Abstract
Practical relevance:
Cytauxzoonosis is a life-threatening hematoprotozoal disease with a rapidly progressive clinical course. Once considered a rare disease only relevant to a small geographic area, it is now recognized in more than about a third of the United States. The geographic range seems likely to increase with expansion of the range of the vector tick.
Clinical challenges:
Both disease diagnosis and treatment offer challenges. The acute illness is often recognized by characteristic parasitic cellular inclusions, but illness may occur before parasites can be identified, and parasitic inclusions may persist long after illness has resolved. Also, while infection was once considered nearly uniformly fatal, subclinical infections are now recognized. Disease prognosis has improved for many cats through implementation of new therapies, but some pathogens are resistant to these therapies and death from disease is still common. Currently, prevention strategies are limited to ectoparasite control.
Global importance:
Cytauxzoonosis caused by Cytauxzoon felis is limited to the Americas, and is especially problematic in southeastern and south central USA. However, other Cytauxzoon species have been recognized in Europe and Asia.
Audience:
This review is aimed at veterinary practitioners and focuses on the diagnosis and treatment of cytauxzoonosis. Disease management is of crucial importance in endemic regions. Furthermore, the expanding geographic range of infection, and the possibility of parasite identification in chronically infected cats with a travel history, make understanding cytauxzoonosis relevant in non-endemic regions as well.
Evidence base:
The authors draw on evidence from prospective clinical trials, experimental infections, retrospective clinical studies and case reports, as well as their own personal experience with the diagnosis and treatment of cytauxzoonosis.
Pathogen and pathogen transmission
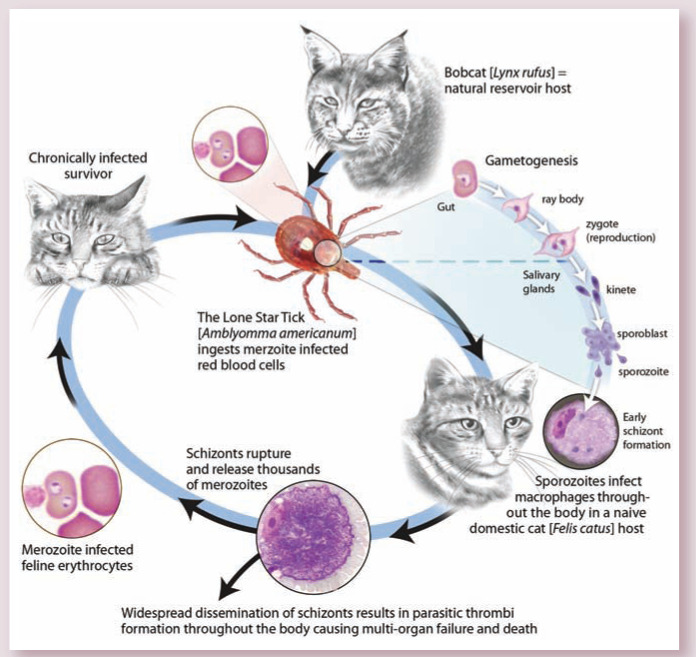
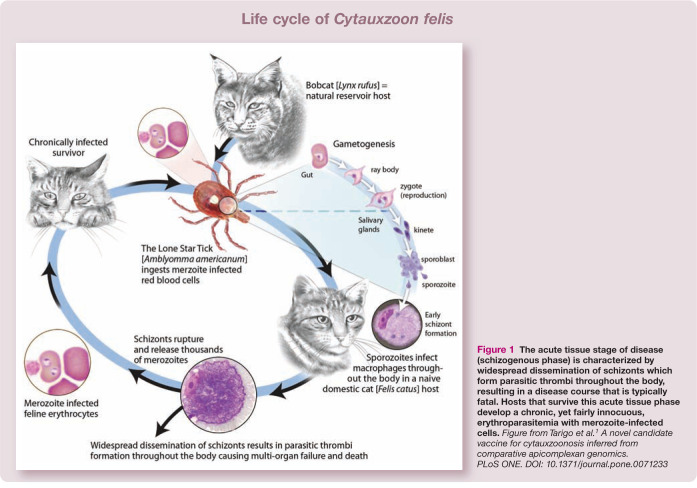
Cytauxzoon felis is a protozoan blood parasite transmitted via the bite of an infected tick (Figure 1).1,2 Bobcats (Lynx rufus) are the reservoir host; infected bobcats are believed to develop short-lived illness followed by clinical recovery and a persistent carrier state.3,4 When a tick feeds on an infected bobcat, the tick acquires the pathogen. If that tick subsequently feeds on a bobcat, the sylvatic cycle continues. However, if the tick feeds on a domestic cat instead, infection typically leads to severe illness (ie, cytauxzoonosis) and often death.5,6 All Felidae are susceptible to infection; infection has not been reported in any non-felid animal.7 –9
Figure 1.
