Abstract
Objective:
Feline gastrointestinal eosinophilic sclerosing fibroplasia (FGESF) is a recently described inflammatory disease of cats affecting stomach or intestines and draining regional lymph nodes. This study presents clinical and laboratory data on 13 newly described cases from Australia (11) and the UK (two).
Observations:
The disease was most often observed in middle-aged cats (median 7 years of age; interquartile range 5–9 years). Ragdolls (7/13) and males (9/13) were overrepresented. Cats generally had a long history of vomiting and/or diarrhoea. Lesions were typically large, hard, non-painful, easily palpable and most commonly situated near the pylorus or ileocaecocolic junction. Lesions were heterogeneous ultrasonographically and on sectioning at celiotomy or necropsy. Masses were hard and ‘gritty’ on fine-needle aspiration due to internal trabeculae made up of mature collagen bundles. Bacteria were commonly detected within masses (9/13 cases) using either culture or conventional light microscopy and a panel of special stains, and/or fluorescence in situ hybridisation (FISH), although detection often required a diligent search of multiple tissue sections. A consistent bacterial morphology could not be appreciated among the different cases.
Outcome:
Patients were treated with a variable combination of cytoreduction (debulking and biopsy, to complete surgical resection), immunosuppressive therapy and antimicrobial agents. Many cats had a poor outcome, which was attributable to late diagnosis combined with suboptimal management. It is hoped that suggestions outlined in the discussion may improve clinical outcomes and long-term survival in future cases.
Feline gastrointestinal eosinophilic sclerosing fibroplasia (FGESF) refers to a clinical and pathological entity of unknown cause or association defined by the presence of eosinophilic mass(es), largely confined to the gastrointestinal tract and associated lymph nodes. 1 It has been diagnosed in cats in the USA, Japan, Europe (including the UK) and, most recently, New Zealand.1 –5 The prognosis for affected cats is considered guarded. This may reflect delays in treatment because the entity is not commonly considered in the differential diagnosis of intra-abdominal mass lesions in cats. Without early diagnosis and intervention, the likelihood of a successful outcome is reduced.
Grossly, lesions can be confused with lymphoma, granuloma or adenocarcinoma, while histologically lesions have been confused with sclerosing mast cell neoplasia, fibrosarcoma or extra-skeletal osteosarcoma.1,6 –8 In a case series of 25 cats, 24% of patients were euthanased due to the gross appearance of the lesion at laparotomy. 1
Although direct evidence is lacking, it has been hypothesised that some cats have an, as yet uncharacterised, genetic predisposition to develop eosinophilic inflammation in response to antigens, possibly from bacteria or parasites, breaching the intestinal mucosa. 1 Bacteria have been isolated from lesions in a substantial proportion of cats, but not in all cases. It is unclear whether the role of bacteria is primary or secondary.1,4,9 Other infectious agents have been found in association with this entity including zygomycetes in a domestic cat and nematodes (most commonly Cylicospirura species) in pumas (Felis concolor).5,10
As the pathogenesis of FGESF is not well understood, the purpose of this study was to contribute insights from additional cases from Australia and the UK. Our aims were (1) to examine trends in the clinical presentation in terms of disease associations (diet, history, breed, gender), and salient physical and imaging findings; (2) determine whether feline coronavirus (FCoV), feline herpesvirus type 1 (FHV-1) or specific bacterial species play a role in the development of this disease through the use of special stains, immunohistochemistry (IHC) and/or fluorescence in situ hybridisation (FISH); and (3) to speculate as to the underlying pathophysiology of this enigmatic entity.
Methods
Case selection and clinical data retrieval
This study was initially retrospective, but developed a prospective arm as eight additional cases were encountered during case analyses. To be included in the study, a histological diagnosis of FGESF had to have been considered by the original pathologist, and confirmed by a second pathologist (JSN). Cases were acquired directly by the authors, or via consultation with colleagues in general, institutional or private referral practice. In addition, we contacted veterinary pathologists in Australia and presented abstracts to the Australian and New Zealand College of Veterinary Scientists and the annual meeting of the Australian Society for Veterinary Pathology to maximise case recruitment and improve awareness of this emerging entity.
On inclusion into the study, case notes, clinical images (photographs, radiographs, ultrasonograms) and formalin-fixed paraffin-embedded (FFPE) tissue blocks were retrieved. Patient characteristics, physical findings, clinicopathological data, procedural reports, treatment regimens, outcomes (when available) and necropsy findings were tabulated into an Excel spreadsheet.
Histology and immunohistochemistry
Cases were classified as FGESF if full thickness gastrointestinal biopsies and/or incisional biopsies of mesenteric lymph nodes showed typical histological features. 1 The key findings were the presence of eosinophilic inflammation within a characteristic fibroplasia, including broad mature organised collagen bundles. Multiple sections were examined to exclude poorly differentiated mast cell neoplasia and atypical lymphoma, and to search for hair or plant material (to exclude trichobezoars). Special stains were employed mainly to determine whether or not infectious agents (bacteria, fungi, protozoa) were present within lesions; these included Giemsa, methamine silver, Ziehl–Neelsen (for mycobacteria and Nocardia species), toluidine blue (for mast cells), Gram Twort, and periodic acid Schiff (for fungi). Trichrome stain was sometimes used to highlight collagen in tissue sections.
IHC for FCoV and FHV-1 was performed as described previously.11,12 Briefly, sections (4 µm) of FFPE tissue were mounted on silane-coated slides and dried for 24 h at 37°C to facilitate tissue adherence to the slide. Slides were deparaffinised and rehydrated by submerging in 100% xylol and graded dilutions of ethanol to water. Antigen retrieval for FCoV IHC was achieved using working dilutions of a commercially available antigen retrieval solution (Target Retrieval Solution, 10 × concentrate; DakoCytomation, CA, USA) and microwaved for 15 mins, according to the manufacturer’s instructions. 12 Antigen retrieval for FHV-1 IHC was achieved using a proteinase K enzymatic method. 11 Thereafter slides were placed in an automated slide processing system (DakoCytomation Autostainer Plus). Endogenous peroxidases were blocked by incubating the slides with 0.03% hydrogen peroxide (Peroxidase Block; DakoCytomation) for 15 mins at room temperature. Antigen detection was then achieved by incubating the slides for 60 mins at room temperature using either a 1/1000 dilution of monoclonal antibody against the nucleocapsid of FCoV or a 1/200 dilution of monoclonal antibody against the gD glycoprotein of FHV-1 (FHV7-5; Custom Monoclonals International, Sacramento, USA). All slides were incubated with the secondary antibody (Envision Labelled Polymer-HRP Anti-mouse; DakoCytomation) for 30 mins at room temperature. Finally, slides were incubated for 5 mins at room temperature with 3,3′-diaminobenzidine (DAB) chromogen solution (DakoCytomation).
Slides were thoroughly rinsed with Tris-buffered saline between each of the above steps. Once the Autostainer had completed the DAB step, slides were rinsed in water, manually counterstained with haematoxylin, and dehydrated through graded alcohol dilutions and xylol; a cover-slip was placed prior to microscopic examination. Positive and negative controls were included in every run.
Fluorescence in situ hybridisation
To determine if bacteria were present within biopsy specimens, FFPE sections (4 µm) were mounted on silane-coated slides and evaluated by FISH using a eubacterial probe (EUB-338; GCT GCC TCC CGT AGG AGT). 13 Paraffin-embedded tissue sections were deparaffinised by passage through 100% xylene and rehydrated by submerging in graded dilutions of ethanol (100% to 70%) to water. Slides were dried at 45°C for 30 mins. FISH probes 5′ labelled with Cy3 or 6-FAM (Sigma-Aldrich, Australia) were reconstituted using sterile water and diluted to a working concentration of 5 ng/ml with hybridisation buffer (20 mM Tris-HCl, 0.1% sodium dodecyl sulphate, 0.9% NaCl [pH 7.5]). Sections were allowed to hybridise with 30 µl of DNA probe mix in a hybridisation chamber at 46°C for 14 h. Slides were rinsed in wash buffer (hybridisation buffer without SDS) at 48°C for 30 mins. Hybridised samples were washed in phosphate-buffered saline, allowed to dry, and mounted with a ProLong Antifade Gold reagent (Molecular Probes, OR, USA). Sections were examined under a fluorescent microscope (Olympus model BX60F-3).
