisidis AV, James T, Smith HV, et al. Snake envenomation in cats and its detection by rapid immunoassay. Aust Vet J 1996; 74: 143–147. [DOI] [PubMed] [Google Scholar]
- 31. Ash RA, Harvey AM, Tasker S. Primary hyperaldosteronism in the cat: a series of 13 cases. J Feline Med Surg 2005; 7: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buffington CAT. Idiopathic cystitis in domestic cats – beyond the lower urinary tract. J Vet Intern Med 2011; 25: 784–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lalioti MD, Zhang J, Volkman HM, et al. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genetics 2006; 38: 1124–1132. [DOI] [PubMed] [Google Scholar]
- 34. Langford Veterinary Services. How to swab your cat. http://www.langfordvets.co.uk/diagnostic-laboratories/diagnostic-laboratories/general-info-breeders/how-swab-your-cat (accessed October 8 2014).
- 35. Lipinski MJ, Amigues Y, Blasi M, et al. An international parentage and identification panel for the domestic cat (Felis catus). Anim Genet 2007; 38: 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lyons LA. DNA mutations of the cat: the good, the bad and the ugly. J Feline Med Surg 2015; 17: 203–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gandolfi B, Alhaddad H. Investigation of inherited diseases in cats: genetic and genomic strategies over three decades. J Feline Med Surg 2015: 17: 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]