Abstract
Practical relevance:
Hyperparathyroidism exists in primary and secondary forms. Primary hyperparathyroidism has typically been considered a disease that uncommonly affects cats, but this condition is more prevalent than previous diagnoses would suggest. Secondary hyperparathyroidism may be caused by either nutritional influences (ie, nutritional secondary hyperparathyroidism) or chronic kidney disease (ie, renal secondary hyperparathyroidism). Tertiary hyperparathyroidism has yet to be documented in veterinary medicine, but it is possible that this condition occurs in some cats following longstanding renal secondary hyperparathyroidism.
Clinical challenges:
Diagnosis of this group of calcium metabolic disorders presents a number of challenges for the clinician. For example, clinical signs can be non-specific and, especially in the case of primary hyperparathyroidism, there is often a low index of suspicion for the disease; careful sample handling is required for testing of parathyroid hormone (PTH) and ionized calcium levels; and there is currently no feline-specific assay for PTH, which has implications for test sensitivity and interpretation of results.
Aims:
This article briefly outlines PTH and calcium physiology by way of introduction to a review of PTH measurement and interpretation. Various forms of feline hyperparathyroidism are then described, encompassing diagnosis and treatment options.
Parathyroid hormone and calcium regulation
Parathyroid hormone (PTH) is the principal hormone involved in the minute-to-minute fine regulation of blood calcium concentration through effects on tubular reabsorption of calcium, intestinal absorption of calcium mediated indirectly via calcitriol, and bone resorption of calcium. PTH is a calcemic hormone secreted by chief cells in the parathyroid glands (PTGs).
The anatomy of the PTGs in the cat is similar to that of dogs and humans. Classically there are two PTGs associated with each lobe of the thyroid gland (one external and one internal) but, in dogs, there is considerable variation in the number of PTGs; 1 detailed dissection studies of this nature have not been reported in the cat. The PTGs are exquisitely sensitive to fluctuations in ionized calcium (iCa), especially when iCa is low. If the PTGs are responding appropriately, PTH secretion will dramatically increase when iCa is low; when iCa is high, PTH secretion will decrease. The effects of PTH can be seen directly in the kidney and bone, and indirectly in the gastrointestinal tract (Figure 1):
Figure 1.
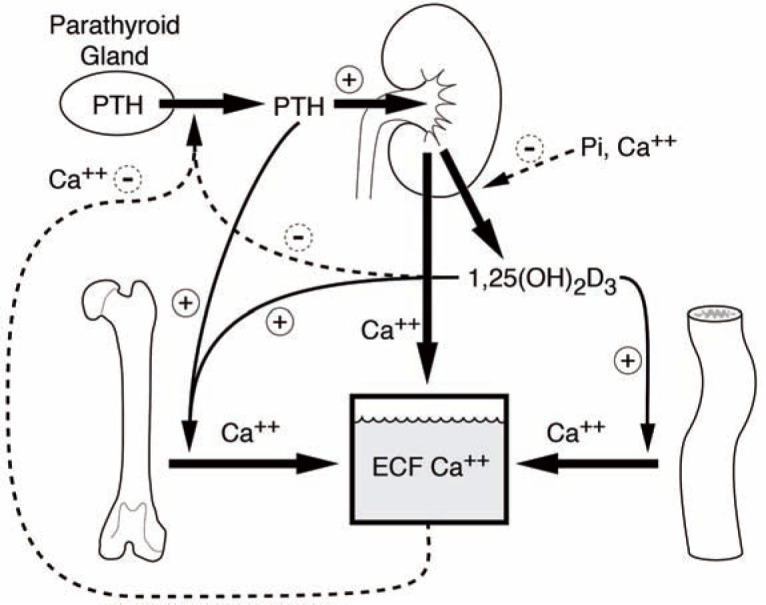
Regulation of extracellular fluid (ECF) calcium concentration by the effects of parathyroid hormone (PTH) and calcitriol (1,25[OH]2D3) on the gut, kidneys, bone and parathyroid gland
In the kidney, PTH will increase calcium reabsorption and phosphorus excretion. It also increases the activity of 1α-hydroxylase, the enzyme responsible for converting 25-hydroxyvitamin D (25OHD) to 1,25-dihydroxyvitamin D3 (1,25[OH]2D3) (calcitriol).
Calcitriol then acts on the gastrointestinal tract to increase calcium and phosphorus absorption.
In bone, PTH induces osteoclastic bone resorption, which increases calcium and phosphorus resorption.
All of these actions result in an increase in the concentration of circulating iCa; this, in turn, provides negative feedback to reduce PTH secretion. When PTH and iCa concentrations are plotted graphically, an inverse sigmoidal curve with a steep slope is generated (Figure 2), as has recently been shown in the cat. 2 This relationship ensures a relatively large change in circulating PTH concentration in reaction to small changes in iCa over the physiological range. The set-point is defined as the concentration of circulating calcium that results in half the maximal PTH secretion that can be achieved. Certain diseases can shift this curve to the right or left. For example, with primary hyperparathyroidism (PHPT), the PTH–iCa curve is shifted to the right as the set-point for calcium increases. 3
Figure 2.
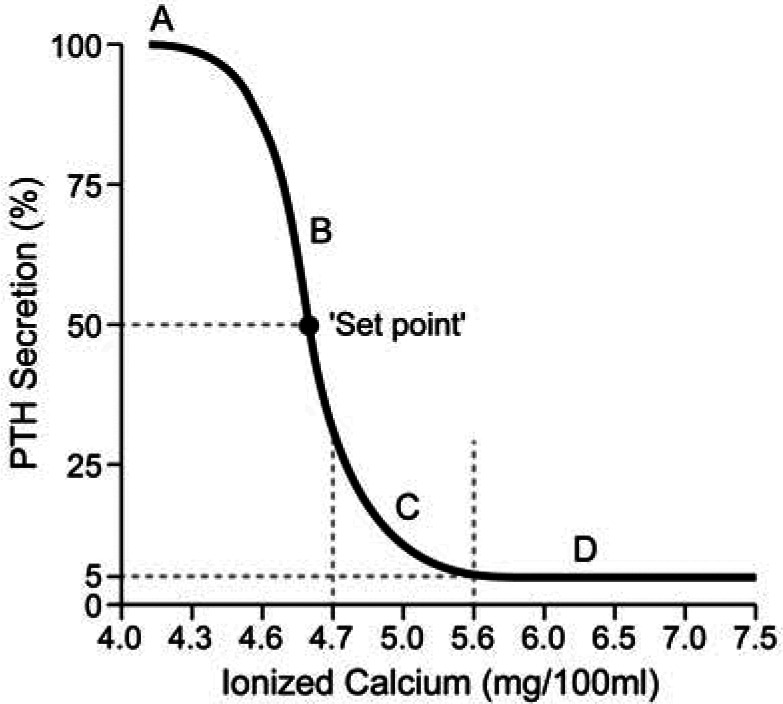
Graph detailing idealized set-point relationships between ionized calcium and the PTH secretory rate. Note that maximal parathyroid hormone (PTH) secretion is achieved as iCa declines as a normal homeostatic mechanism – this occurs as an attempt to prevent further decline in circulating iCa that could otherwise be lethal
Total serum calcium cannot be reliably used to predict the metabolically active iCa fraction in cats.4,5 In one study that evaluated 434 feline serum samples, there was an overall diagnostic discordance of 40% when using tCa to predict iCa. Ionized hypercalcemia and normocalcemia were underestimated and ionized hypocalcemia was overestimated. 5 Thus, screening cats for calcium metabolic disorders is better served by measurement of iCa (see box).