Control samples consisted of FFPE tissues prepared by making a ‘bacterial sandwich’ using lung tissue. 14 Control bacteria in these preparations included Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli or Clostridium perfringens. Probe specificity was additionally evaluated using tissue sections treated with ribonuclease and also using the irrelevant probe non-EUB-338 (ACT CCT ACG GGA GGC AGC).
Following evaluation by FISH with eubacterial probes, new slides were prepared for examination with specific probes to characterise fluorescent bacteria further as being either Clostridium species (5′ GTT ATC CGT GTG TAC AGG G-3′; 5′ TTA TGC GGT ATT AAT CTY CCT TT -3′) or E coli (5′ GCA AAG GTA TTA ACT TTA CTC CC 3′) using adjusted hybridisation and wash solutions. 15
Statistical analysis
Fisher’s exact tests were conducted to determine whether the observed gender or breed of cats with FGESF differed significantly from expected frequencies. Expected breed frequencies were based on the Companion Animals Register for New South Wales (NSW) for 2011 (containing breed data for 439,145 registered cats). Results were considered significant if P <0.05.
Results
Thirteen cats met the inclusion criteria and were recruited into the study. All cases were neutered. There was a preponderance of male cats (9/13 cases; P = 0.10) compared with the reference population in Toribio and colleagues’ survey. 16 The median age of cases was 7 years (range 2–11 years; interquartile range 5–9 years). There were seven Ragdolls, one Persian and five domestic shorthair cats. The observed frequency of breeds diagnosed with FGESF differed significantly from that expected based on the NSW Companion Animals Register data for 2011. Ragdolls were significantly overrepresented (P <0.0001; Fisher’s exact test) compared with the reference population (Figure 1). Cats had been fed various diets, typically a mixture of commercial canned and extruded dry food; raw meaty bones had not been fed regularly to any of the patients.
Figure 1.
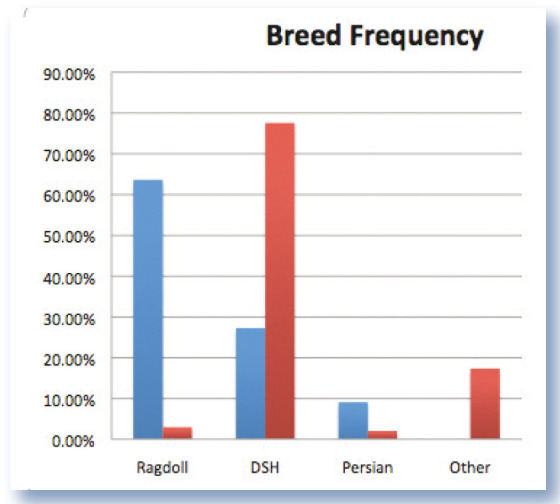
Observed frequency of each breed for cats with confirmed FGESF (blue bars). Red bars show expected breed frequencies based on registration data from the Companion Animals Register, New South Wales, Australia. DSH = domestic shorthair
The reported clinical signs and physical examination findings are listed in Tables 1 and 2. Pertinent findings included weight loss and a history of chronic vomiting and/or diarrhoea of at least 3 months’ duration (usually greater than 12 months). When owners identified antecedent clinical signs, they were observed between 2 days and up to 5 months prior to presentation. There was no history of previous foreign body or hairball obstruction in any of the patients.
Table 1.
Historical findings in 13 cats with FGESF
| Owner-reported clinical signs | Number (percentage) of patients |
|---|---|
| Chronic vomiting and diarrhoea | 11/12 (91%) |
| Weight loss | 10/13 (77%) |
| Lethargy | 8/13 (62%) |
| Acute vomiting | 7/13 (54%) |
| Excessive grooming | (5/10) 50% |
| Anorexia | 6/13 (46%) |
| Acute diarrhoea | 5/13 (38%) |
| Coughing | 1/13 (8%) |
| No clinical signs reported | 1/13 (8%) |
FGESF = feline gastrointestinal eosinophilic sclerosing fibroplasia
Table 2.
Physical examination findings in 13 cats with FGESF
| Abnormal physical exam findings | Number (percentage) of patients |
|---|---|
| Abdominal mass | 11/13 (85%) |
| Abdominal pain | 3/12 (25%) |
| Pyrexia | 2/11 (18%) |
| Dyspnoea | 1/13 (8%) |
FGESF = feline gastrointestinal eosinophilic sclerosing fibroplasia
A firm, irregular, fixed abdominal mass in the cranial and/or mid-abdomen was the most common physical finding (Figures 2 and 3). One cat (case 2) had an atypical presentation with dyspnoea due to pleural effusion (Figure 4). Two cats did not have a palpable abdominal mass at presentation but were lethargic, anorexic and had a history of vomiting. Partial serum biochemistry analyses were available for 12 cats; findings are summarised in Table 3. Hyperproteinaemia referable to hyperglobulinaemia was present in 7/11 cats. Hypoalbuminaemia was present in 5/11 cases, with the albumin/globulin ratio <0.6 in 7/11 cases. Protein serum electrophoresis showed a polyclonal gammopathy in case 3, with beta–gamma bridging, the alpha 1 and alpha 2 globulins being normal. Elevated urea and creatinine concentrations were identified in case 5. Two cats had hypocalcaemia, most likely due to hypoalbuminaemia. Hyperbilirubinaemia was present in one patient (case 6).
Figure 2.
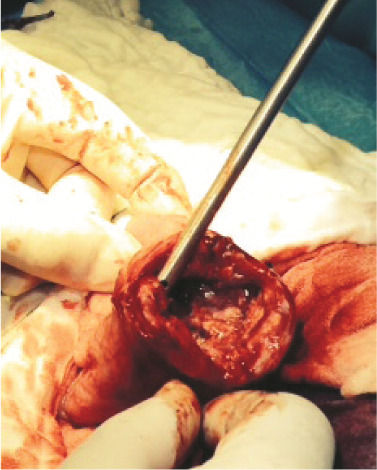
Intraoperative photograph of the pyloric lesion in case 11. Marked thickening of the pyloric wall is evident
Figure 3.
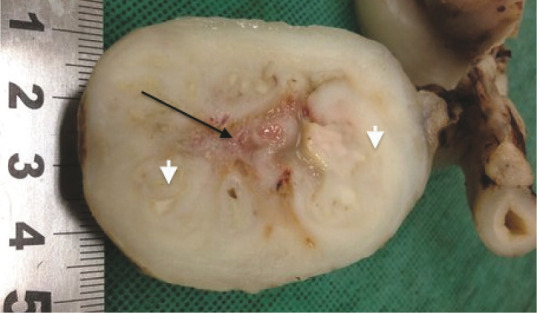
Photograph of the resected jejunal mass from case 10. The intestinal lumen has been completely obliterated, resulting in signs of intestinal obstruction. The arrow denotes a region of caseous necrosis; arrowheads identify foci of mature collagen
Figure 4.