The acute tissue stage of disease (schizogenous phase) is characterized by widespread dissemination of schizonts which form parasitic thrombi throughout the body, resulting in a disease course that is typically fatal. Hosts that survive this acute tissue phase develop a chronic, yet fairly innocuous, erythroparasitemia with merozoite-infected cells. Figure from Tarigo et al. 1 A novel candidate vaccine for cytauxzoonosis inferred from comparative apicomplexan genomics. PLoS ONE. DOI: 10.1371/journal.pone.0071233
As for other apicomplexan protozoa, the life cycle of C felis is complex (Figure 1) and various stages of the parasite are associated with different clinical consequences. 1 During feeding, the tick inoculates sporozoites that enter host mononuclear cells and multiply.10,11 Infected mononuclear cells distend with organisms known as schizonts (Figure 2), and these cells can act as thrombi, resulting in vascular occlusion (Figure 3) with resultant multi-organ failure.12 –15 It is during the schizogenous stage of parasite replication, usually about 2 weeks after the bite of an infected tick, that infected cats develop clinical illness.2,16
Figure 2.
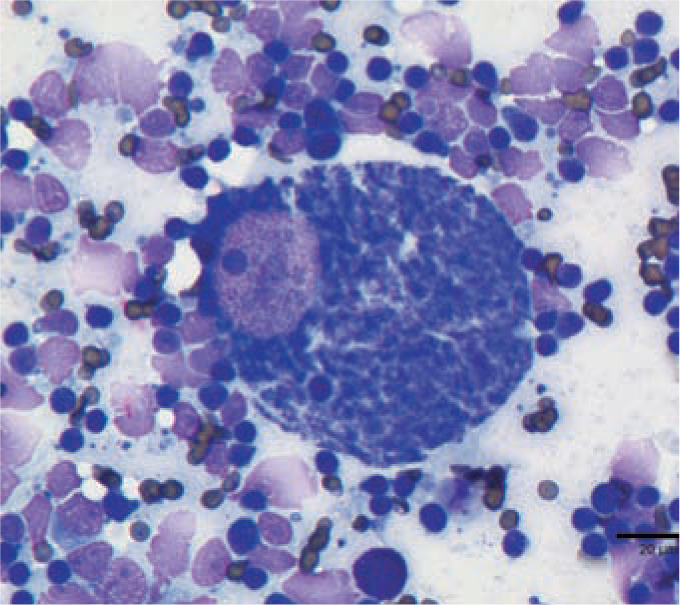
Cytauxzoon felis schizont-laden macrophage in a peripheral lymph node. Wright–Giemsa. Schizonts are variably sized basophilic, foamy, amorphous protozoal bodies in the phagocytic cell cytoplasm. Courtesy of Dr Erin Burton, University of Missouri Veterinary Medical Diagnostic Laboratory
Figure 3.
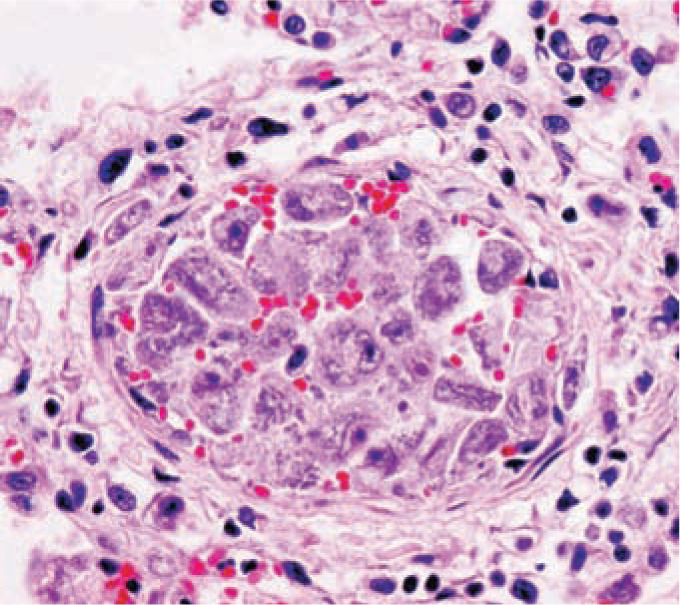
Vascular occlusion secondary to Cytauxzoon felis-infected macrophages. A medium-sized pulmonary vein is markedly distended and almost completely occluded by a large quantity of severely enlarged schizont-laden monocytes. Courtesy of Dr Dae Young Kim, University of Missouri Veterinary Medical Diagnostic Laboratory
The multinucleated schizonts divide into merozoites. Within days, merozoites rupture from the mononuclear cells and are taken up by red blood cells (RBCs) where they appear as piroplasms (Figure 4). The duration of illness is brief, lasting between 2 and 12 days in both natural and experimental infection.2,14,17
Figure 4.