Measurement and interpretation of PTH
PTH is released from the PTGs as an 84 amino acid single-chain peptide. This intact active form of the hormone (PTH1–84) is inactivated by hepatic and renal metabolism (plasma half-life approximately 2–4 mins). The 1–34 N-terminal region is essential for the biological activity of PTH and cleavage at that site by endoproteases renders the hormone inactive. In this process, fragments of various lengths are produced and released into the blood. These fragments are then cleared from the circulation by renal excretion.
Development of immunoassay methods for PTH analysis
Development of the first radioimmunoassay for PTH pre-dated the identification of truncated PTH forms. 9 The lack of specificity of the single-site antibody used in these early assays (typically directed towards the mid-region of the PTH1–84 sequence – the region of greatest antigenicity) meant significant cross-reactivity from circulating PTH fragments, and thus overestimation of the intact active PTH1–84 concentration. The discovery of circulating PTH fragments in the late 1960s and 1970s was the main driver for the development of increasingly accurate second- and third-generation immunoassay methods for PTH analysis.
Second-generation (or ‘intact’), two-site immunoassays use two antibodies: one directed at the C-terminal region (amino acids 39–84) and one directed at the N-terminal region of the intact 1–84 PTH molecule. Most second-generation assays use an N-terminal antibody directed at amino acids 12–24, although some use an antibody directed at amino acids 26–32. These assays were thought to be far more specific since they avoided cross-reactivity with C-terminal PTH fragments (PTH 34–84). 7 However, description of these assays as ‘intact’ PTH assays is a misnomer; these assays were unable to differentiate the entire molecule (1–84) from fragments cleaved between amino acids 1–12.
In the mid-1990s, it was recognized that such fragments do exist, consisting of different lengths of the biologically active N-terminal region. This led to the development of third-generation assays that use a primary antibody directed at the C-terminal region together with a secondary antibody directed at amino acids 1–4 on the N-terminal region to detect ‘whole’ PTH (ie, only the full length [all 84 amino acids] chain of PTH was detected by these assays). 10
Assay validation and precision
While specificity was improved through the development of second- and third-generation immunoassays, the sensitivity of these assays is still an unresolved problem. The amino acid sequence varies between species, and post-translational modifications (eg, phosphorylation, cleavage, etc) vary between physiological and pathophysiological states.7,11 The extent of any post-translational modifications is unknown in the cat. Post-translational modifications can affect the affinity of the PTH molecule for the anti-PTH antibody used in an assay, even though the amino acid sequence remains the same. Furthermore, other molecules present in the blood might interfere with detection of PTH and these could be species-specific or associated with certain pathological conditions. All of these factors may affect the antigenicity of PTH and reduce the sensitivity of a given assay in particular conditions and across species.
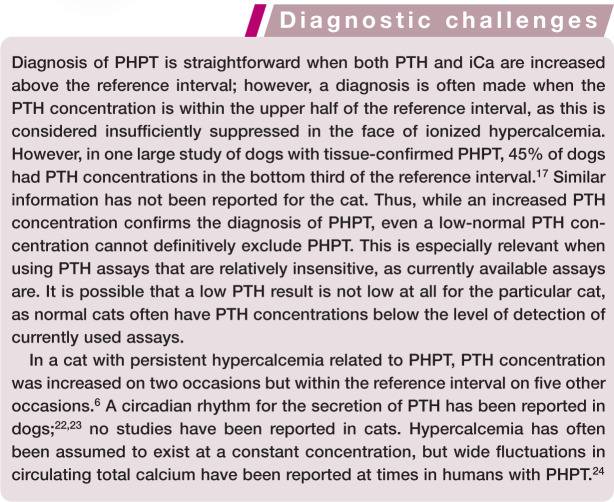
A second-generation (‘intact’) human PTH assay (Allegro Intact PTH immunoradiometric assay; Nichols Institute Diagnostics, CA, USA) that has been validated for use in cats is no longer commercially available. 12 This assay had been the standard in feline medicine for many years and most studies investigating PTH in cats have utilized it.4,13,14 This assay, however, had several limitations. The validation process of this assay did not include accuracy assessment because purified feline PTH was not available to the researchers. 12 The specificity (based on dilution parallelism study) and precision of this assay were limited. Furthermore, the adequacy of this assay at the lower limit of detection was questioned by the original researchers since the normal feline PTH concentration was below the apparent sensitivity of the assay (3.9 pg/ml [37.1 pmol/l]). The overlap between the reference range and assay sensitivity reduces the ability of any assay to detect PTH concentration that has been suppressed below normal. This is crucial in conditions in which a low PTH concentration is the key finding for diagnosis (eg, differentiating PHPT from idiopathic hypercalcemia).
Recently, a third-generation assay (Duo PTH IRMA Kit; Scantibodies Laboratory, CA, USA) has been validated for use in cats and compared with a second-generation assay. 2 Overall, the performance of both assays was acceptable; however, the lower limit of detection still overlapped with the lower limit of the calculated reference interval. Interestingly, in this study of normal cats, the ‘intact’ PTH assay yielded values that were similar to what has been previously reported; 12 but, surprisingly, greater PTH values were detected when using the third-generation assay (whole PTH). Assuming lesser cross-reactivity with PTH fragments, the whole PTH assay should in theory yield lower values. However, differences in amino acid sequence, post-translational modifications and external factors could affect the affinity of the molecule for tracer antibodies.
Based on the results of this study, it seems that the affinity of the tracer antibody against 1–4 PTH in the whole PTH assay is higher than the affinity of the antibody against human 7–34 PTH, used by the ‘intact’ PTH assay. 2 This is consistent with the finding that the amino acid sequence in positions 1–4 is identical in the feline and human PTH molecules but there are differences in amino acid numbers 7, 16, 18 and 26 between the two molecules. 15 Values measured by the whole PTH assay are also more in line with the ‘true’ concentration of PTH in other species and they are more likely to reflect the concentrations of the biologically relevant compound since the 1–4 region is most crucial for activation of the PTH receptor.
An important component of the assay validation in this study was the investigation of its biological relevance (ie, generating a PTH–iCa curve at both high and low concentrations of circulating iCa). Overall, both the second- and third-generation assays yielded the expected sigmoid PTH–iCa curve. However, in the hypercalcemia range, concentrations of PTH were only slightly higher than the lower limit of detection of the assays and were still within the reference interval for ‘normal’ cats. This again means that determination of ‘inappropriately suppressed’ PTH concentration in a hypercalcemic cat would be problematic with both assays. The same second-generation assay that was used in this study 2 was also validated by another group and their results were similar. 16 Again, PTH concentrations in healthy cats were sometimes measured below the lower limit of detection of the assay (5.2 pg/ml in this study).
Current best option
To develop an assay that accurately measures feline PTH, the hormone would have to be purified from cats and then used either to raise specific antibodies or at least validate the specificity and sensitivity of available antibodies. This process is expensive and unlikely to be commercially viable. Currently, it seems that the third-generation ‘whole’ PTH assay works best in cats, 2 but caution should be used when interpreting the results of the assay, especially when concentrations at the low end of the reference interval are measured.