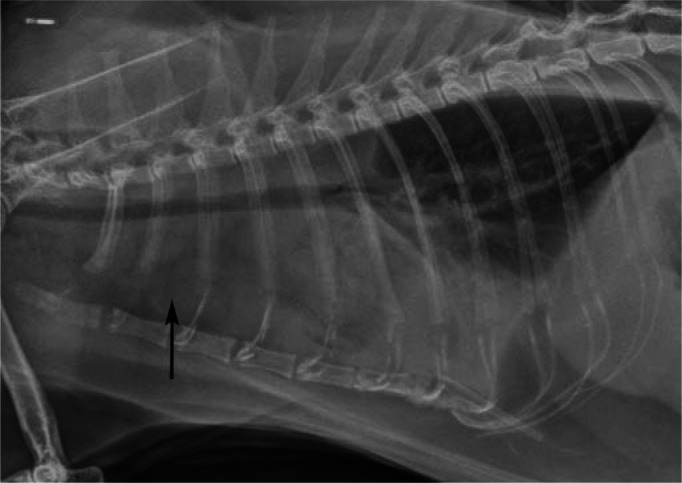
Lateral thoracic radiograph from case 2 presented for respiratory distress. Note the pleural effusion (most evident cranioventrally) and prominent sternal lymph node (arrow). Although this is an atypical presentation, it is important to emphasise that some cases of FGESF can present with bicavitary involvement, the disease process starting in the abdomen and spreading to the thoracic cavity, presumably due to drainage of abdominal lymphatics to the sternal lymph node. A similar observation was reported recently3
Table 3.
Serum biochemical findings in 13 cats with FGESF. Abnormally high values are highlighted in yellow; abnormally low values in red
| Analyte | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 | Case 12 | Case 13 | Reference interval |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium | 151.0 | 144.0 | 146.0 | 149.0 | 205.0 | N/A | N/A | 152.0 | 153.0 | N/A | 165.0 | 147.0 | N/A | 144.0–158.0 mmol/l |
| Potassium | 4.1 | 4.2 | 4.9 | 4.4 | 4.7 | N/A | N/A | 4.2 | 4.0 | N/A | 3.4 | 4.4 | N/A | 3.7–5.4 mmol/l |
| Chloride | 118.0 | 113.0 | 116.0 | 112.0 | 178.0 | N/A | N/A | 115.0 | N/A | N/A | 131.0 | 118.0 | N/A | 106.0–123.0 mmol/l |
| Bicarbonate | 15.0 | 21.0 | 21.0 | 25.0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 17.0 | N/A | 12.0–24.0 mmol/l |
| Na/K ratio | 36.8 | 34.3 | 29.8 | 30.0 | 45.6 | N/A | N/A | 36.2 | N/A | N/A | 48.5 | 33.4 | N/A | >29.0 |
| Anion gap | 22.1 | 14.2 | 13.9 | 16.4 | N/A | N/A | N/A | 19.1 | N/A | N/A | N/A | 16.4 | N/A | 15.0–31.0 mmol/l |
| Glucose (FlOx) | 5.6 | 5.0 | N/A | N/A | N/A | N/A | N/A | 4.7 | 8.7 | N/A | N/A | 5.3 | N/A | 3.2–7.5 mmol/l |
| Glucose (serum) | 6.4 | 4.8 | 5.4 | 5.4 | 4.7 | 6.3 | N/A | N/A | N/A | 7.3 | N/A | 4.7 | 4.9 | 3.2–7.5 mmol/l |
| Urea | 10.3 | 8.2 | 6.1 | 8.8 | 20.3 | 7.5 | N/A | 13.0 | 5.7 | 9.0 | N/A | 5.2 | 6.1 | 5.0–15.0 mmol/l |
| Creatinine | 100 | 100 | 100 | 160 | 239 | 200 | N/A | 200 | 100 | 70 | N/A | 100 | 126 | 80–200 µmol/l |
| Calcium | 2.4 | 2.0 | 2.2 | 2.4 | 2.4 | 2.1 | N/A | 2.4 | 2.3 | N/A | N/A | 2.0 | 2.2 | 2.1–2.8 mmol/l |
| Phosphate | 1.7 | 1.3 | 2.0 | 1.1 | 1.8 | 1.3 | N/A | 1.5 | 1.6 | N/A | N/A | 1.4 | 1.2 | 1.0–2.3 mmol/l |
| Protein (total) | 138.0 | 135.0 | 96.0 | 62.0 | 120.0 | 78.0 | N/A | 71.0 | 57.0 | 105.0 | N/A | 113.0 | 67.0 | 60.0–84.0 g/l |
| Albumin | 25.0 | 18.0 | 20.0 | 30.0 | 25.0 | 24.0 | N/A | 34.0 | 26.0 | N/A | 21.0 | 20.0 | 27.0 | 25.0–38.0 g/l |
| Globulin | 113.0 | 117.0 | 76.0 | 32.0 | 95.0 | 55.0 | N/A | 37.0 | 31.0 | N/A | 61.0 | 93.0 | 40.0 | 31.0–52.0 g/l |
| A/G ratio | 0.2 | 0.2 | 0.3 | 0.9 | 0.3 | 0.4 | N/A | 0.9 | 0.8 | N/A | 0.3 | 0.2 | 0.7 | |
| Bilirubin | 4.0 | 3.0 | 6.0 | 2.0 | 7.0 | 11.0 | N/A | 2.0 | N/A | N/A | N/A | 3.0 | 5.0 | <7 µmol/l |
| ALP | 13.0 | 10.0 | 5.0 | 34.0 | 27.0 | 20.0 | N/A | 33.0 | 24.0 | 15.0 | 25.0 | 9.0 | 22.0 | 5.0–50.0 IU/l |
| AST | 88.0 | 30.0 | 39.0 | 23.0 | N/A | N/A | N/A | 34.0 | N/A | N/A | N/A | 31.0 | N/A | 0–62.0 IU/l |
| ALT | 26.0 | 20.0 | 14.0 | 48.0 | 10.0 | <10 | N/A | 61.0 | 17.0 | 34.0 | 23.0 | 24.0 | N/A | 0–100.0 IU/l |
| CK | 188.0 | 74.0 | 93.0 | 170.0 | N/A | N/A | N/A | 197.0 | N/A | N/A | N/A | 105.0 | N/A | 64–400.0 IU/l |
| Cholesterol | 2.9 | 1.1 | 2.5 | 3.8 | 3.3 | 2.9 | N/A | 3.6 | N/A | N/A | 2.8 | 2.8 | 2.8 | 2.2–5.5 mmol/l |
| GGT | N/A | N/A | <2 | N/A | N/A | N/A | N/A | 5.0 | 1.0 | N/A | <0 | 6.0 | <0 | <6.0 IU/l |
FGESF = feline gastrointestinal eosinophilic sclerosing fibroplasia, N/A = not available, Na/K ratio = sodium/potassium ratio, A/G ratio = albumin/globulin ratio, ALP = alkaline phosphatase, AST = aspartate aminotransferase, ALT = alkaline transaminase, CK = creatine kinase, GGT = gamma-glutamyl transferase
Partial haematology results were available for 11 cats (Table 4). Peripheral eosinophilia was present in 5/10 cases. Case 3 had eosinophilia 2 days after initial presentation, the eosinophil count having been within the reference interval on presentation. Two cats had a mild non-regenerative anaemia (cases 5 and 9), one of which also had a disproportionately elevated serum urea concentration (case 5) compared with the creatinine concentration (Table 3), consistent with gastrointestinal bleeding, dehydration or concurrent renal disease; unfortunately urine specific gravity was not recorded. Serological testing for feline leukaemia virus antigen and feline immunodeficiency virus antibody was conducted in two patients and was negative in both instances. Where haematology and biochemistry were monitored in surviving animals (after therapy), both eosinophilia and hyperglobulinaemia had resolved.
Table 4.