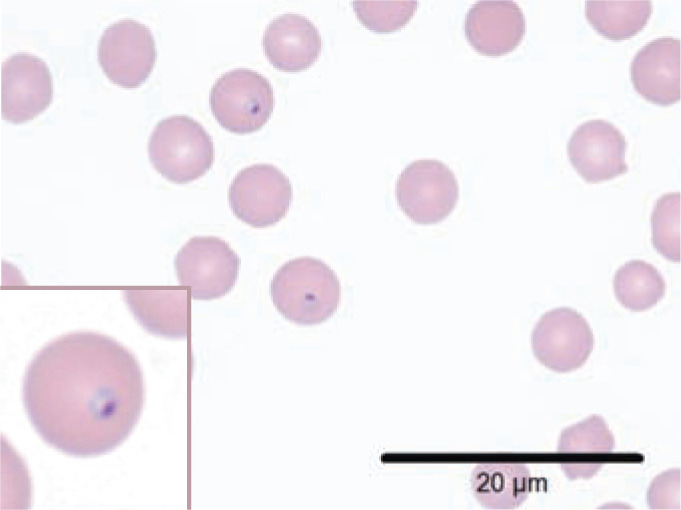
Cytauxzoon felis piroplasms. Wright–Giemsa stained preparation showing two dark purple staining intraerythrocytic piroplasms with the classic ‘signet ring’ appearance (inset). Piroplasms may alternatively appear as biopolar oval structures, round anaplasmoid bodies, or as tetrads; typically they are 1–2 μm in diameter. Feline erythrocytes are approximately 5 μm in diameter. Courtesy of Dr Erin Burton, University of Missouri Veterinary Medical Diagnostic Laboratory
Many cats die within 24 h of presentation to a veterinary clinic for treatment. 17 In cats that survive initial infection, piroplasms persist for months, years, or even for life, although schizonts can no longer be found.18 –21 While clinically relevant hemolysis may occur in the acute phase of infection, chronic erythroparasitemia is relatively benign.18 –21
The parasite life cycle is perpetuated when a tick takes a blood meal containing merozoite-infected RBCs. It has now been documented that recovered domestic cats are competent to transmit the pathogen to feeding ticks.2,16
C felis cannot be transmitted through physical contact between infected cats. 22 Although experimental inoculation of naive cats with schizonts results in cytauxzoonosis and death, inoculation of RBCs containing piroplasms does not lead to schizogeny or illness.10,22,23 Vertical transmission, common in other apicomplexan infections, has not been documented. 24
Geography and epidemiology
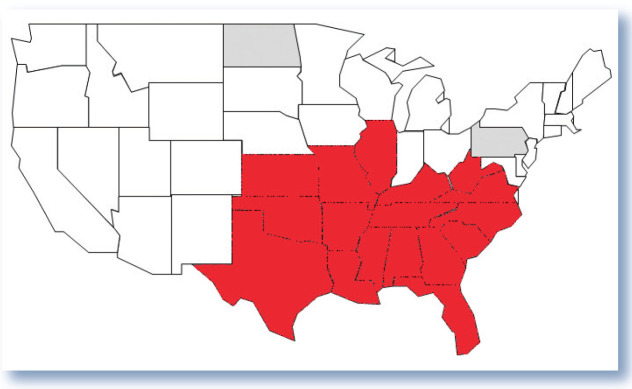
Since the first reported case of cytauxzoonosis was recognized in Missouri in 1976, there has been a progressive expansion in the geographic range in which disease has been identified. 5 To date, cytauxzoonosis has been confirmed in domestic cats from 17 US states, and the pathogen has been documented in bobcats from Pennsylvania and North Dakota, states where ill domestic cats have not yet been reported (Figure 5).14,15,18,21,25 –29 The increase in geographic range is likely due to expansion in the range of the vector Amblyomma americanum (lone star tick) population, hypothesized to be linked to an expanding white-tailed deer population. 30 The prevalence of C felis in bobcats is higher in geographic regions with established A americanum populations. 8 Veterinarians in areas where lone star ticks are found but cytauxzoonosis has not yet been recognized, including much of the northeastern and central USA, must remain vigilant for the disease.
Figure 5.