Primary hyperparathyroidism
PHPT has been described in a few small case series and case reports in cats. Increased serum total calcium concentration on routine clinical chemistry testing is usually the pivotal finding that initiates a diagnostic work-up to determine if the hypercalcemia is PTG dependent or independent. 5
Signalment
Affected cats are typically middle-aged to older (8–15 years old). There are no reported feline breed predispositions; domestic shorthair cats are most commonly affected. In dogs, Keeshonds are over-represented.17,18
Clinical signs and differentials
Clinical signs tend to be non-specific, with anorexia and lethargy most commonly reported in cats with PHPT. Vomiting, polyuria, polydipsia and weight loss may also be observed in advanced cases. Calcium-containing urinary stones occur in some cats. 19 A cervical mass was palpated in 4/7 cats in one report. 20 It is unlikely that PTG enlargement alone will produce a palpable cervical mass, as the masses are quite small; however, cyst-like structures associated with the PTG mass are often palpable. Masses in the cervical region must be differentiated from thyroid enlargement, which is more readily palpated.
Idiopathic hypercalcemia, chronic kidney disease (CKD) and neoplasia are the most common and important differential diagnoses to exclude; hypervitaminosis D and hypoadrenocorticism are other, far less common causes of parathyroid-independent total serum hypercalcemia.4,14 Measurement of parathyroid hormone-related peptide (PTHrP) may be useful for diagnosis of hypercalcemia of malignancy. 21
Anecdotally, idiopathic hypercalcemia appears to be the most commonly diagnosed cause of serum total hypercalcemia in cats in North America, although no epidemiological survey on the causes of hypercalcemia in the cat have been reported since 2000. PHPT was infrequently diagnosed as the cause of serum total hypercalcemia at one teaching hospital (4/71 cats), 4 but this diagnosis is far more frequently made by veterinary endocrine referral laboratories.6,21
Diagnosis
Review of the chemistry profile and urinalysis may yield indirect support for a diagnosis of PHPT. Due to PTH’s effect of increasing renal phosphorus excretion, low or low-normal serum phosphorus is anticipated. However, in one study of 210 dogs, only 65% of cases had hypophosphatemia. 17 If hyperphosphatemia is noted, a diagnosis of PHPT is unlikely and other differential diagnoses should be considered (eg, CKD, vitamin D toxicosis; see Table 1). Hypercalcemia in dogs commonly induces polyuria due to the effect of calcium on the responsiveness of the renal tubule to antidiuretic hormone, with many dogs elaborating urine that is hyposthenuric. In cats without CKD, a surprising preservation of ability to concentrate urine in the face of hypercalcemia has been noted in two reports.4,14 In one study, which included cats with CKD, the mean urine specific gravity was 1.032 (range 1.012–1.060). 14
Table 1.
Expected biochemical changes with various causes of hypercalcemia
| Disease | Total calcium | Ionized calcium | Phosphorus | PTH | PTHrP | PTG imaging |
|---|---|---|---|---|---|---|
| Primary hyperparathyroidism | ↑ | N or ↑ | N or ↓ | ↑ or upper half of reference range | N or not detectable | ↑ One gland typical |
| Hypercalcemia of malignancy | ↑ | ↑ | N or ↓ | ↓ or lower half of reference range | N or ↑, depends on tumor type | N or ↓ |
| Chronic kidney disease | ↓ , N, ↑ | ↓ , N, ↑ | ↑ or N | ↑ | N or not detectable | ↑ Multiple glands |
| Idiopathic hypercalcemia | ↑ | ↑ | N | N or ↓ | N or not detectable | N or ↓ |
| Acute vitamin D toxicity | ↑↑ | ↑↑ | ↑↑ | ↓ | N or not detectable | N |
| Nutritional secondary hyperparathyroidism | N or ↓ | N or ↓ | N or ↓ | ↑ | N or not detectable | N or ↑ |
| Hypoadrenocorticism | ↑ | N or ↑ | ↑ | N or ↓ | N or not detectable | N |
PHPT is characterized by excessive secretion of PTH by one or more PTGs, resulting in hypercalcemia by the time of diagnosis. Definitive diagnosis of PHPT requires measurement and interpretation of paired PTH and iCa test results in order to analyze whether the PTH concentration is appropriate to the degree of ionized calcemia. Most cats with PHPT are affected by single PTG adenomas. Fewer cats are diagnosed with parathyroid hyperplasia, carcinomas or cystadenomas.18,20 High-frequency ultrasonography of the thyroid and PTG region is highly supportive of a diagnosis of PHPT when a single enlarged PTG is found. 17
A diagnosis of PHPT can sometimes be challenging (see box).
Treatment
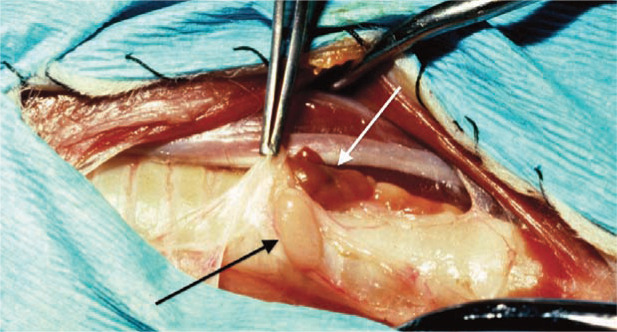
Parathyroidectomy of one or more PTGs is most commonly recommended for definitive treatment. Ultrasonography of the neck identifies the location of the PTG tumor as internal or external and allows presurgical planning (Figure 3). Surgical exploration of the cervical region in patients with PTG adenoma or carcinoma usually reveals enlargement of one PTG; the remaining three tend to be small or impossible to identify because hypercalcemia results in atrophy of normal parathyroid tissue (Figure 4). Primary PTG hyperplasia may affect more than one gland, and clinical signs can recur if only the largest gland is removed surgically. PTG tumors may be difficult to identify if the tumor is embedded in fat or if it arises from an internal PTG.
Figure 3.
Parathyroid mass on ultrasound examination in a 9-year-old domestic medium haired spayed female cat with hypercalcemia and mild depression. Note the hypoechogenic mass (solid arrows) below the thyroid (open arrow), which is the abnormal parathyroid gland (PTG; height 1.4 mm, length 2.8 mm). Even with the high frequency ultrasonographic technique available today, the normal feline PTG is too small to be seen. This mass was confirmed to be a PTG adenoma following surgical removal and histopathology. Courtesy of Dr John Mattoon, Washington State University College of Veterinary Medicine, USA
Figure 4.
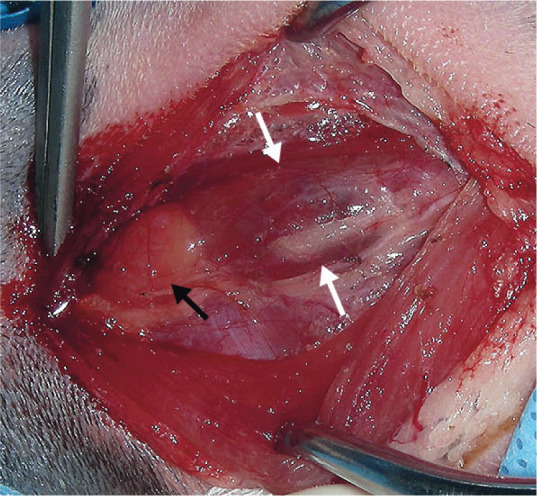
Surgical exploration of the cervical region in a cat with hypercalcemia. Note the enlarged parathyroid gland (black arrow) next to the thyroid gland (between white arrows). Courtesy of Dr Daniel Smeak, Colorado State University College of Veterinary Medicine, USA
Parathyroidectomy of an adenoma or cystadenoma was associated with a good prognosis for long-term survival in a subset of cats in one study. 20 Percutaneous ultrasound-guided ethanol ablation and percutaneous ultrasound-guided heat ablation, as performed to treat PHPT in dogs, 25 have not been reported in cats.
