Abstract
Global importance:
The two feline retroviruses, feline immunodeficiency virus (FIV) and feline leukaemia virus (FeLV), are global and widespread, but differ in their potential to cause disease.
Viral infection – FIV:
FIV, a lentivirus that shares many properties with human immunodeficiency virus (HIV), can cause an acquired immune deficiency syndrome, which predisposes cats to other infections, stomatitis, neurological disorders and tumours. Although secondary infections are common, specific opportunistic infections or acquired immunodeficiency virus-defining infections, such as those that occur with HIV, are not commonly reported in FIV-infected cats. In most naturally infected cats, FIV does not cause a severe clinical syndrome; with appropriate care, FIV-infected cats can live many years before succumbing to conditions unrelated to their FIV infection. Thus, overall survival time is not necessarily shorter than in uninfected cats, and quality of life is usually high over many years or lifelong.
Viral infection – FeLV:
FeLV, an oncornavirus, is more pathogenic than FIV. Historically, it was considered to account for more disease-related deaths and clinical syndromes in cats than any other infectious agent. Recently, the prevalence and importance of FeLV have been decreasing, mainly because of testing and eradication programmes and the use of FeLV vaccines. Progressive FeLV infection can cause tumours, bone marrow suppression and immunosuppression, as well as neurological and other disorders, and leads to a decrease in life expectancy. However, with appropriate care, many FeLV-infected cats can also live several years with a good quality of life.
Practical relevance:
A decision regarding treatment or euthanasia should never be based solely on the presence or absence of a retrovirus infection. Antiviral chemotherapy is of increasing interest in veterinary medicine, but is still not used commonly.
Evidence base:
This article reviews the current literature on antiviral chemotherapy in retrovirus-infected cats, focusing on drugs that are currently available on the market and, thus, could potentially be used in cats.

Retroviral infections and the indication for antiviral chemotherapy
Feline leukaemia virus (FeLV) and feline immunodeficiency virus (FIV) are among the most common infectious agents of cats.1 –5 Retroviral tests diagnose only infection, and not clinical disease,1,2 and cats infected with FeLV or FIV can live for many years.6,7 Therefore, a decision regarding treatment or euthanasia should never be based solely on the presence of a retrovirus infection. 8 FIV- and FeLV-infected cats suffer from the same diseases that occur in cats free of retrovirus infections and, as such, the clinical signs in an individual cat might not be related to retrovirus infection at all.9,10
The retrovirus status of all cats should be known (Figure 1). 8 If a cat is diagnosed with a retrovirus infection in a multi-cat household, all cats in that household need to be tested to determine their retrovirus status. 8 If positive and negative cats are identified in the same household, the owner must be informed of the potential risk to uninfected cats and be advised that the best method of avoiding spread is to isolate the infected cat(s) and prevent them from interacting with housemates. 8 The risk of transmission, however, is not very high for either infection.3 –5
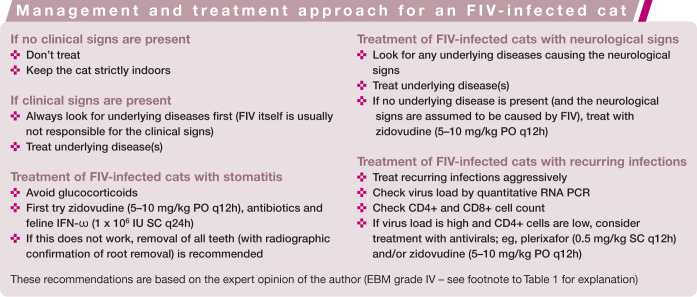
Figure 1.
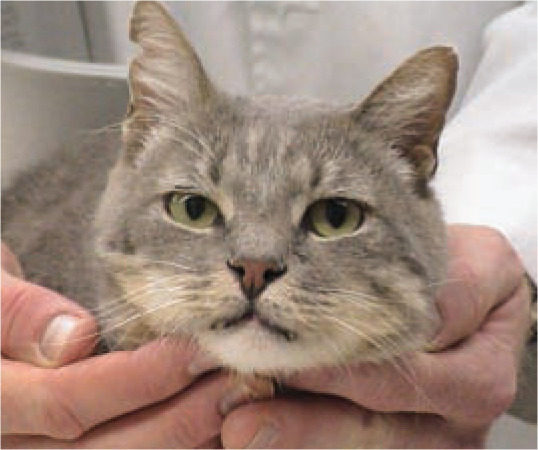
This cat with dual FIV and FeLV infection is in the high risk group of cats with outdoor access and a history of fighting
Retrovirus-infected cats need special management and care, and provided they receive this can live for many years in good health. Most retrovirus-infected cats are well managed with symptomatic therapy.3 –5 Specific treatment recommendations for individual cats are summarised in the boxes on page 926. Immune modulators are commonly used in retrovirus-infected cats. Results of uncontrolled studies sometimes suggest dramatic clinical improvement with these compounds, but these effects are usually not observed in properly designed trials, and clear evidence of efficacy is lacking. 4
Antiviral chemotherapy is only indicated in exceptional cases of FIV and FeLV infection, due to lack of proven efficacy of many antivirals and also their toxicity. 4 An update on published treatment studies is provided in this review, focusing on those drugs that are available on the market and, thus, could potentially be used in cats.