Haematological findings in 13 cats with FGESF. Abnormally high values are highlighted in yellow; abnormally low values in red
| Haematological value | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 | Case 12 | Case 13 | Reference interval |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RBC | 10.0 | 5.4 | 7.93 | 8.2 | N/A | 7.6 | N/A | 9.61 | 4.7 | 7.3 | 7.7 | 7.7 | N/A | 4.9–10.0 × 1012/l |
| Haemoglobin | 129 | 80 | 103 | 124 | 83 | 111 | N/A | 122 | 76 | N/A | 122 | 95 | N/A | 77–156 g/l |
| Haematocrit | 0.39 | 0.27 | 0.34 | 0.38 | 0.24 | 0.38 | N/A | 0.41 | 0.23 | 0.30 | 0.35 | 0.32 | N/A | 0.25-0.48 l/l |
| MCV | 39.0 | 50.0 | 43.0 | 46.0 | N/A | 50.0 | N/A | 42.8 | 49.1 | 40.0 | 45.4 | 42.0 | N/A | 43.0–55.0 fl |
| MCH | 13.0 | 15.0 | 13.0 | 15.0 | N/A | 14.58 | N/A | 12.7 | N/A | 13.0 | 14.4 | 12.0 | N/A | 13.0–17.0 pg |
| MCHC | 331 | 296 | 304 | 330 | 324 | 292 | N/A | 296 | 327 | 322 | 317 | 297 | N/A | 282–333 g/l |
| Reticulocytes | N/A | N/A | N/A | N/A | N/A | 1.0 | N/A | 0.1 | N/A | N/A | 0.2 | 0.4 | N/A | 0.0–0.4% |
| Platelets | Clumped | Clumped and adequate | Clumped and adequate | N/A | N/A | Clumped and adequate | N/A | N/A | Clumped | N/A | Clumped and adequate | Clumped | N/A | |
| Platelet count | 312 | 226 | N/A | 359 | N/A | 215 | N/A | N/A | 365 | 322 | 82 | 331 | N/A | 300–800 × 109/l |
| Total white cell count | 15.3 | 7.6 | 6.3 | 6.5 | 13.1 | 19.5 | N/A | 9.1 | 53.4 | 21.16 | 36.4 | 14.3 | N/A | 5.5–19.0 × 109/l |
| Neutrophils | 8.0 | 5.2 | 4.2 | 3.6 | N/A | 8.9 | N/A | 6.9 | 21.8 | N/A | 15.0 | 7.3 | N/A | 2.0–3.0 × 109/l |
| Lymphocytes | 2.4 | 1.1 | 1.1 | 2.7 | N/A | 3.3 | N/A | 1.7 | N/A | 1.4 | 3.6 | 2.9 | N/A | 0.9–7.0 × 109/l |
| Monocytes | 0.3 | 0.5 | 0.2 | 0.1 | N/A | 0.8 | N/A | 0.2 | 2.7 | 1.3 | 0.5 | 0.6 | N/A | <0.7 × 109/l |
| Eosinophils | 4.4 | 0.8 | 0.9 | 0.2 | N/A | 6.4 | N/A | 0.3 | 26.3 | N/A | 16.0 | 3.6 | N/A | <1.1 × 109/l |
| Basophils | 0.2 | <0.1 | N/A | N/A | N/A | <0.1 | N/A | <0.1 | <0.1 | N/A | 1.4 | <0.1 | N/A | <0.1 × 109/l |
| Granulocytes | N/A | N/A | N/A | N/A | 13.3 | N/A | N/A | N/A | N/A | 18.16 | N/A | N/A | N/A | 2.5–12.5 × 109/l |
FGESF = feline gastrointestinal eosinophilic sclerosing fibroplasia, RBC = red blood cells, MCV = mean cell volume, MCH = mean cell haemoglobin, MCHC = mean cell haemoglobin concentration, N/A = not available
Histological examination was performed in all 13 cases (Figure 5). Histopathology in all gastrointestinal sections showed areas of sclerosing fibroplasia characterised by broad trabeculae of fibrous tissue interspersed by fibroblastic cells and dotted with foci of inflammation and necrosis. Sometimes these necrotic areas contained bacteria. Examination revealed that inflammation was mixed but predominantly eosinophilic and often of varying degrees of intensity. In 2/13 cases, the initial histopathological diagnosis (extraskeletal osteosarcoma and fibrosarcoma) was revised after review of the original sections in concert with clinical and surgical features (cases 2 and 5). In three cases there was some initial debate as to whether the lesions were due to inflammation or a fibrosarcoma; however, all lesions were eventually classified as being inflammatory in nature. There was no evidence of eosinophilic enteritis or other forms of inflammatory bowel disease (IBD) in cases where normal gastrointestinal tissue adjacent to the lesion was available for microscopic assessment.
Figure 5.
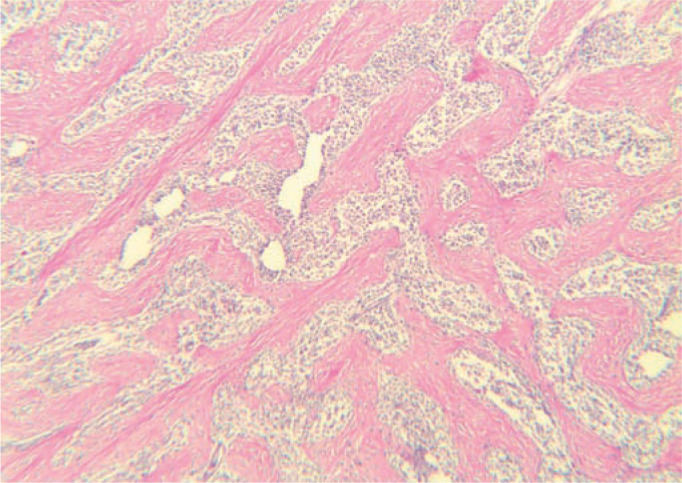
Histology of the resected lesion from case 10. The characteristic network of coarse collagen trabeculae (staining pink) throughout the lesion is an important distinguishing feature of FGESF. This abundance of collagen causes the hard, gritty texture of these lesions, which is most obvious during biopsy procedures (or during dissection at necropsy). Haematoxylin and eosin, × 400 magnification
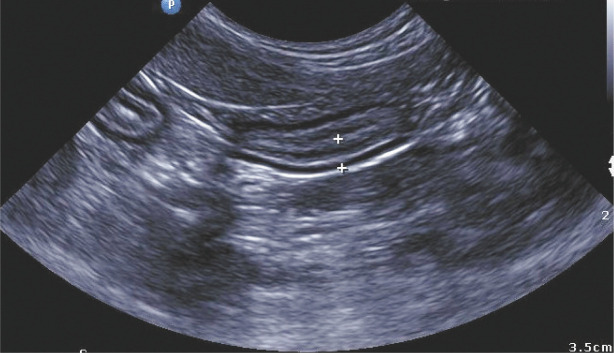
Lesions were situated at the ileocaecocolic junction (eight cats), pylorus (two cats; Figure 6) and greater curvature of the stomach (one cat). In two patients, the exact site could not be established from the case notes. Mesenteric lymphadenomegaly was noted in 10/13 patients (Figures 7 and 8); microscopically, the lymph nodes exhibited lymphoid hyperplasia and eosinophilia. Bacteria were identified in 9/13 cases using a combination of cytology, histology using special stains, FISH and/or culture of representative tissue specimens obtained surgically or with a spring-loaded core biopsy device (Table 5). In 4/13 cases, one pathologist (JSN) identified bacteria using conventional light microscopy whereas the original pathologist did not. In two of these four cases, bacteria were identified using special stains, while they were not originally detected in haematoxylin and eosin stained sections. In the two other discordant cases, the discrepancy may have been due to sampling bias, as the reference pathologist examined material re-cut from tissue blocks supplied by the original laboratory.
Figure 6.