Secondary hyperparathyroidism
Nutritional secondary hyperparathyroidism
Nutritional secondary hyperparathyroidism (NSHP) may arise as a consequence of disturbances in the dietary factors calcium, phosphorus and vitamin D. In veterinary medicine, NSHP is reported to occur when a cat consumes a diet that is either relatively or absolutely deficient in calcium (Figure 5). The relative calcium concentration is affected by the dietary phosphorus concentration, as diets high in phosphorus will have an altered calcium:phosphorus (Ca:P) ratio. It is recommended that growing kittens eat a diet with a Ca:P ratio of approximately 1:1. 26
Figure 5.
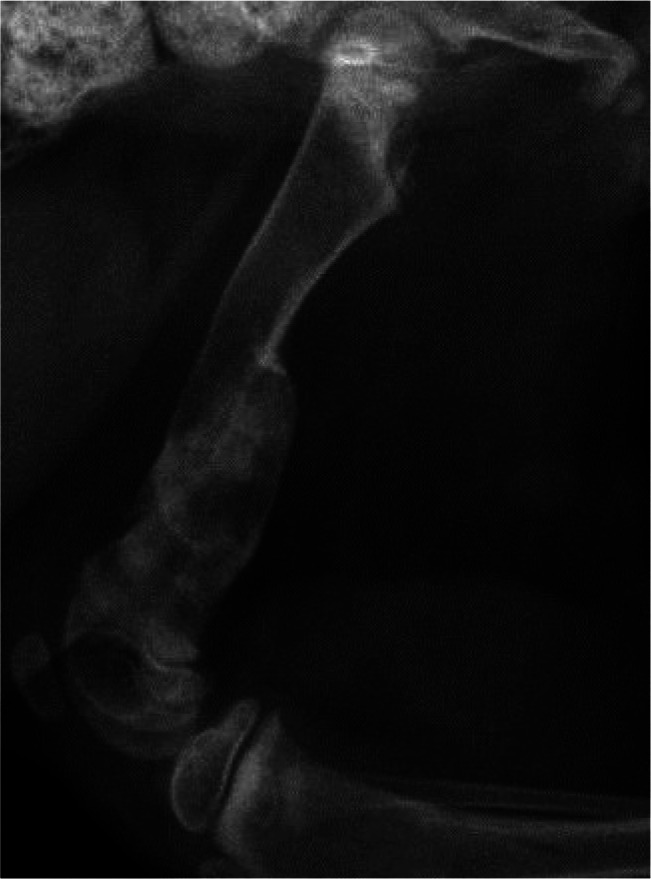
Distal femoral fracture in a 2-month-old kitten eating an all-meat diet. Note that the bone has already healed as a functional malunion. The cortical thickness and bone mineral density are reduced, as is expected in cats with nutritional secondary hyperparathyroidism. Folding fractures of long bones are typical with this condition; pathological fractures can also occur in the pelvis and spine. This kitten additionally presented with humeral and pelvic fractures. After transitioning on to a complete and balanced diet, all fractures healed without surgical intervention and no new fractures were acquired. Courtesy of Dr Jonathan Dyce, The Ohio State University College of Veterinary Medicine, USA
The development of NSHP has most commonly been associated with consumption of all-meat diets. The low calcium concentration in meat in combination with the high phosphorus concentration results in a very low Ca:P ratio. Young animals are at higher risk of developing NSHP, given the increased demand for calcium necessary for bone growth coupled with their minimal calcium stores.13,27
Clinical signs
In reports of kittens with NSHP, clinical signs are most commonly related to consequences of hypocalcemia and osteopenia. The clinical signs associated with hypocalcemia included tremors and seizure activity. Long bones and vertebrae are often affected, resulting in painful ambulation, limb deformity or paresis. Vertebral body fractures can lead to profound neurological deficits. Radiographs may reveal diffuse osteopenia, bony deformities and fractures.13,28
Biochemistry findings
There are no findings on a minimum database pathognomonic for NSHP. Both total calcium and iCa concentrations may be normal or decreased. They will be normal if PTH secretion is great enough to correct the hypocalcemia; with partial correction, hypocalcemia may occur. Serum phosphorus concentrations are typically normal or decreased. Since young, growing animals often have higher total calcium and phosphorus concentrations than adult animals, these ‘normal’ values may be relatively abnormal. Hypophosphatemia may be noted due to decreased renal reabsorption and thus increased excretion. Elevated alkaline phosphatase concentrations are often observed due to the elevated bone isoenzyme associated with growth.13,28
By definition, circulating PTH concentration is elevated in response to hypocalcemia. This causes chronic calcium resorption from bones and can lead to fibrous osteodystrophy. In a case series of cats with NSHP, a few animals had decreased 25OHD and increased calcitriol concentrations. This may be due to the effect of PTH on activation of 25OHD to calcitriol or to changes in 1α-hydroxylase activity in the kidney if serum calcium is trending low. 13
Differential diagnoses
Differential diagnoses for NSHP include renal secondary hyperparathyroidism (RSHP) (see later), osteogenesis imperfecta and rickets.
Osteogenesis imperfecta is a genetic disorder characterized by brittle bones that is caused by a defect in collagen production. Although rarely described in animals, it has been reported in Dachshunds and a kitten.29 –31 These animals should have normal blood calcium, phosphorus, vitamin D and PTH concentrations. The disease should be considered in young animals with spontaneous fractures despite eating complete and balanced diets. Abnormal dentine development, characterized by severe thinning of the dentine layer, translucency and pink discoloration, may also be observed. 31
Rickets describes defective bone mineralization in young animals due to hypovitaminosis D. In people, nutritional rickets is defined as either a vitamin D deficiency or dietary calcium deficiency. The primary feature of vitamin D deficiency rickets is a reduction in intestinal calcium absorption due to inadequate calcitriol concentrations in the face of an otherwise normal dietary calcium intake. Comparing biochemical abnormalities in people, those with calcium deficiency rickets typically have greater 25OHD concentrations than those with vitamin D deficiency, as well as consistently elevated calcitriol concentrations. 32 Rickets has also been described in animals that have normal dietary vitamin D intake but excessive calcium intake early in life, causing hypercalcemia, secondary hypoparathyroidism and decreased calcitriol production. 27
Hereditary rickets, also known as pseudo-vitamin D deficiency rickets, is slightly different. This genetic form of rickets is classified into two types: vitamin D-dependent rickets types I and II (VDDR-I and VDDR-II). VDDR-I results from a deficiency in 1α-hydroxylase, the enzyme responsible for conversion of 25OHD to calcitriol. VDDR-II results from end-organ resistance to calcitriol, typically due to a defect in the vitamin D receptor. VDDR-I has been described in a kitten. 33 VDDR-II has been described in a Pomeranian. 34 PTH concentrations were elevated in both cases.
Treatment
Treatment of NSHP entails feeding a complete and balanced diet. For animals with acute signs of hypocalcemia, parenteral calcium or oral calcium supplements may be administered short-term. Cage rest may be implemented to reduce the risk of development or disruption of fractures. Analgesia may be required for animals with fractures or other painful bony deformities.