States of the USA where Cytauxzoon felis has been identified. Red fill color represents states from which domestic cats with cytauxzoonosis have been identified. Gray fill color represents states in which bobcats with C felis infection have been recognized, but where no cases of cytauxzoonosis have yet been reported in domestic cats
It is difficult to determine disease incidence, and few reports on prevalence exist. Cytauxzoonosis is not a reportable disease, and infected cats may not receive veterinary care, making incidence determination problematic. However, cytauxzoonosis accounted for 1.5% of all feline admissions to the Boren Veterinary Medical Teaching Hospital at Oklahoma State University between 1998 and 2006. 31 Practitioners in endemic regions of southern Missouri commonly report seeing a dozen or more cases per year. Prevalence of infection in domestic cats has rarely been reported, and estimates of prevalence are impacted by the fact that most cats die soon after infection. However, in some geographic areas survivors are not uncommon. 19 Prevalence rates of 28% have been reported for healthy feral cats in regions of Arkansas and Georgia, and a prevalence of just over 6% for healthy cats from endemic areas of Oklahoma, Missouri and Arkansas.21,32 The authors have encountered multi-cat households (>20 cats) in Arkansas with as high as 50% prevalence of chronic infection. 33
As expected for the better-adapted reservoir host, the prevalence of C felis in free-ranging bobcats is higher. In a 2011 study, 79% of bobcats from Missouri were infected. 8
Historical and physical findings
Infection is far more likely in cats with outdoor exposure in rural (particularly unmanaged) areas where tick contact is more common. 31 Despite tick transmission, owners often deny known tick bite. 17 Temporal occurrence of the disease corresponds with the active questing season of A americanum, with most infections recognized between April and September.31,34 Cats of any age and either sex may be infected, although young adults are infected most commonly.14,31
Cytauxzoonosis is a rapidly progressive illness with no specific historical or physical findings. The most common presenting complaints are anorexia and lethargy. 17 Fever, often with marked temperature elevation, and icterus are the most consistent findings on examination of ill cats. 17 Although fever is characteristic, hypothermia occurs in moribund cats, and cyclical fluctuations in daily temperatures have been reported in experimental infection.2,35 Other findings consistent with cytauxzoonosis include: dehydration, tachypnea, tachycardia, dyspnea, pale mucous membranes, pain on abdominal palpation, lymphadenomegaly, splenomegaly, vocalization, elevated third eyelid, abnormal mentation or a moribund state, and ecchymoses.11,14,15,17,27,35,36
Disease diagnosis
In endemic areas, cytauxzoonosis becomes a likely differential diagnosis for any acutely ill, febrile cat with outdoor exposure during the spring, summer or early autumn. Confirmation of the diagnosis may be simple when characteristic hemoparasites are observed on blood smear evaluation in an animal with typical signs of disease, but difficult when hemoparasites are absent, or confusing when hemoparasites are seen but the clinical presentation is not characteristic of cytauxzoonosis.
There are no pathognomonic findings on serum chemistry or urinalysis. Hyperbilirubinemia, hyperglycemia, hypoproteinemia and hypocalcemia are often noted, but azotemia is uncommon.11,14,17 There are no specific findings on imaging studies, but hepatomegaly and splenomegaly due to parasitic vascular occlusion are common.35,37 Interstitial to alveolar lung patterns are sometimes seen, and pleural effusion may be documented on thoracic radiographs. 38
Complete blood count
A complete blood count with smear examination is the single most useful diagnostic test for cytauxzoonosis, but is subject to limitations. Pancytopenia is often, but not invariably, identified in infected cats. 17 A low white blood cell count may be associated with a worsened prognosis. 17 Thrombocytopenia is a frequent finding and may be due in part to disseminated intravascular coagulation (DIC), a common complication of cytauxzoonosis.39,40 Lymphopenia is part of a stress leukogram, and lymphocytes may have a large granular morphology. 16 Although anemia occurs commonly during acute illness, typically at about the same time as piroplasms are seen in the circulation, recovered carrier cats do not have anemia despite the persistence of piroplasms.9,17 –20 Anemia is typically hemolytic, but may not appear regenerative initially due to the acute nature of the illness.
Morphologic identification of piroplasms (intraerythyrocytic merozoites)
Morphologic identification of intraerythrocytic merozoites (ie, piroplasms) on blood smears is the single most commonly used method for confirmation of infection. Although piroplasms of other Cytauxzoon species and Babesia felis have identical morphology, those pathogens have not been reported to cause natural infection in the USA. 25
Piroplasms are seen on light microscopy after Wright or Wright–Giemsa staining.10,11 Caution is required to avoid confusing stain artefact, Howell-Jolly bodies or even other red cell parasites for C felis. C felis piroplasms are most commonly described as ‘signet ring’ shaped (Figure 4); the blue nuclear chromatin is peripheralized with a small amount of pink cytoplasm, resulting in this classic appearance. Piroplasms may also appear as bipolar oval structures, round anaplasmoid bodies, or as tetrads. Typically they are 1–2 µm in diameter, but may range from 0.2–2.5 µm.10,11
Even accurate recognition of piroplasms is neither 100% sensitive nor specific for disease diagnosis. Because disease is related to the schizogenous phase of infection, rather than the later piroplasm phase, piroplasms may be absent early in disease. Blood smears can be completed on consecutive days to screen for parasites, but in the authors’ experience piroplasms may not be seen for as long as 6 days after infection is identified by more sensitive means (ie, PCR).5,41 Initially, only very low numbers of RBCs may be infected; but, with disease progression, up to 50% of RBCs may become parasitized.5,11,42
Because cats that develop subclinical infection or recover from disease often maintain low numbers of piroplasms for months, years or for life, recognition of piroplasms does not confirm that illness is due to cytauxzoonosis.19 –21 This can be the source of confusion when a recovered cat is subsequently evaluated for another disease process. Cats with low level parasitemia and either chronic illness or atypical clinical signs may simply be incidentally discovered carriers of the parasite.