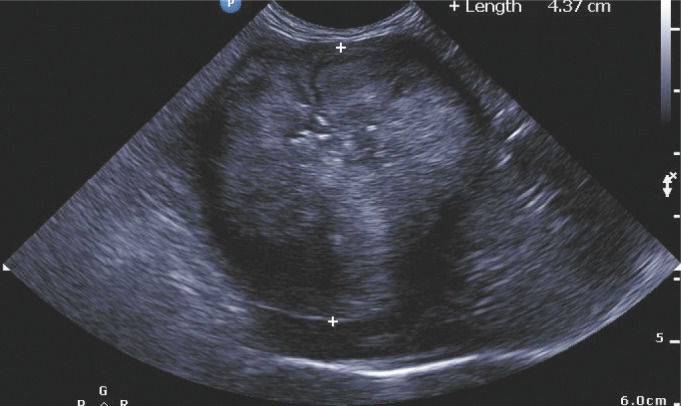
Ultrasound image of the pyloric lesion (denoted by +) of case 11. Note the loss of layering with the mixture of hypoechoic and hyperechoic regions within the tissue. It is thought that the hyperechoic regions correspond with the fibrotic zones described histologically 4
Figure 7.
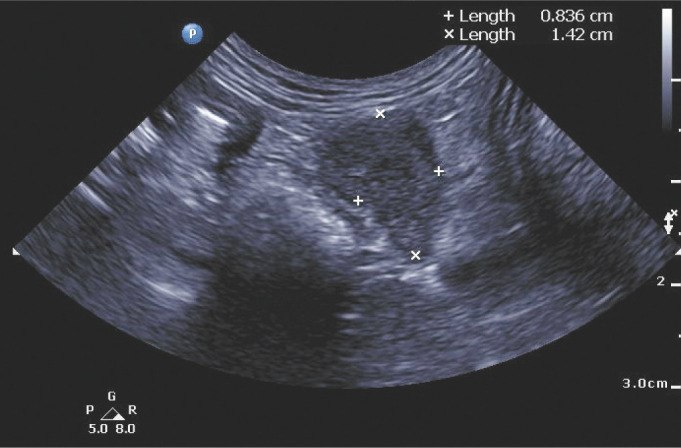
Ultrasound image from case 11, demonstrating an enlarged mesenteric lymph node (8 mm × 14 mm)
Figure 8.
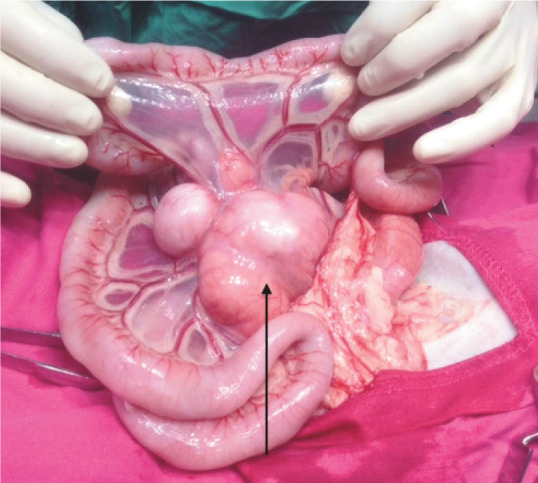
Intraoperative photograph from case 13. Note the marked enlargement of the lymph nodes (arrow) at the root of the mesentery. This is a common feature of FGESF as well as other disease entities such as lymphoblastic B cell lymphoma 5
Table 5.
Summary of microbiological and gastrointestinal histopathological findings in 13 cats with FGESF
| Case | Bacterial morphology detected on histology | Presumptive microbial diagnosis based on special stains or histology* | Culture of tissue | FISH |
|---|---|---|---|---|
| 1 | Gram +ve bacilli Gram +ve cocci Gram –ve bacilli | Clostridium species | N/A† | Bacilli detected with EUB probe
Probe for Clostridium detected |
| 2 | Gram +ve bacilli | Clostridium species | N/A† | None detected with EUB probe or specific probes |
| 3 | Filamentous and beaded bacilli | Actinomyces species |
E coli, Bacteroides species, Enterococcus
durans, Fusobacterium species |
Bacilli detected with EUB probe but not with specific probes |
| 4 | None identified | N/A | N/A† | None detected with EUB probe or specific probes |
| 5 | Club colony cocci | Staphylococcus Actinomyces | N/A† | None detected with EUB probe or specific probes |
| 6 | Gram +ve bacilli Filamentous and beaded bacilli | Not reported | N/A† | Bacilli detected with EUB probe but not with specific probes |
| 7 | Coccobacilli | Not reported | N/A† | Bacilli detected with EUB probe but not with specific probes |
| 8 | None identified | N/A | N/A† | None detected with EUB probe or specific probes |
| 9 | None identified | N/A | N/A† | None detected with EUB probe or specific probes |
| 10 | Gram +ve cocci Gram –ve bacilli Gram +ve bacilli | E coli Mixed anaerobes | N/A† | Bacilli detected with EUB probe. Specific probe for E coli detected |
| 11 | Gram +ve bacilli | Clostridium species | E coli, plus a light growth of mixed anaerobes‡ | Bacilli detected with EUB probe
Probe for Clostridium detected |
| 12 | None identified | N/A | Providencia stuartii | No tissue specimen available |
| 13 | None identified | N/A | N/A† | None detected with EUB probe or specific probes |
FGESF = feline gastrointestinal eosinophilic sclerosing fibroplasia, FISH = fluorescence in situ hybridisation, EUB probe = eubacterial probe, N/A = not available
Immunohistochemistry was negative for feline coronavirus (FCoV) and feline herpesvirus-type 1 (FHV-1) in 12/12 cats tested
Insufficient tissue available
Culture and susceptibility testing of tissue from lumen of pylorus suggestive of bacterial contamination
In all three cases in which tissue specimens were submitted for microbiology, bacteria were isolated (Table 5). Specifically, E coli and a light mixed growth of anaerobes were isolated from the pyloric lesion in case 11; Providencia stuartii was cultured from a mesenteric lesion in case 12; and a combination of E coli, a Bacteroides species, Enterococcus durans and a Fusobacterium species was cultured from pus aspirated from mesenteric lymph nodes (on different occasions) in case 3. Toxoplasma gondii tachyzoites were identified from case 7 after the administration of chlorambucil and prednisolone; no evidence of toxoplasmosis had been identified in the original sections. FISH confirmed cases 1 and 11 to have Clostridium species present and case 10 to have E coli, while cases 3, 6 and 7 had bacilli detected via the eubacterial probe but no specific identification was possible using the specific probes selected. FISH and histopathology (including special stains) produced discordant findings in two instances where histology and special stains identified bacteria but FISH did not (cases 2 and 5). Finally, FISH and conventional histology (including special stains) identified bacteria of different morphology in case 7.
IHC was conducted on representative tissue specimens from 12 cases. FHV-1 and FCoV antigen could not be detected in any of the tissue specimens examined.
Case outcome and/or results of treatment
Eight of 13 cats died or were euthanased. Three cats died perioperatively (cases 1, 5 and 11); two had lesions at the pylorus and one in the ileocaecocolic region. One cat (case 1) developed sepsis, one was found dead in its cage 24 h post-surgery, while another (case 11) was speculated to have died of anaesthetic complications attributable to hypertrophic cardiomyopathy. One cat was euthanased due to poor response to therapy and an incorrect histological diagnosis (case 2). Mean survival time for cats that died or were euthanased was 3–152 days.
Five surviving cats were alive at the time of writing, with survival after diagnosis ranging from 1–10 years (Figure 9a). The impact of treatment selection on survival is summarised in Figure 9b,c. The number of cats receiving different treatment regimens precluded meaningful statistical analysis. When information was available, most surviving cats had complete resolution of clinical signs (ie, vomiting and diarrhoea) with no recurrence of a palpable intra-abdominal mass. The exception was case 3, which was subjected to multiple surgeries and antibiotic regimens over a 10 year period and only appeared to be in remission through a combination of immunomodulatory treatment (prednisolone) and antibiotics (amoxicillin and clavulanic acid).
Figure 9.