The prognosis for recovery from uncomplicated cases (ie, without severe bony abnormalities) is good. Although the exact timeframe for normalization of calcium, phosphorus and PTH concentrations is unknown, in one cat, iCa had returned to reference interval levels at the 10-day recheck. 28 Generally, the authors anticipate that that these values should return to normal within 2 weeks. As long as the cat is doing well clinically, it is not mandatory to recheck PTH concentrations. Appropriate bony mineralization may occur within 4–8 weeks of dietary modification.
For animals with spinal fractures or more severe bony changes, the prognosis may be guarded to poor.
Renal secondary hyperparathyroidism
RSHP is a complex disease that occurs secondarily to CKD (Figure 6). Diagnosis is based on an elevated PTH concentration in a cat with CKD.
Figure 6.
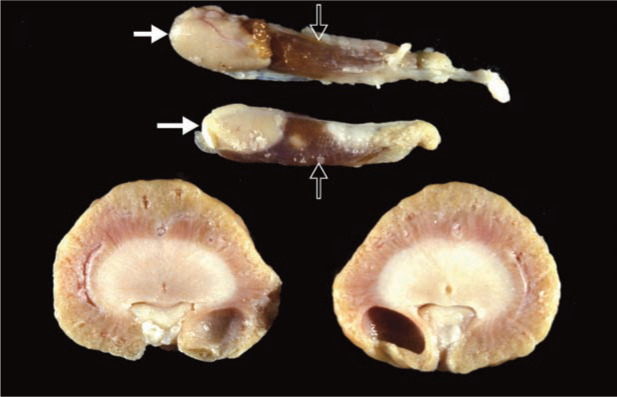
Thyroid glands, parathyroid glands (PTGs) and kidneys at necropsy from a 7-year-old domestic shorthair cat with a 3 year history of chronic kidney disease (CKD). The cat’s CKD was acquired secondarily to potassium deficit nephropathy (kaliopenic nephropathy). Note that the renal cortices are irregular and pale. Also there is a large retention cyst near the renal pelvis. This cat’s PTH level was increased to 6 x the upper limit of the reference interval. Solid arrows denote the greatly enlarged PTGs that occur as part of renal secondary hyperparathyroidism.
Open arrows denote the thyroid glands. The PTG enlargement is largely due to chief cell hyperplasia, although hypertrophy of chief cells also contributes. Courtesy of Dr Larry Nagode, The Ohio State University College of Veterinary Medicine, USA
Evolving understanding of pathophysiology
The original ‘trade-off hypothesis’ for the development of RSHP was thought to be a fairly simple process, beginning with the onset of CKD. The progressive loss of functional nephrons leads to increased phosphorus (via a decreased glomerular filtration rate) and decreased activation of 25OHD to calcitriol (via decreased 1α-hydroxylase activity), both of which will cause a reduction in iCa. Hyperphosphatemia, ionized hypocalcemia and decreased calcitriol all result in secretion of PTH. PTH has been recognized as a uremic toxin that may contribute to the development of renal osteodystrophy, bone marrow suppression, anemia and the progression of CKD in people and dogs;35,36 this has not been specifically studied in cats. It is likely that increased PTH secretion in patients with RSHP is primarily caused by PTG hyperplasia, as each cell contributes a portion to PTH secretion that cannot be suppressed.
Diagnosis of RSHP is made by documenting an increased PTH concentration in an animal with CKD. Calcium and phosphorus concentrations are variable. The prevalence of RSHP in cats has been reported to be as high as 84%. 37 Similar to what has been described in dogs, 38 PTH concentrations may increase early in feline CKD.39,40 In one study, PTH concentrations were higher in non-azotemic cats (creatinine <2.0 mg/dl; 176.8 mmol/l) that subsequently developed azotemia within 12 months compared with cats that remained non-azotemic. This increase in PTH was noted to occur before changes in plasma calcium or phosphorus concentrations were detected. 39 However, in another study, PTH concentrations were only significantly different between control cats and those with International Renal Interest Society (IRIS) stage 4 CKD. 41 Similar to what is observed in human medicine, PTH concentrations have been shown to be positively correlated with age in cats without known CKD. 39
More recent evidence has shown that there are other hormonal factors that influence the relationships between calcium, phosphorus and vitamin D metabolites. Fibroblast growth factor-23 (FGF-23) plays an important role in phosphorus regulation. It has been independently associated with progression of CKD, development of RSHP and higher mortality rates in people.42 –44 Stimulated by hyperphosphatemia and increased calcitriol concentrations, FGF-23 is a phosphatonin, synthesized and secreted by osteoblasts and osteocytes, which promotes renal phosphorus excretion. The effects of FGF-23 on the development of RSHP are complicated. While in the early stages of CKD, FGF-23 directly decreases PTH, it also downregulates 1α-hydroxylase, leading to reduced calcitriol concentrations, which further contribute to lack of inhibition of PTH transcription and secretion. It is unclear whether increased FGF-23 concentrations contribute to the development of RSHP or if increased FGF-23 concentrations fail to inhibit PTH secretion due to the lack of PTG Klotho expression.
Klotho, a protein that exists in both membrane-bound and soluble forms, has also been shown to affect the progression of CKD and the development of RSHP in people. 45 Expressed in the kidneys and PTG, membrane-bound Klotho is a co-receptor required for FGF-23 activity. Soluble Klotho affects calcium homeostasis by suppressing 1α-hydroxylase in the kidney. It also plays a role in PTH synthesis in the PTGs. Klotho expression is decreased in the kidneys of CKD patients. Thus, although FGF-23 secretion is increased in CKD, there is end-organ resistance to FGF-23 due to a deficiency of the Klotho cofactor. As a result of the subsequent downregulation of the Klotho/FGF-23 receptor complex in the PTGs, PTH concentrations increase. In people, Klotho has been studied as an early biomarker in CKD and RSHP as it has been identified as an independent risk factor for the progression of CKD. 45 There are currently no veterinary studies that have evaluated Klotho.
Concentrations of FGF-23 have been measured in healthy non-azotemic cats and cats with CKD. In one study that monitored geriatric, non-azotemic cats for 12 months, FGF-23 concentrations at baseline were significantly increased in cats that developed azotemia (creatinine >2.0 mg/dl; 176.8 mmol/l) as compared with cats that did not develop azotemia. There was a weak positive linear relationship between FGF-23 and PTH concentrations in those cats and a moderate negative exponential relationship between FGF-23 and the glomerular filtration rate. 46 In cats with CKD, FGF-23 concentrations were significantly different between the group of healthy control cats and cats with IRIS stage 2–4 CKD. As IRIS stage increased, FGF-23 concentrations increased. Additionally, within IRIS stages 2 and 3, cats with hyperphosphatemia (phosphate >5.0 mg/dl; 1.6 mmol/l) had greater FGF-23 concentrations than their normophosphatemic (phosphate <4.5 mg/dl; 1.5 mmol/l) counterparts. Serum phosphorus concentrations were independent predictors of FGF-23 concentrations. 41 Targeted circulating serum phosphorus concentrations below 4.5 mg/dl (1.5 mmol/l) have been reported in dogs with CKD to be a surrogate for PTH that is not increased. 38
Implications for treatment
Treatment of RSHP includes controlling serum phosphorus concentrations by feeding a reduced phosphorus diet ± administration of a dietary phosphorus binder. In one study, increased survival of cats with CKD was attributed in part to control of hyperphosphatemia and reduced PTH concentrations. 47 Plasma FGF-23 concentrations declined in cats with CKD fed a reduced phosphorus diet. 48
Calcitriol has also been used in an attempt to reduce PTH concentrations by preventing or reversing PTG hyperplasia and inhibiting transcription of PTH messenger RNA. 49 The effects of calcitriol therapy have been variable. Two studies have reported improved clinical signs and survival in dogs and cats treated with calcitriol.36,50 Another study showed no reduction in PTH concentrations in cats receiving calcitriol; however, these cats did not have obvious increases in PTH at the outset. Variability in the PTH assay used at the time may also have contributed to these findings. 35
One potential disadvantage of the use of calcitriol is the risk of inducing hypercalcemia, but this risk is minimal when calcitriol is used in the low dose range (ie, 2.5–3.5 ng/kg/day or 9 ng/kg/week, twice weekly). If there is concurrent hyperphosphatemia, the risk of soft tissue mineralization increases. 49 The use of 22-oxacalcitriol, an analogue of calcitriol with less calcemic activity, has been investigated in dogs with induced RSHP. It was concluded that this may be a useful alternative treatment for RSHP.51,52 This vitamin D analogue has not been investigated in cats.