Morphologic identification of schizonts
Schizont-infected macrophages provide a pathognomonic morphologic diagnosis of cytauxzoonosis since, unlike piroplasms, schizonts are not identified in carrier cats.5,10,13 Due to their unique appearance and large size, they are not easily mistaken for any other cell type. Schizonts are variably sized basophilic, foamy, amorphous protozoal bodies in the cell cytoplasm; a single schizont is identified per host macrophage.5,10,13 The infected macrophages can vary in size (15–250 µm diameter) and are generally larger as the disease progresses. 10
Occasionally, macrophages with intracellular C felis schizonts can be identified on blood smears.10,12 Because they become lodged in blood vessels, infected macrophages are more commonly identified cytologically from fine-needle tissue aspirates (lymph node, spleen, liver) or by histopathology after death (Figure 3).10,12 Aspirate cytology with the goal of schizont identification might be appropriate if piroplasms are not identified but cytauxzoonosis is still suspected, or to differentiate between acute cytauxzoonosis and a chronic C felis infection in a cat that is ill for some unrelated reason.
Molecular testing
PCR testing for C felis infection is commercially available, and is the most sensitive and specific method for recognition of infection. 25 However, just as for piroplasm observation, a positive result could be found in a recovered carrier cat and thus PCR cannot be used to differentiate between cytauxzoonosis (ie, acute disease) and chronic C felis infection (ie, carrier status). 20 The most important limitation of PCR assay is related to timing; PCR is a ‘mail out’ test, and any delay in results is important for this rapidly progressive disease. Treatment should not be delayed while waiting for results if cytauxzoonosis is clinically suspected.
Unfortunately, development of an antibody-based test would not be useful for this acute disease since antibodies take time to develop, and at present there are no antigen-based in-clinic testing options. 4
Disease treatment
Despite the fact that a proportion of domestic cats can be infected without developing illness (ie, cytauxzoonosis), most infected cats do become ill.19 –21 Cytauxzoonosis causes death in an estimated 90% of infected cats;11,14,19,23,42 at present, there are few useful predictors of survival (see box below). 17 Because death occurs so soon after initial illness, treatment should be initiated as soon as possible for all cats suspected of having cytauxzoonosis, even if a definitive diagnosis has not been confirmed.
Antiprotozoal therapy
Various antiprotozoal therapies have been used to treat either natural or experimental infections. Paravaquone and buparavaquone, two antiprotozoal drugs used successfully in the therapy of bovine theileriosis, were completely ineffective against experimentally induced cytauxzoonosis. 23 Diminazene aceturate is an antiprotozoal drug not approved for any use in the USA, but was reported as a part of successful treatment in 5/6 cats with naturally occurring cytauxzoonosis. 39 Although no prospective trials have evaluated its efficacy in disease treatment, its ability to eliminate the parasite in recovered cats has been investigated. Unfortunately, it did not prove beneficial.43,44
Recently, a prospective open-label parallel-group study was undertaken to compare outcome in naturally infected cats treated with either a combination of the antimalarial drug atovaquone and the antibiotic azithromycin, or with the antiprotozoal drug imidocarb dipropionate. 17 Survival in cats treated with the combination therapy (60%) was significantly improved compared with survival in cats treated with imidocarb (26%). 17 Despite dramatic improvement in outcome with the drug combination, some C felis pathogens appear to possess a cytochrome b genotype that is associated with atovaquone resistance and a reduced chance of survival despite treatment. 45 Nevertheless, at present the combination of atovaquone and azithromycin should be considered the standard of care for cats with cytauxzoonosis (see box below).
Because atovaquone is expensive, difficult to obtain and difficult to administer, alternative therapies for cytauxzoonosis are desirable, and studies investigating alternatives are ongoing.
Supportive care
Cytauxzoonosis is a critical illness, and cats could benefit from aggressive supportive care for sepsis regardless of the type of antiprotozoal used. Unfortunately, there is no evidence to support the utility or futility of any specific supportive measure. The recommendations in the box below are based largely on the authors’ personal preference and experience.
Intravenous crystalloid fluid therapy, with potassium chloride supplementation as needed, should be given to provide maintenance fluid therapy, correct dehydration and address any ongoing losses. Overly aggressive fluid administration should be avoided as pneumonitis may be exacerbated by fluid overload. Cats with cytauxzoonosis often seem to be in pain, and analgesic therapy such as buprenorphine is appropriate. Although the use of heparin as prophylaxis or treatment for DIC is controversial, the authors have used it in most treated cats, and have additionally administered plasma when bleeding complications have been observed. Transfusion of packed RBCs or whole blood is indicated for cats with clinical signs referable to anemia. For cats with respiratory distress or tachypnea, oxygen supplementation is indicated, as is thoracocentesis if pleural effusion is documented.
Placement of a nasoesophageal feeding tube permits administration of enteral nutrition and provides a less stressful route to administer drugs, including the thick, viscous citrus-flavored liquid atovaquone that must be given q8h. Although vomiting is not a common complication of cytauxzoonosis, maropitant can be helpful for cats with vomiting or hypersalivation. In the authors’ experience, antipyretic agents including NSAIDs seem to be associated with a worsened, not improved, prognosis and are thus avoided. Additional antimicrobial drugs have no demonstrated beneficial effect, but if infections such as tularemia remain part of the differential diagnosis, other antibiotics may be indicated until a specific diagnosis is confirmed.