Classification of antiviral drugs
Most antivirals used in cats are licensed for humans and are specifically intended for treatment of human immunodeficiency virus (HIV) infection. Some of these drugs can be used to treat FIV infection because most enzymes of FIV and HIV have similar sensitivities to a range of inhibitors. Many drugs, such as nucleoside analogues, are less effective against FeLV, 4 as FeLV is not as closely related to HIV.
Antiviral compounds interfere with the viral replication cycle and can be grouped into different classes depending on the specific step at which they exert their activity.11 –13 These drug classes are summarised in Table 1.
Table 1.
Treatment options (antiviral drugs) for retrovirus-infected cats, with EBM grading of available efficacy data
| Drug | Infection | Efficacy in vitro | Controlled study in vivo | Efficacy in vivo | Author’s personal opinion | EBM grade (I–IV)* |
|---|---|---|---|---|---|---|
| Nucleoside analogue reverse transcriptase inhibitors | ||||||
| Zidovudine (AZT) | FIV | Yes | Yes | Yes | Effective in some cats (eg, with stomatitis, neurological disorders) | I |
| FeLV | Yes | Yes | No | Not very effective | I | |
| Stavudine (d4T) | FIV | Yes | No | ND | Possibly effective, but no data in cats | IV |
| FeLV | ND | No | ND | Possibly effective, but no data in cats | IV | |
| Didanosine (ddI) | FIV | Yes | Yes | Yes | Effective in one experimental study, but neurological side effects | II |
| FeLV | Yes | No | ND | Possibly effective, but no data in cats | IV | |
| Zalcitabine (ddC) | FIV | Yes | No | ND | Possibly effective, but toxic | IV |
| FeLV | Yes | Yes | No | Not very effective, and toxic | II | |
| Lamivudine (3TC) | FIV | Yes | Yes | No | Not very effective, and toxic in high dosages | II |
| FeLV | No | No | ND | Possibly effective, but toxic | IV | |
| Nucleotide analogue reverse transcriptase inhibitors | ||||||
| Adefovir (PMEA) | FIV | Yes | Yes | No | Effective in some cats, but relatively toxic | I |
| FeLV | Yes | Yes | No | Poorly effective, and relatively toxic | I | |
| Tenofovir (PMPA) | FIV | Yes | No | ND | Possibly effective, but likely also relatively toxic | IV |
| FeLV | Yes | No | ND | Possibly effective, but likely also relatively toxic | IV | |
| Non-nucleoside reverse transcriptase inhibitors | ||||||
| Suramin | FIV | No | No | ND | Possibly effective, but too toxic | IV |
| FeLV | No | No | ND | Weak efficacy in uncontrolled experimental studies | III | |
| Nucleotide synthesis inhibitors | ||||||
| Foscarnet (PFA) | FIV | Yes | No | ND | Effective in vitro, but too toxic | IV |
| FeLV | Yes | No | ND | Effective in vitro, but too toxic | IV | |
| Ribavirin (RTCA) | FIV | Yes | No | ND | Possibly effective, but too toxic | IV |
| FeLV | Yes | No | ND | Possibly effective, but too toxic | IV | |
| Receptor homologues/antagonists | ||||||