Historically, calcitriol measurements have been considered pivotal in the diagnosis and treatment of RSHP, with lesser emphasis on measurements of 25OHD. Given its half-life, 25OHD is a better marker of systemic vitamin D status than calcitriol, which has a half-life of 4–6 h. Circulating concentrations of 25OHD may be of greater importance than previously thought. Decreased 25OHD concentrations are frequently found in people with CKD, and 25OHD has been shown to be independently associated with CKD disease progression and mortality. 53 Dogs with CKD have low circulating 25OHD concentrations as well. 54 This might have previously been attributed to low dietary vitamin D intake or decreased vitamin D absorption. It is now known that low circulating 25OHD can result from CKD because filtered 25OHD and vitamin D-binding protein are inadequately reabsorbed by the proximal tubules, such that less 25OHD is returned to the circulation. A low 25OHD concentration in a cat with CKD could be the result of some combination of nutritional or renal factors, based on observations in humans and dogs with CKD. 49
Since 25OHD concentrations are frequently decreased in people with CKD, it is often recommended that parent vitamin D supplementation (eg, ergocalciferol, cholecalciferol) is administered prior to activated vitamin D therapy (eg, calcitriol). 55 There are species differences in the ability to metabolize various vitamin D sources; cats utilize ergocalciferol (vitamin D2) with 70% efficiency as compared with cholecalciferol (vitamin D3). 56 Additional medical therapy may be attempted using calcimimetics, which work to inhibit PTH secretion by modulating the calcium-sensing receptor in the PTGs. There is some evidence to suggest that this may be effective in reducing calcium in people with tertiary hyperparathyroidism (THPT). 57 There is no published experience of the use of calcimimetics in cats.
Hyperthyroidism and hyperparathyroidism
There has been an association made between hyperthyroidism and hyperparathyroidism, with 57–77% of cats with hyperthyroidism having concurrent hyperparathyroidism (Figure 7).16,58 Total and iCa concentrations have been variably affected in these cats, but tend to be either normal or slightly decreased. Hyperphosphatemia has been more frequently observed. These findings are most likely due to the effects of thyroid hormone on bone turnover and renal excretion. Hypocalcemia and/or hyperphosphatemia would then cause a compensatory increase in PTH secretion.
Figure 7.
Surgical exploration of the cervical region in a cat undergoing thyroidectomy for treatment of hyperthyroidism. Note the enlarged parathyroid gland (black arrow) to the left of the thyroid gland (white arrow). Courtesy of Dr Steven Birchard and Dr Mark Peterson, The Animal Medical Center, New York, USA
In one study of hyperthyroid cats, PTH concentrations were quite variable, ranging from normal to 19 times the upper limit of the reference interval. 58 Although these cats had creatinine concentrations within the reference interval, it is possible that RSHP could be contributing to the hyperparathyroidism associated with hyperthyroidism since PTH concentrations may increase before azotemia is observed.16,39 In one study, treatment of hyperthyroidism led to increased calcium concentrations, decreased phosphorus concentrations and return of PTH concentrations to normal in non-azotemic cats. 16
Tertiary hyperparathyroidism
THPT develops as a consequence of prolonged RSHP and is characterized by increased PTH levels and hypercalcemia. With this syndrome, the PTGs act in an almost autonomous fashion, similar to what is seen with PHPT. One might wonder how to distinguish between RSHP and THPT. One distinction between the two syndromes is that while serum calcium is typically normal to low with RSHP, hypercalcemia will be observed with THPT. It is theorized that THPT occurs due to a monoclonal expansion of parathyroid cells that have an altered set-point of their calcium-sensing receptor, likely as a consequence of downregulation of vitamin D receptor and calcium-sensing receptor expression within the PTGs as CKD advances (secondary to decreasing calcitriol dynamics).
A long-standing history of CKD in which the serum calcium was normal for some time before it became elevated helps in the diagnosis of THPT. It is essential that the increase in calcium is associated with ionized hypercalcemia; total calcium may increase in cats with CKD at times when iCa is not increased, likely caused by increased circulating complexes that are binding calcium. 59
THPT has been described in people, mostly with severe CKD or after renal transplantation. This is not a well-described phenomenon and has never been reported in cats. Treatment in people is variable. Cinacalcet, a calcimimetic that upregulates the calcium-sensing receptor, has been effective in some cases in reducing serum PTH, calcium and phosphorus concentrations; 60 although people with severely enlarged PTGs may not be as responsive. 61 With prolonged, persistent hypercalcemia, surgical management may be recommended to reduce the number of functional parathyroid cells, although high dose pulse therapy with activated vitamin D metabolites (eg, calcitriol) to upregulate vitamin D receptor and calcium-sensing receptor expression can also be effective. High dose pulse calcitriol protocols were reportedly effective in lowering iCa when PTH was suppressed, presumably due to upregulation of the vitamin D receptor in the PTGs. 57
Key Points
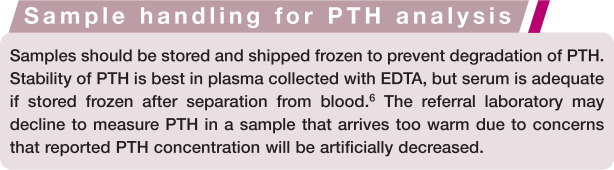
Serum or plasma samples for PTH measurement should be frozen shortly after collection and shipped frozen to prevent degradation of PTH. Samples that arrive too warm at the laboratory are not suitable for analysis. Temperature of the sample upon arrival at the laboratory should be noted on the report form.
Third-generation assays for measurement of PTH that detect ‘whole’ PTH are recommended for use in cats, as they appear to have greater affinity for feline PTH.
PTH must always be interpreted along with ionized calcium in the same sample in order to determine appropriateness of the PTH concentration.
High concentrations of PTH frequently occur in cats with CKD (even at IRIS stage 1) and may be associated with a poor outcome. PTH itself must be measured, since surrogates for PTH, such as serum phosphorus, fail to accurately predict whether PTH is increased in cats with CKD.
High concentrations of PTH are typically encountered in cats with nutritional secondary hyperparathyroidism and primary hyperparathyroidism (PHPT). Some cats with PHPT will be documented to have PTH concentrations within the reference interval.