Disease prevention
Currently, prophylactic ectoparasite control is the only effective method to prevent disease. 46 Keeping cats indoors reduces tick exposure and should be advocated in endemic regions. Ectoparasite control products may also be useful, but the owners of several infected cats reported regular use of fipronil prior to infection. 17 Because the duration of tick feeding necessary for pathogen transmission is unknown, it would be ideal to prevent tick bites rather than kill ticks once they have bitten. In a recent study, cats wearing a 10% imidacloprid/4.5% flumethrin collar could not be infected with C felis using a tick-feeding model that resulted in infection in all but one of 10 control cats. 46 Recovered cats are apparently immune to repeat illness.22,41 A novel candidate vaccine antigen, based on the C felis gene cf76, was identified using comparative apicomplexan genomics, 1 but thus far there are no commercially available vaccines.
Recovered or subclinically infected cats are competent to transmit infection to ticks, and therefore indirectly to other domestic cats.2,16 It would be ideal to eliminate parasitemia to reduce the risk posed by recovered cats. Neither diminazene diaceturate nor imidocarb dipropionate have demonstrated any ability to reduce parasite burden in carriers.43,44 Although a combination of atovaquone and azithromycin may not eliminate parasitemia completely, it did dramatically reduce parasite burden in treated cats. 33 Recovered cats could also transmit infection via blood transfusion, although transfusion of piroplasms results only in erythroparasitemia of the recipient cat and not in cytauxzoonosis.10,22,23 Transfusion of schizonts can induce disease, but since only sick cats harbor schizonts, it is extremely unlikely that such a cat would be used as a blood donor. The 2005 ACVIM consensus statement on blood donor screening recommended use of indoor cats using ectoparasite prophylaxis; 47 C felis PCR was not yet available at the time these guidelines were developed. In endemic regions, it would be reasonable to use PCR screening for donor cats from an outdoor environment.
Key Points
Cytauxzoon felis, an apicomplexan protozoan parasite, is transmitted by the vector Amblyomma americanum (lone star tick). The disease is not transmitted by direct cat-to-cat contact.
In endemic regions, cytauxzoonosis should be a differential diagnosis for cats exhibiting acute lethargy, anorexia, fever, icterus, respiratory distress, central nervous system signs and/or hemolytic anemia.
The disease is most commonly diagnosed between April and September in cats from non-urban areas with outdoor exposure, with or without a history of tick attachment.
Identification of schizonts from tissue aspirates or on peripheral blood smears is diagnostic for cytauxzoonosis.
Identification of piroplasms is suggestive of cytauxzoonosis in cats with compatible clinical signs, but occasional piroplasms may reflect past infection rather than current disease.
PCR is a more sensitive method for diagnosis than identification of either piroplasms or schizonts, but is associated with a delay in results compared with in-clinic microscopic identification of parasites.
Without therapy, over 90% of cats with cytauxzoonosis die, but as many as 60% of cats treated with atovaqone, azithromycin and supportive care survive.
Cats can remain persistent carriers of C felis, but there are no confirmed reports of recurrent illness.
Persistently infected carrier cats may serve as reservoirs for infection via a tick vector.
Stringent ectoparasite control is the only method of disease prevention at the present time.
Footnotes
Funding: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
The authors received no financial support for the research, authorship and/or publication of this article.
Contributor Information
Meredith K Sherrill, 201 W 67th Ct, Loveland, CO 80538, USA.
Leah A Cohn, 900 E Campus Drive, College of Veterinary Medicine, University of Missouri, Columbia, MO 65211, USA.
References
- 1. Tarigo JL, Scholl EH, Mc KBD, et al. A novel candidate vaccine for cytauxzoonosis inferred from comparative apicomplexan genomics. PloS ONE 2013; 8: e71233. DOI: 10.1371/journal.pone.0071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reichard MV, Edwards AC, Meinkoth JH, et al. Confirmation of Amblyomma americanum (Acari: Ixodidae) as a vector for Cytauxzoon felis (Piroplasmorida: Theileriidae) to domestic cats. J Med Entomol 2010; 47: 890–896. [DOI] [PubMed] [Google Scholar]