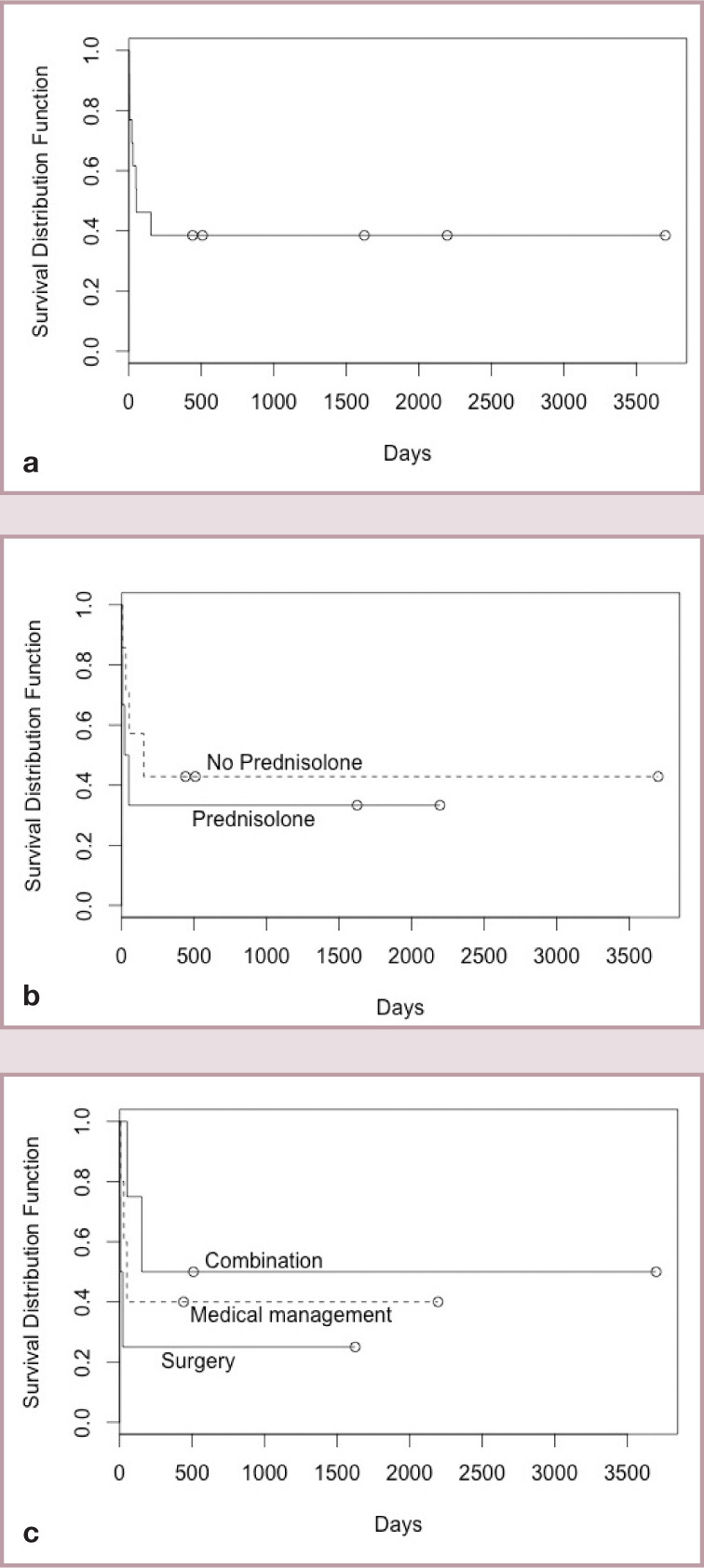
(a) Kaplan-Meier plot of survival for all 13 cats with FGESF. (b) Comparison of survival between cats treated with and without prednisolone. (c) Comparison of survival between cats treated with complete surgical resection and antibiotics (no immunomodulatory therapy) – labelled ‘Surgery’; surgical resection (complete or incomplete resection) with immunomodulatory therapy and antibiotics – labelled ‘Combination’; and no surgery (cases diagnosed on incisional biopsy and treated with antibiotics, dietary management ± immunomodulatory therapy) – labelled ‘Medical management’
Discussion
Clinical features
FGESF is a newly recognised entity. It is being diagnosed more often because of increased recognition by clinicians and pathologists following definitive diagnosis via biopsy. 1 This concept is supported by one case from 2004 being diagnosed retrospectively in the current series, and another from 1996 which could not be included because the detailed case notes had not been preserved.
In this study, most cats were mature adults (median 7 years), although cats of almost any age (2–11 years) were affected. So far, the entity has not been described in kittens. Longhaired cats and specifically the Ragdoll breed were overrepresented in this study. Such a preponderance has not been recorded previously. Study of the genetic relationship between affected cats may be informative, but pedigrees were not retrievable in most instances. DNA from affected cats has been archived to facilitate future molecular genetic investigations. Alternately, there might be another feature of Ragdolls, such as their long hair coat (with increased ingestion of hair, associated allergens and entrapped plant material), which predisposes to the development of FGESF.
Most cats had a palpable mass in the abdomen, which was typically fixed in position at presentation. Often there was a peripheral eosinophilia. Mass lesions often contained bacteria. This is hardly surprising, as the lesions were frequently ulcerated and communicated with the lumen of the gastrointestinal tract, as observed in this study. Critically, when the mass lesions are subjected to aspiration, core biopsy or excision at laparotomy, the tissue is hard, and on many occasions ‘gritty’ on advancing the needle, biopsy device or scalpel blade, due to abundant trabeculae of mature organised collagen. This is a useful and inexpensive point of differentiation from other common intra-abdominal masses of cats, such as large cell lymphoma, mast cell neoplasia and non-scirrhous adenocarcinoma, and it is conceivable that this would give a distinctive appearance on computed tomography.
The most consistent historical features were chronic vomiting and/or diarrhoea, which were typically ascribed to dietary hypersensitivity, food intolerance or IBD (Figure 10). However, in normal gastrointestinal tract adjacent to lesions, there was no histological evidence of eosinophilic enteritis or IBD. It might be informative to examine other portions of the gut in future cases to confirm or refute the presence of more widespread alimentary disease.
Figure 10.
Ultrasound image of the jejunum of case 11. Note the generalised wall thickening (3.3 mm), denoted by (+). Normal layering of the intestinal wall has been preserved. It is thought that this cat was also suffering from inflammatory bowel disease, namely eosinophilic enteritis, although this was not confirmed histopathologically
Aetiopathogenesis
It has been hypothesised that affected cats suffer from immunological dysregulation triggered by one or more factors. The trigger might be dietary (food allergy or intolerance), dysbiosis of the gut microbiota or other predisposing factors (ingestion of ectoparasites, endoparasites, excessive ingested hair or plant material). In pumas, nematodes have been reported as a possible trigger, with these felids showing a similar histopathological reaction pattern to domestic cats with FGESF; it is conceivable that ascarids or other helminths might do the same in cats. 10 In one case series, lymphoma was identified at the site of an FGESF lesion. 4 In the present study, cases were seen at different stages in the evolution of the lesions, which hinders an accurate determination of disease chronology. Secondary infection with bacteria, protozoa, fungi or other infectious agents is facilitated by the abnormal tissue architecture associated with a breach in mucosal integrity.17,18 Secondary infections presumably further perpetuate the whole process, causing a vicious cycle of eosinophilic inflammation, fibroplasia and eventually fibrosis.