| Plerixafor (AMD3100) | FIV | Yes | Yes | Yes | Some effect in a study in privately owned cats; thus, can be considered | I |
| FeLV | ND | No | ND | Very likely ineffective | IV | |
| Integrase inhibitors | ||||||
| Raltegravir | FIV | Yes | No | ND | Possibly effective | IV |
| FeLV | Yes | No | ND | Mild effect in one uncontrolled experimental study | III | |
| Interferons | ||||||
| Human interferon-α (IFN-α) | ||||||
| SC high dose | FIV | Yes | No | ND | Likely ineffective | IV |
| FeLV | Yes | Yes | No | Ineffective | I | |
| PO low dose | FIV | Yes | Yes | Yes | Some effect (more likely on secondary infections) | I |
| FeLV | Yes | Yes | No | Ineffective | I | |
| Feline interferon-ω (IFN-ω) | ||||||
| SC high dose | FIV | Yes | Yes | Yes | Some effect (more likely on secondary infections) | I |
| FeLV | Yes | Yes | Yes | Some effect (more likely on secondary infections) | I | |
| PO low dose | FIV | Yes | No | ND | Potentially some effect (more likely on secondary infections) | III |
| FeLV | Yes | No | ND | Potentially some effect (more likely on secondary infections) | IV | |
FIV = feline immunodeficiency virus; FeLV = feline leukaemia virus; ND = not determined; SC = subcutaneous; PO = oral
EBM grades used according to the European Advisory Board on Cat Diseases (ABCD):
I = Best evidence, comprising data obtained from properly designed, randomised, controlled clinical trials in the target species (cats)
II = Data obtained from properly designed, randomised, controlled studies in the target species (cats) with spontaneous disease in an experimental setting
III = Data based on non-randomised clinical trials, multiple case series, other experimental studies, and dramatic results from uncontrolled studies
IV = Expert opinion, case reports, studies in other species, pathophysiological justification
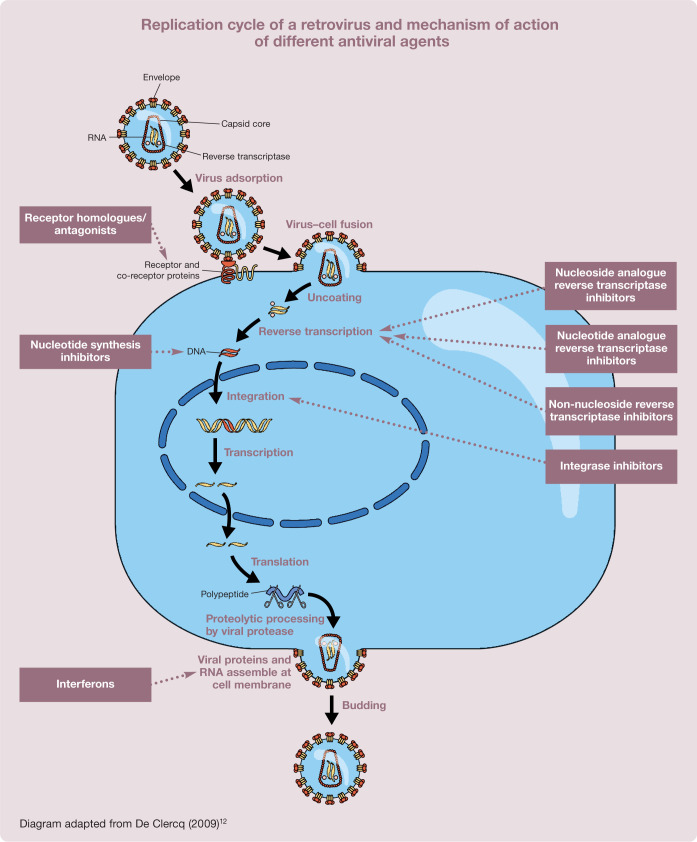
The most common antiretroviral drugs interfere with reverse transcription by inhibiting the retroviral enzyme reverse transcriptase (RT). Three classes of these RT inhibitors can be distinguished: nucleoside analogues (the most widely used antiviral compounds); nucleotide analogue RT inhibitors; and non-nucleoside RT inhibitors. 12 The last are usually highly selective for HIV and, thus, not useful in veterinary medicine.14,15 Drugs with a broader spectrum inhibit other viral enzymes, such as DNA or RNA polymerases, and thus interfere with virus genome replication or inhibit proteinases that are important for the splitting of precursor proteins during viral assembly. Other classes of drugs target viral entry by binding to specific receptors that the virus uses for adsorption; by acting as fusion inhibitors, preventing conformational changes by the virus necessary for the fusion process; or by interfering with viral uncoating.4,11 These various mechanisms of action are illustrated below.