- 3. Blouin EF, Kocan AA, Kocan KM, et al. Evidence of a limited schizogonous cycle for Cytauxzoon felis in bobcats following exposure to infected ticks. J Wildl Dis 1987; 23: 499–501. [DOI] [PubMed] [Google Scholar]
- 4. Cowell RL, Fox JC, Panciera RJ, et al. Detection of anticytauxzoon antibodies in cats infected with a Cytauxzoon organism from bobcats. Vet Parasitol 1988; 28: 43–52. [DOI] [PubMed] [Google Scholar]
- 5. Wagner JE. A fatal cytauxzoonosis-like disease in cats. J Am Vet Med Assoc 1976; 168: 585–588. [PubMed] [Google Scholar]
- 6. Blouin EF, Kocan AA, Glenn BL, et al. Transmission of Cytauxzoon felis Kier, 1979 from bobcats, Felis rufus (Schreber), to domestic cats by Dermacentor variabilis (Say). J Wildl Dis 1984; 20: 241–242. [DOI] [PubMed] [Google Scholar]
- 7. Lewis KM, Cohn LA, Downey ME, et al. Evaluation of Cytauxzoon felis infection status in captive-born wild felids housed in an area endemic for the pathogen. J Am Vet Med Assoc 2012; 241: 1088–1092. [DOI] [PubMed] [Google Scholar]
- 8. Shock BC, Murphy SM, Patton LL, et al. Distribution and prevalence of Cytauxzoon felis in bobcats (Lynx rufus), the natural reservoir, and other wild felids in thirteen states. Vet Parasitol 2011; 175: 325–330. [DOI] [PubMed] [Google Scholar]
- 9. Rotstein DS, Taylor SK, Harvey JW, et al. Hematologic effects of cytauxzoonosis in Florida panthers and Texas cougars in Florida. J Wildl Dis 1999; 35: 613–617. [DOI] [PubMed] [Google Scholar]
- 10. Kier AB, Wagner JE, Kinden DA. The pathology of experimental cytauxzoonosis. J Comp Pathol 1987; 97: 415–432. [DOI] [PubMed] [Google Scholar]
- 11. Hoover JP, Walker DB, Hedges JD. Cytauxzoonosis in cats: eight cases. J Am Vet Med Assoc 1994; 205: 455–460. [PubMed] [Google Scholar]
- 12. Kocan AA, Kocan KM, Blouin EF, et al. A redescription of schizogony of Cytauxzoon felis in the domestic cat. Ann N Y Acad Sci 1992; 653: 161–167. [DOI] [PubMed] [Google Scholar]
- 13. Simpson CF, Harvey JW, Lawman MJ, et al. Ultrastructure of schizonts in the liver of cats with experimentally induced cytauxzoonosis. Am J Vet Res 1985; 46: 384–390. [PubMed] [Google Scholar]
- 14. Birkenheuer AJ, Le JA, Valenzisi AM, et al. Cytauxzoon felis infection in cats in the mid-Atlantic states: 34 cases (1998–2004). J Am Vet Med Assoc 2006; 228: 568–571. [DOI] [PubMed] [Google Scholar]
- 15. Jackson CB, Fisher T. Fatal cytauxzoonosis in a Kentucky cat (Felis domesticus). Vet Parasitol 2006; 139: 192–195. [DOI] [PubMed] [Google Scholar]
- 16. Reichard MV, Meinkoth JH, Edwards AC, et al. Transmission of Cytauxzoon felis to a domestic cat by Amblyomma americanum. Vet Parasitol 2009; 161: 110–115. [DOI] [PubMed] [Google Scholar]
- 17. Cohn LA, Birkenheuer AJ, Brunker JD, et al. Efficacy of atovaquone and azithromycin or imidocarb dipropionate in cats with acute cytauxzoonosis. J Vet Intern Med 2010; 25: 55–60. [DOI] [PubMed] [Google Scholar]
- 18. Haber MD, Tucker MD, Marr HS, et al. The detection of Cytauxzoon felis in apparently healthy free-roaming cats in the USA. Vet Parasitol 2007; 146: 316–320. [DOI] [PubMed] [Google Scholar]
- 19. Meinkoth J, Kocan AA, Whitworth L, et al. Cats surviving natural infection with Cytauxzoon felis: 18 cases (1997–1998). J Vet Intern Med 2000; 14: 521–525. [DOI] [PubMed] [Google Scholar]
- 20. Brown HM, Latimer KS, Erikson LE, et al. Detection of persistent Cytauxzoon felis infection by polymerase chain reaction in three asymptomatic domestic cats. J Vet Diagn Invest 2008; 20: 485–488. [DOI] [PubMed] [Google Scholar]
- 21. Brown HM, Lockhart JM, Latimer KS, et al. Identification and genetic characterization of Cytauxzoon felis in asymptomatic domestic cats and bobcats. Vet Parasitol 2010; 172: 311–316. [DOI] [PubMed] [Google Scholar]
- 22. Wagner JE, Ferris DH, Kier AB, et al. Experimentally induced cytauxzoonosis-like disease in domestic cats. Vet Parasitol 1980; 6: 305–311. [Google Scholar]
- 23. Motzel SL, Wagner JE. Treatment of experimentally induced cytauxzoonosis in cats with parvaquone and buparvaquone. Vet Parasitol 1990; 35: 131–138. [DOI] [PubMed] [Google Scholar]
- 24. Lewis KM, Cohn LA, Birkenheuer AJ. Lack of evidence for perinatal transmission of Cytauxzoon felis in domestic cats. Vet Parasitol 2012; 188: 172–174. [DOI] [PubMed] [Google Scholar]
- 25. Birkenheuer AJ, Marr H, Alleman AR, et al. Development and evaluation of a PCR assay for the detection of Cytauxzoon felis DNA in feline blood samples. Vet Parasitol 2006; 137: 144–149. [DOI] [PubMed] [Google Scholar]