Eosinophilic inflammation is a defining feature of this condition. Eosinophils produce numerous mediators that lead to tissue destruction (major basic protein [MBP], transforming growth factor beta [TGF-β], interleukin-1 beta [IL-1b], etc), while elaboration of IL-6 and MBP can result in fibroplasia and fibrosis.19 –22 This self-perpetuating process presumably gives rise to an abdominal mass, while structural alterations to the stomach, intestines, mesenteric lymph nodes, enteric nervous system and lymphatic drainage result in vomiting, diarrhoea and poor appetite.23,24 It would be informative to perform cytokine profiling from affected cats. 25
Eosinophilia, as in previous studies, was the most consistent haematological abnormality in this series of cats with FGESF, being evident in 5/11 cases. Eosinophils are often specifically linked with the presence of metazoan pathogens, fungi, viruses and sometimes bacteria that release unusual antigens into tissues, or diseases affecting mast cells (typically mast cell tumours and hypersensitivity disorders).11,26 –29 The reason why some cats have normal eosinophil numbers in peripheral blood remains unknown, although both endogenous and exogenous glucocorticoids could play a role in reducing counts to normal levels. 30 FGESF should be considered in cats with peripheral eosinophilia and an abdominal mass, as cats with neoplasia tend to have a stress-induced eosinopenia. Paraneoplastic eosinophilia can also be seen with intra-abdominal lymphoma. 31 Additionally, FGESF cannot be excluded when eosinophilia is absent.
IHC was performed to exclude the involvement of FHV-1 in FGESF because herpetic disease is linked enigmatically with an eosinophilic response in cats, specifically eosinophilic dermatitis, conjunctivitis and keratitis.11,32 Hyperglobulinaemia was the most common biochemical abnormality in FGESF cases. Protein electrophoresis showed a polyclonal gammopathy with beta–gamma bridging in one patient (case 3), non-specific changes consistent with inflammation. The secondary hypoalbuminaemia present in some cats was presumably due to hepatic down-regulation of albumin synthesis associated with elevated globulin levels, and compounded by albumin loss into the gastrointestinal lumen. The albumin/globulin ratio was <0.6 in 7/11 cats tested. Hyperglobulinaemia and a low albumin/ globulin ratio are frequently associated with feline infectious peritonitis (FIP). 33 This is important because pyogranulomatous lesions reminiscent of FGESF can occur in young adult cats with non-effusive FIP with massive mesenteric lymphadenomegaly or ileocaecocolic lesions in association with high globulin and low albumin concentrations.34 –36 FCoV IHC was, therefore, performed to exclude the possibility of atypical non-effusive FIP.
In 12 tissue specimens tested, IHC was negative for both FHV-1 and FCoV/FIP viral antigens. Therefore, it appears unlikely that FHV-1 or FCoV contribute to the pathogenesis of FGESF.
Diagnostic imaging and exploratory laparotomy
Non-invasive tests such as imaging (radiology, ultrasonography, computed tomography) combined with needle aspiration are required to make a provisional diagnosis of FGESF and exclude alternative possibilities such as neoplasia and non-effusive FIP. Ultrasonographic changes are non-specific, consisting of solitary masses with mural thickening and loss of layering in the stomach, duodenum, jejunum or colon. 4 One report suggests that endoscopy may be insensitive where lesions fail to extend to the mucosa. 3
Given the extent of the disease process, size of the lesions (up to 10 cm diameter) and the heterogeneous nature of the pathological processes within lesions (especially the distribution of bacteria), definitive staging and therapy requires laparotomy in most instances. Possibly this is not the case when disease is detected earlier, as lesions are more focal and thus limited in extent.
Histopathology
Inflammation in gastrointestinal tissue was generally mixed but predominantly eosinophilic. The extent that other inflammatory cells were admixed might be affected by factors such as the presence or absence of (secondary) bacteria, and indeed which bacteria were involved. Histology, although typically characteristic, can be misleading. For example, in one study, five cases were initially diagnosed as mast cell tumours, two as osteosarcomas and one as a haematopoietic neoplasm. 1 In this series, case 2 was initially misdiagnosed as an extra-skeletal osteosarcoma and case 5 as a fibrosarcoma. The differentiation of FGESF from sclerosing mast cell tumours is still debated.6 –8 In our series, toluidine blue staining failed to show any consistency in the number or distribution of mast cells. Mast cells varied from scant to moderately numerous, but were always in a multifocal inflammatory distribution, and never formed mass lesions. Furthermore, the biological behavior of FGESF is not consistent with a mast cell malignancy. For this reason, it is prudent that clinician and pathologist liaise to ensure special stains are employed to make a definitive diagnosis in patients with atypical intra-abdominal mass lesions.
Optimal therapy
Due to the largely retrospective nature of this study, a variety of therapeutic regimens were trialled: surgery, and various combinations of antimicrobial agents, corticosteroids, alkylating agents, gastrointestinal protectants and analgesics. It is, therefore, difficult to make definitive statements concerning which regimens worked best.
In spite of these limitations, there was a trend towards improved survival times when prednisolone was included in the therapeutic regimen and when surgery was not the sole mode of therapy (Figure 9b,c). However, this latter statement is logically flawed because not all surgical cases survived sufficiently long to receive systemic medications. Specifically, one case died in the perioperative period due to an unrelated comorbidity (cardiomyopathy), one case developed postoperative sepsis and was euthanased, one case treated in general practice was found dead 24 h postoperatively and one case was euthanased due to having been given an incorrect diagnosis. Additionally, a standardised treatment protocol was not in place and so treatment was at the discretion of the individual clinician; thus, different cats received different doses of a variety of antimicrobial and immunosuppressive agents.
In our opinion a multimodal approach to therapy is ideal, consisting of prednisolone, additional immunomodulatory agents and antibiotics following surgical resection (see box on page 403).
Prognosis
Due to the largely retrospective nature of the study, cases were presented at variable stages of disease and treatment regimens were inconsistent. For this reason, survival data must be appraised cautiously. What is clear, however, is that, if treated appropriately, survival times can be good, with most cats surviving the perioperative period remaining well for several years (Figure 9a).
Key Points
FGESF is primarily a condition of middle-aged cats with a chronic history of vomiting, diarrhoea and weight loss.
Ragdolls were significantly overrepresented in the 13 cases investigated in this study.
Abdominal lesions were usually palpable and localised to the ileocaecocolic region or stomach.
There is no evidence that FCoV, FHV-1 or a single specific bacterial species played a role in the development ofthis disease.
A multimodal approach to therapy of FGESF would seem ideal.
Acknowledgments
The authors thank the following veterinary practices for contributing cases: Castle Hill Veterinary Hospital, Hampton Park Veterinary Clinic, Fountain Gate Veterinary Clinic, Cambridge Cat Clinic UK, Your Vets, UK, Peninsula Animal Hospital, Hobart Animal Hospital, Greencross Vets Sandringham, North Shore Veterinary Specialist Centre, Rose Bay Veterinary Hospital, Leslie Street Veterinary Hospital, University of Melbourne Veterinary Clinic and Hospital, and Ferntree Gully Veterinary Hospital. Professor Niels Pedersen kindly donated monoclonal antibody against the nucleocapsid of FCoV. Professor Kenny Simpson generously taught Jacqui Norris the FISH technique during sabbatical leave. The authors also thank Tal Bergman for creating the Kaplan-Meier plot for survival data. Richard Malik is supported by the Valentine Charlton Bequest of the Centre for Veterinary Education, University of Sydney.
Footnotes
Date accepted: 17 December 2014
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
The authors do not have any potential conflicts of interest to declare.