The development of a PTH assay using antibodies directed against the amino acid sequence specific to feline PTH would likely result in an increased ability to detect changes in circulating PTH with greater sensitivity early in disease processes affecting calcium and phosphorus metabolism.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article.
The authors have no conflict of interest to declare.
References
- 1. Krook L. Spontaneous hyperparathyroidism in the dog; a pathological-anatomical study. Acta Pathol Microbiol Scand Suppl 1957; 122: 1–88. [PubMed] [Google Scholar]
- 2. Pineda C, Aguilera-Tejero E, Raya AI, et al. Feline parathyroid hormone: validation of hormonal assays and dynamics of secretion. Domest Anim Endocrinol 2012; 42: 256–264. [DOI] [PubMed] [Google Scholar]
- 3. Malberti F, Farina M, Imbasciati E. The PTH-calcium curve and the set point of calcium in primary and secondary hyperparathyroidism. Nephrol Dial Transplant 1999; 14: 2398–2406. [DOI] [PubMed] [Google Scholar]
- 4. Savary K, Price GS, Vaden SL. Hypercalcemia in cats: a retrospective study of 71 cases (1991–1997). J Vet Intern Med 2000; 14: 184–189. [DOI] [PubMed] [Google Scholar]
- 5. Schenck PA, Chew DJ. Prediction of serum ionized calcium concentration by serum total calcium measurement in cats. Can J Vet Res 2010; 74: 209–213. [PMC free article] [PubMed] [Google Scholar]
- 6. Schenck PA, Chew DJ, Nagode LA, et al. Disorders of calcium: hypercalcemia and hypocalcemia. In: DiBartola SP. (ed). Fluid, electrolyte, and acid-base disorders in small animal practice. 4th ed. St Louis, MO: WB Saunders, 2012, pp 120–194. [Google Scholar]
- 7. Couchman L, Taylor DR, Krastins B, et al. LC-MS candidate reference methods for the harmonisation of parathyroid hormone (PTH) measurement: a review of recent developments and future considerations. Clin Chem Lab Med 2014; 52: 1251–1263. [DOI] [PubMed] [Google Scholar]
- 8. D’Amour P. Circulating PTH molecular forms: what we know and what we don’t. Kidney Int Suppl 2006: S29–33. [DOI] [PubMed] [Google Scholar]
- 9. Berson SA, Yalow RS, Aurbach GD, et al. Immunoassay of bovine and human parathyroid hormone. Proc Natl Acad Sci USA 1963; 49: 613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao P, Scheibel S, D’Amour P, et al. Development of a novel immunoradiometric assay exclusively for biologically active whole parathyroid hormone 1-84: implications for improvement of accurate assessment of parathyroid function. J Bone Miner Res 2001; 16: 605–614. [DOI] [PubMed] [Google Scholar]
- 11. Toribio RE, Kohn CW, Chew DJ, et al. Comparison of serum parathyroid hormone and ionized calcium and magnesium concentrations and fractional urinary clearance of calcium and phosphorus in healthy horses and horses with enterocolitis. Am J Vet Res 2001; 62: 938–947. [DOI] [PubMed] [Google Scholar]
- 12. Barber PJ, Elliott J, Torrance AG. Measurement of feline intact parathyroid hormone: assay validation and sample handling studies. J Small Anim Pract 1993; 34: 614–620. [Google Scholar]
- 13. Tomsa K, Glaus T, Hauser B, et al. Nutritional secondary hyperparathyroidism in six cats. J Small Anim Pract 1999; 40: 533–539. [DOI] [PubMed] [Google Scholar]
- 14. Midkiff AM, Chew DJ, Randolph JF, et al. Idiopathic hypercalcemia in cats. J Vet Intern Med 2000; 14: 619–626. [DOI] [PubMed] [Google Scholar]
- 15. Toribio RE, Kohn CW, Chew DJ, et al. Cloning and sequence analysis of the complementary DNA for feline preproparathyroid hormone. Am J Vet Res 2002; 63: 194–197. [DOI] [PubMed] [Google Scholar]
- 16. Williams TL, Elliott J, Syme HM. Calcium and phosphate homeostasis in hyperthyroid cats: associations with development of azotaemia and survival time. J Small Anim Pract 2012; 53: 561–571. [DOI] [PubMed] [Google Scholar]
- 17. Feldman EC, Hoar B, Pollard R, et al. Pretreatment clinical and laboratory findings in dogs with primary hyperparathyroidism: 210 cases (1987–2004). J Am Vet Med Assoc 2005; 227: 756–761. [DOI] [PubMed] [Google Scholar]
- 18. Bonczynski J. Primary hyperparathyroidism in dogs and cats. Clin Tech Small Anim Pract 2007; 22: 70–74. [DOI] [PubMed] [Google Scholar]
- 19. Marquez GA, Klausner JS, Osborne CA. Calcium oxalate urolithiasis in a cat with a functional parathyroid adenocarcinoma. J Am Vet Med Assoc 1995; 206: 817–819. [PubMed] [Google Scholar]
- 20. Kallet AJ, Richter KP, Feldman EC, et al. Primary hyperparathyroidism in cats: seven cases. J Am Vet Med Assoc 1991; 199: 1767–1771. [PubMed] [Google Scholar]
- 21. Bolliger AP, Graham PA, Richard V, et al. Detection of parathyroid hormone-related protein in cats with humoral hypercalcemia of malignancy. Vet Clin Pathol 2002; 31: 3–8. [DOI] [PubMed] [Google Scholar]
- 22. Domingo V, Lopez I, Mendoza FJ, et al. Circadian variation of the Ca2+–PTH curve during hypercalcaemia in dogs. J Vet Med A Physiol Pathol Clin Med 2007; 54: 545–548. [DOI] [PubMed] [Google Scholar]
- 23. Lopez I, Aguilera-Tejero E, Estepa JC, et al. Diurnal variations in the plasma concentration of parathyroid hormone in dogs. Vet Rec 2005; 157: 344–347. [DOI] [PubMed] [Google Scholar]
- 24. Wood GM, Sidhu B, Saunders WA, et al. Disappearing hypercalcaemia. Postgrad Med J 1987; 63: 741–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rasor L, Pollard R, Feldman EC. Retrospective evaluation of three treatment methods for primary hyperparathyroidism in dogs. J Am Anim Hosp Assoc 2007; 43: 70–77. [DOI] [PubMed] [Google Scholar]
- 26. National Research Council Ad Hoc Committee on Dog and Cat Nutrition. Minerals. In: Nutrient requirements of dogs and cats. Washington DC: National Academies Press, 2006, pp 145–192. [Google Scholar]
- 27. Towell TL. Nutrition-related skeletal disorders. In: Ettinger S, Feldman EC. (ed). Textbook of veterinary internal medicine. 7th ed. St Louis, MO: Elsevier, 2010; 668–672. [Google Scholar]
- 28. Dimopoulou M, Kirpensteijn J, Nielsen DH, et al. Nutritional secondary hyperparathyroidism in two cats: evaluation of bone mineral density with dual-energy X-ray absorptiometry and computed tomography. Vet Comp Orthop Traumatol 2010; 23: 56–61. [DOI] [PubMed] [Google Scholar]
- 29. Seeliger F, Leeb T, Peters M, et al. Osteogenesis imperfecta in two litters of dachshunds. Vet Pathol 2003; 40: 530–539. [DOI] [PubMed] [Google Scholar]