- 26. Hauck WN, Snider TG, 3rd, Lawrence JE. Cytauxzoonosis in a native Louisiana cat. J Am Vet Med Assoc 1982; 180: 1472–1474. [PubMed] [Google Scholar]
- 27. Meier HT, Moore LE. Feline cytauxzoonosis: a case report and literature review. J Am Anim Hosp Assoc 2000; 36: 493–496. [DOI] [PubMed] [Google Scholar]
- 28. Mueller EK, Baum KA, Papeş M, et al. Potential ecological distribution of Cytauxzoon felis in domestic cats in Oklahoma, Missouri, and Arkansas. Vet Parasitol 2012; 192: 104–110. [DOI] [PubMed] [Google Scholar]
- 29. Birkenheuer AJ, Marr HS, Warren C, et al. Cytauxzoon felis infections are present in bobcats (Lynx rufus) in a region where cytauxzoonosis is not recognized in domestic cats. Vet Parasitol 2008; 153: 126–130. [DOI] [PubMed] [Google Scholar]
- 30. Springer YP, Eisen L, Beati L, et al. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol 2014; 51: 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reichard MV, Baum KA, Cadenhead SC, et al. Temporal occurrence and environmental risk factors associated with cytauxzoonosis in domestic cats. Vet Parasitol 2008; 152: 314–320. [DOI] [PubMed] [Google Scholar]
- 32. Rizzi TE, Cohn LA, Birkenhauer AJ, et al. Prevalence of Cytauxzoon felis infection in healthy cats from enzootic areas in Arkansas, Missouri, and Oklahoma. Parasit Vectors 2015; 8: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cohn LA, Birkenheuer AJ, Ratcliff E. Comparison of two drug protocols for clearance of Cytauxzoon felis infections. J Vet Intern Med 2008; 22: 704 [Google Scholar]
- 34. Miller J, Davis CD. Increasing frequency of feline cytauxzoonosis cases diagnosed in western Kentucky from 2001 to 2011. Vet Parasitol 2013; 198: 205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cohn LA, Birkenheuer AJ. Cytauxzoonosis. In: Greene C. (ed). Infectious diseases of the dog and cat. St Louis, MO: Saunders Elsevier, 2011, pp 764–770. [Google Scholar]
- 36. Bondy PJ, Jr, Cohn LA, Kerl ME. Feline cytauxzoonosis. Compend Contin Educ Pract Vet 2005; 27: 69. [Google Scholar]
- 37. Aschenbroich SA, Rech RR, Sousa RS, et al. Pathology in practice. Cytauxzoon felis infection. J Am Vet Med Assoc 2012; 240: 159–161. [DOI] [PubMed] [Google Scholar]
- 38. Snider TA, Confer AW, Payton ME. Pulmonary histopathology of Cytauxzoon felis infections in the cat. Vet Pathol 2010; 47: 698–702. [DOI] [PubMed] [Google Scholar]
- 39. Greene CE, Latimer K, Hopper E, et al. Administration of diminazene aceturate or imidocarb dipropionate for treatment of cytauxzoonosis in cats. J Am Vet Med Assoc 1999; 215: 497–500. [PubMed] [Google Scholar]
- 40. Conner BJ, Hanel RM, Brooks M, et al. Coagulation abnormalities in 5 cats with naturally occurring cytauxzoonosis. J Vet Emerg Crit Care 2015; 254: 538–545. [DOI] [PubMed] [Google Scholar]
- 41. Ferris DH. A progress report on the status of a new disease of American cats: cytauxzoonosis. Comp Immunol Microbiol Infect Dis 1979; 1: 269–276. [DOI] [PubMed] [Google Scholar]
- 42. Walker DB, Cowell RL. Survival of a domestic cat with naturally acquired cytauxzoonosis. J Am Vet Med Assoc 1995; 206: 1363–1365. [PubMed] [Google Scholar]
- 43. Lewis KM, Cohn LA, Marr HS, et al. Diminazene diaceturate for treatment of chronic Cytauxzoon felis parasitemia in naturally infected cats. J Vet Intern Med 2012; 26: 1490–1493. [DOI] [PubMed] [Google Scholar]
- 44. Lewis KM, Cohn LA, Marr HS, et al. Failure of efficacy and adverse events associated with dose-intense diminazene diaceturate treatment of chronic Cytauxzoon felis infection in five cats. J Feline Med Surg 2014; 16: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schreeg ME, Marr HS, Tarigo J, et al. Pharmacogenomics of Cytauxzoon felis cytochrome b: implications for atovaquone and azithromycin therapy in domestic cats with cytauxzoonosis. J Clin Microbiol 2013; 51: 3066–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reichard MV, Thomas JE, Arther RG, et al. Efficacy of an imidacloprid 10 %/flumethrin 4.5 % collar (Seresto®, Bayer) for preventing the transmission of Cytauxzoon felis to domestic cats by Amblyomma americanum. Parasitol Res 2013; 112 Suppl 1: 11–20. [DOI] [PubMed] [Google Scholar]
- 47. Wardrop KJ, Reine N, Birkenheuer A, et al. Canine and feline blood donor screening for infectious disease. J Vet Intern Med 2005; 19: 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]