References
- 1. Craig LE, Hardam EE, Hertzke DM, et al. Feline gastrointestinal eosinophilic sclerosing fibroplasia. Vet Pathol 2009; 46: 63–70. [DOI] [PubMed] [Google Scholar]
- 2. Suzuki M, Onchi M, Ozaki M. A case of feline gastrointestinal eosinophilic sclerosing fibroplasia. J Toxicol Pathol 2013; 26: 51–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Munday J, Martinez A, Soo M. A case of feline gastrointestinal eosinophilic sclerosing fibroplasia mimicking metastatic neoplasia. N Z Vet J 2014; 62: 356–360. [DOI] [PubMed] [Google Scholar]
- 4. Weissman A, Penninck D, Webster C, et al. Ultrasonographic and clinicopathological features of feline gastrointestinal eosinophilic sclerosing fibroplasia in four cats. J Feline Med Surg 2013; 15: 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Catro-Lopez J, Fernandez M, de Sousa AR, et al. A case of feline gastrointestinal eosinophilic sclerosing fibroplasia associated with zygomycetes fungi [abstract, ISFM Feline Congress. Budapest]. J Feline Med Surg 2012; 14: 650. [Google Scholar]
- 6. Gamble DA. Letters to the editor and rebuttal regarding the paper recently published in Veterinary and Comparative Oncology, ‘Feline intestinal sclerosing mast cell tumour: 50 cases (1997–2008) 2010; 8: 72–79’ by C. H. C. Halsey, B. E. Powers and D. A. Kamstock. Letter to the editor #2. Vet Comp Oncol 2010; 8: 235–236; author reply 236–242. [DOI] [PubMed] [Google Scholar]
- 7. Halsey CH, Powers BE, Kamstock DA. Feline intestinal sclerosing mast cell tumour: 50 cases (1997–2008). Vet Comp Oncol 2010; 8: 72–79. [DOI] [PubMed] [Google Scholar]
- 8. Schulman FY, Lipscomb TP. Letters to the editor and rebuttal regarding the paper recently published in Veterinary and Comparative Oncology, ‘Feline intestinal sclerosing mast cell tumour: 50 cases (1997–2008) 2010; 8: 72–79’ by C. H. C. Halsey, B. E. Powers and D. A. Kamstock. Letter to the editor #1. Vet Comp Oncol 2010; 8: 234–235; author reply 236–242. [DOI] [PubMed] [Google Scholar]
- 9. Ozaki K, Yamagami T, Nomura K, et al. Abscess-forming inflammatory granulation tissue with Gram-positive cocci and prominent eosinophil infiltration in cats: possible infection of methicillin-resistant Staphylococcus. Vet Pathol 2003; 40: 283–287. [DOI] [PubMed] [Google Scholar]
- 10. Eckstrand CD, Barr BC, Woods LW, et al. Nematode-associated intramural alimentary nodules in pumas are histologically similar to gastrointestinal eosinophilic sclerosing fibroplasia of domestic cats. J Comp Pathol 2013; 148: 405–409. [DOI] [PubMed] [Google Scholar]
- 11. Lee M, Bosward KL, Norris JM. Immunohistological evaluation of feline herpesvirus-1 infection in feline eosinophilic dermatoses or stomatitis. J Feline Med Surg 2010; 12: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Worthing KA, Wigney DI, Dhand NK, et al. Risk factors for feline infectious peritonitis in Australian cats. J Feline Med Surg 2012; 14: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simpson KW, Dogan B, Rishniw M. Adherent and invasive Escherichia coli is associated with granulomatous colitis in Boxer dogs. Infect Immun 2006: 4778–4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Recordati C, Radaelli E, Simpson KW, et al. A simple method for the production of bacterial controls for immunohistochemistry and fluorescent in situ hybridization. J Mol Histol 2008; 39: 459–462. [DOI] [PubMed] [Google Scholar]
- 15. Janeczko S, Atwater D, Bogel E, et al. The relationship of mucosal bacteria to duodenal histopathology, cytokine mRNA, and clinical disease activity in cats with inflammatory bowel disease. Vet Microbiol 2008; 128: 178–193. [DOI] [PubMed] [Google Scholar]
- 16. Toribio JA, Norris JM, White JD, et al. Demographics and husbandry of pet cats living in Sydney, Australia: results of cross-sectional survey of pet ownership. J Feline Med Surg 2009; 11: 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brandtzaeg P. Gate-keeper function of the intestinal epithelium. Benef Microbes 2013; 4: 67–82. [DOI] [PubMed] [Google Scholar]
- 18. Kurashima Y, Goto Y, Kiyono H. Mucosal innate cells regulate both gut homeostasis and intestinal inflammation. Eur J Immunol 2013; 43: 3108–3115. [DOI] [PubMed] [Google Scholar]
- 19. Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol 2000; 105: 651–663. [DOI] [PubMed] [Google Scholar]
- 20. Gomes I, Mathur SK, Espenshade BM, et al. Eosinophil-fibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: implications in fibrogenesis. J Allergy Clin Immunol 2005; 116: 796–804. [DOI] [PubMed] [Google Scholar]
- 21. Rochester CL, Ackerman SJ, Zheng T, et al. Eosinophil-fibroblast interactions. Granule major basic protein interacts with IL-1 and transforming growth factor-beta in the stimulation of lung fibroblast IL-6-type cytokine production. J Immunol 1996; 156: 4449–4456. [PubMed] [Google Scholar]
- 22. Noguchi H, Kephart GM, Colby TV, et al. Tissue eosinophilia and eosinophil degranulation in syndromes associated with fibrosis. Am J Pathol 1992; 140: 521–528. [PMC free article] [PubMed] [Google Scholar]
- 23. Batchelor DJ, Devauchelle P, Elliott J, et al. Mechanisms, causes, investigation and management of vomiting disorders in cats: a literature review. J Feline Med Surg 2013; 15: 237–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elwood C, Devauchelle P, Elliott J, et al. Emesis in dogs: a review. J Small Anim Pract 2010; 51: 4–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gelain ME, Meli M, Paltrinieri S. Whole blood cytokine profiles in cats infected by feline coronavirus and healthy non-FCoV infected specific pathogen-free cats. J Feline Med Surg 2006; 8: 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bloom PB. Canine and feline eosinophilic skin diseases. Vet Clin North Am Small Anim Pract 2006; 36: 141–160, vii. [DOI] [PubMed] [Google Scholar]
- 27. Svensson L, Wenneras C. Human eosinophils selectively recognize and become activated by bacteria belonging to different taxonomic groups. Microbes Infect 2005; 7: 720–728. [DOI] [PubMed] [Google Scholar]
- 28. Uri N, Ronen O, Marshak T, et al. Allergic fungal sinusitis and eosinophilic mucin rhinosinusitis: diagnostic criteria. J Laryngol Otol 2013; 127: 867–871. [DOI] [PubMed] [Google Scholar]
- 29. Howl JH, Peterson MG. Intestinal mast cell tumor in a cat: presentation as eosinophilic enteritis. J Am Anim Hosp Assoc 1995; 31: 457–461. [DOI] [PubMed] [Google Scholar]
- 30. Lilliehook I, Tvedten H. Investigation of hypereosinophilia and potential treatments. Vet Clin North Am Small Anim Pract 2003; 33: 1359–1378, viii. [DOI] [PubMed] [Google Scholar]
- 31. Takeuchi Y, Takahashi M, Tsuboi M, et al. Intestinal T-cell lymphoma with severe hypereosinophilic syndrome in a cat. J Vet Med Sci 2012; 74: 1057–1062. [DOI] [PubMed] [Google Scholar]
- 32. Dean E, Meunier V. Feline eosinophilic keratoconjunctivitis: a retrospective study of 45 cases (56 eyes). J Feline Med Surg 2013; 15: 661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pedersen NC. An update on feline infectious peritonitis: diagnostics and therapeutics. Vet J 2014; 201: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Montali RJ, Strandberg JD. Extraperitoneal lesions in feline infectious peritonitis. Vet Pathol 1972; 9: 109–121. [DOI] [PubMed] [Google Scholar]
- 35. Pedersen NC. A review of feline infectious peritonitis virus infection: 1963–2008. J Feline Med Surg 2009; 11: 225–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harvey CJ, Lopez JW, Hendrick MJ. An uncommon intestinal manifestation of feline infectious peritonitis: 26 cases (1986–1993). J Am Vet Med Assoc 1996; 209: 1117–1120. [PubMed] [Google Scholar]
- 37. Bouvier T, Giorgio PA. Factors influencing the detection of bacterial cells using fluorescence in situ hybridization (FISH): a quantitative review of published reports. FEMS Microbial Ecol 2003; 44: 3–15. [DOI] [PubMed] [Google Scholar]