- 30. Eckardt J, Kluth S, Dierks C, et al. Population screening for the mutation associated with osteogenesis imperfecta in dachshunds. Vet Rec 2013; 172: 364. [DOI] [PubMed] [Google Scholar]
- 31. Evason MD, Taylor SM, Bebchuk TN. Suspect osteogenesis imperfecta in a male kitten. Can Vet J 2007; 48: 296–298. [PMC free article] [PubMed] [Google Scholar]
- 32. Glorieux FH, Pettifor JM. Vitamin D/dietary calcium deficiency rickets and pseudo-vitamin D deficiency rickets. Bonekey Rep 2014; 3: 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Geisen V, Weber K, Hartmann K. Vitamin D-dependent hereditary rickets type I in a cat. J Vet Intern Med 2009; 23: 196–199. [DOI] [PubMed] [Google Scholar]
- 34. LeVine DN, Zhou Y, Ghiloni RJ, et al. Hereditary 1,25-dihydroxyvitamin D-resistant rickets in a Pomeranian dog caused by a novel mutation in the vitamin D receptor gene. J Vet Intern Med 2009; 23: 1278–1283. [DOI] [PubMed] [Google Scholar]
- 35. Hostutler RA, DiBartola SP, Chew DJ, et al. Comparison of the effects of daily and intermittent-dose calcitriol on serum parathyroid hormone and ionized calcium concentrations in normal cats and cats with chronic renal failure. J Vet Intern Med 2006; 20: 1307–1313. [DOI] [PubMed] [Google Scholar]
- 36. Nagode LA, Chew DJ, Podell M. Benefits of calcitriol therapy and serum phosphorus control in dogs and cats with chronic renal failure. Both are essential to prevent or suppress toxic hyperparathyroidism. Vet Clin North Am Small Anim Pract 1996; 26: 1293–1330. [DOI] [PubMed] [Google Scholar]
- 37. Barber PJ, Elliott J. Feline chronic renal failure: calcium homeostasis in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract 1998; 39: 108–116. [DOI] [PubMed] [Google Scholar]
- 38. Cortadellas O, Fernandez del Palacio MJ, Talavera J, et al. Calcium and phosphorus homeostasis in dogs with spontaneous chronic kidney disease at different stages of severity. J Vet Intern Med 2010; 24: 73–79. [DOI] [PubMed] [Google Scholar]
- 39. Finch NC, Syme HM, Elliott J. Parathyroid hormone concentration in geriatric cats with various degrees of renal function. J Am Vet Med Assoc 2012; 241: 1326–1335. [DOI] [PubMed] [Google Scholar]
- 40. Giovaninni LH. Serum intact parathyroid hormone evaluation in cats with chronic kidney disease. Pesq Vet Bras 2013; 33: 229–235. [Google Scholar]
- 41. Geddes RF, Finch NC, Elliott J, et al. Fibroblast growth factor 23 in feline chronic kidney disease. J Vet Intern Med 2013; 27: 234–241. [DOI] [PubMed] [Google Scholar]
- 42. Juppner H. Phosphate and FGF-23. Kidney Int Suppl 2011; 79 Suppl 121: S24–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kuro OM. Phosphate and Klotho. Kidney Int Suppl 2011; 79 Suppl 121: S20–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shaikh A, Berndt T, Kumar R. Regulation of phosphate homeostasis by the phosphatonins and other novel mediators. Pediatr Nephrol 2008; 23: 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hu MC, Kuro-o M, Moe OW. Klotho and chronic kidney disease. Contrib Nephrol 2013; 180: 47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Finch NC, Geddes RF, Syme HM, et al. Fibroblast growth factor 23 (FGF-23) concentrations in cats with early nonazotemic chronic kidney disease (CKD) and in healthy geriatric cats. J Vet Intern Med 2013; 27: 227–233. [DOI] [PubMed] [Google Scholar]
- 47. Elliott J, Rawlings JM, Markwell PJ, et al. Survival of cats with naturally occurring chronic renal failure: effect of dietary management. J Small Anim Pract 2000; 41: 235–242. [DOI] [PubMed] [Google Scholar]
- 48. Geddes RF, Elliott J, Syme HM. The effect of feeding a renal diet on plasma fibroblast growth factor 23 concentrations in cats with stable azotemic chronic kidney disease. J Vet Intern Med 2013; 27: 1354–1361. [DOI] [PubMed] [Google Scholar]
- 49. de Brito Galvao JF, Nagode LA, Schenck PA, et al. Calcitriol, calcidiol, parathyroid hormone, and fibroblast growth factor-23 interactions in chronic kidney disease. J Vet Emerg Crit Care 2013; 23: 134–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Polzin D, Ross SJ, Osborne CA, et al. Clinical benefits of calcitriol in canine chronic kidney disease [abstract]. J Vet Intern Med 2005; 19: 433. [Google Scholar]
- 51. Monier-Faugere MC, Geng Z, Friedler RM, et al. 22-Oxacalcitriol suppresses secondary hyperparathyroidism without inducing low bone turnover in dogs with renal failure. Kidney Int 1999; 55: 821–832. [DOI] [PubMed] [Google Scholar]
- 52. Takahashi F, Furuichi T, Yorozu K, et al. Effects of i.v. and oral 1,25-dihydroxy-22-oxavitamin D(3) on secondary hyperparathyroidism in dogs with chronic renal failure. Nephrol Dial Transplant 2002; 17 Suppl 10: 46–52. [DOI] [PubMed] [Google Scholar]
- 53. Kim SM, Choi HJ, Lee JP, et al. Prevalence of vitamin D deficiency and effects of supplementation with cholecalciferol in patients with chronic kidney disease. J Ren Nutr 2014; 24: 20–25. [DOI] [PubMed] [Google Scholar]
- 54. Galler A, Tran JL, Krammer-Lukas S, et al. Blood vitamin levels in dogs with chronic kidney disease. Vet J 2012; 192: 226–231. [DOI] [PubMed] [Google Scholar]
- 55. Foundation NK. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39: S1–S266. [PubMed] [Google Scholar]
- 56. Morris JG. Cats discriminate between cholecalciferol and ergocalciferol. J Anim Phys Anim Nutr 2002; 86: 229–238. [DOI] [PubMed] [Google Scholar]
- 57. Jamal SA, Miller PD. Secondary and tertiary hyperparathyroidism. J Clin Densitom 2013; 16: 64–68. [DOI] [PubMed] [Google Scholar]
- 58. Barber PJ, Elliott J. Study of calcium homeostasis in feline hyperthyroidism. J Small Anim Pract 1996; 37: 575–582. [DOI] [PubMed] [Google Scholar]
- 59. Schenck PA, Chew DJ. Determination of calcium fractionation in dogs with chronic renal failure. Am J Vet Res 2003; 64: 1181–1184. [DOI] [PubMed] [Google Scholar]
- 60. Jean G, Vanel T, Terrat JC, et al. [Treatment of secondary hyperparathyroidism resistant to conventional therapy and tertiary hyperparathyroidism with Cinacalcet: an efficiency strategy] (article in French). Nephrol Ther 2010; 6: 105–110. [DOI] [PubMed] [Google Scholar]
- 61. Okada M, Tominaga Y, Izumi K, et al. Tertiary hyperparathyroidism resistant to cinacalcet treatment. Ther Apher Dial 2011; 15: 33–37. [DOI] [PubMed] [Google Scholar]