Nucleoside analogue reverse transcriptase inhibitors
Nucleoside analogues are similar molecules to the ‘true’ nucleosides; equally, they have to be phosphorylated intracellularly to become active compounds. Because of their structural similarities, they can bind to the active centre of enzymes (eg, RT, other polymerases) and block enzyme activity. Many of these analogues are also integrated into elongating DNA or RNA strands, but because of small differences in molecular structure, this leads to chain termination or non-functional nucleic acids.12,16,17 Nucleoside analogues are accepted as false substrates not only by viral enzymes, but also by cellular enzymes, which largely accounts for their toxicity. 18
Zidovudine
Zidovudine (3′-azido-2′,3′-dideoxythymidine, AZT) was first synthesised in the 1960s 19 as a potential anticancer drug. In 1985 it was shown to be effective against HIV, 20 and 2 years later it was the first drug to be approved for the treatment of HIV infection. 21
Zidovudine not only inhibits RT, but also cellular polymerases, and this leads to bone marrow suppression. Thus, regular blood cell counts are necessary during zidovudine treatment because non-regenerative anaemia is a common side effect. 22 A complete blood count should be performed weekly for the first month. If values are stable after the first 4 weeks, a monthly recheck is recommended. Cats with bone marrow suppression should not be treated with zidovudine. A study in which FIV-infected cats were treated with zidovudine for 2 years has shown that the drug is well tolerated in most cats. 23 Haematocrit can decline within 3 weeks of initiating treatment to approximately 60% of baseline levels, but recovers in most cases, even without discontinuation of treatment. If the haematocrit drops below 20%, discontinuation is recommended, and anaemia usually resolves within a few days. 23 Other side effects in cats, including vomiting or anorexia, are rare.
FIV Zidovudine inhibits FIV replication in vitro22,24 –36 and in vivo;22,37,38 it reduces plasma viral load, improves the immunological and clinical status of FIV-infected cats, increases quality of life, and prolongs life expectancy. 39 In placebo-controlled trials, zidovudine improved stomatitis (Figure 2) and increased the CD4/CD8 ratio in naturally FIV-infected cats (EBM grade I).22,23,37 Neurological abnormalities also tend to respond favourably to treatment with zidovudine. In some cats with FIV-associated neurological signs, a marked improvement occurs within the first few days of therapy. As is the case in HIV, evidence exists that FIV can become resistant to nucleoside analogues. Zidovudine-resistant FIV mutants can arise after only 6 months’ use, with a single point mutation in the FIV gene being responsible for the resistance. 32
FeLV Zidovudine is effective against FeLV in vitro.40 –45 It has also been shown to be effective in treating cats with early experimental infection. When treated less than 1 week after challenge, cats were protected from bone marrow infection and persistent viraemia (EBM grade II).45,46 In a study in which cats naturally infected with FeLV were treated with zidovudine for 6 weeks, however, treatment did not lead to a significant improvement in clinical, laboratory, immunological or virological parameters (EBM grade I). 47 Thus, overall therapeutic efficacy of zidovudine is less promising in FeLV-infected cats than in FIV-infected cats.
Figure 2.
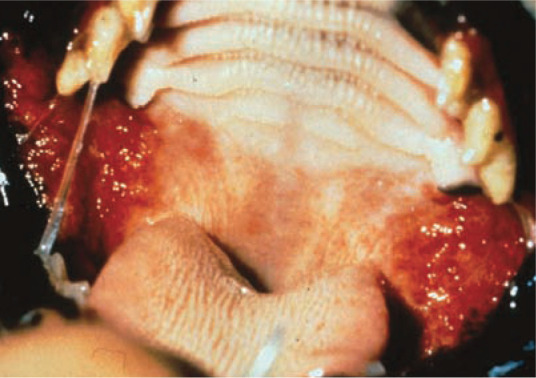
Severe stomatitis due to FIV infection. The nucleoside reverse transcriptase inhibitor, zidovudine, has a proven (EBM grade 1) effect on stomatitis in FIV-infected cats
Stavudine
Stavudine (2′,3′-didehydro-2′,3′-dideoxythymidine, d4T) is another drug with efficacy against HIV,48 –51 and was approved for treatment of HIV infection in 1994.
FIV Stavudine is active against FIV in vitro.28 –30,33,36,44,52 Mutants of FIV that are resistant to stavudine and cross-resistant to several other antivirals, including zidovudine, have been detected. Resistance is caused by a single point mutation in the RT-encoding region of the pol gene. 36 No in vivo data in FIV-infected cats are available.
FeLV The activity of stavudine against FeLV has not been determined.
Didanosine
Didanosine (2′,3′-dideoxyinosine, ddI) is also used to treat HIV infection in humans. It was shown to be active against HIV in 1986, 53 and was the second drug to be approved for treatment of HIV infection. It has been marketed since 1991. 12
FIV Didanosine is active against FIV in vitro.25,28,30 –34,36,54 In one experimental study, FIV replication was significantly suppressed in animals treated with didanosine, but treatment contributed to the development of antiretroviral toxic neuropathy (EBM grade II). 55
FeLV Didanosine is also active against FeLV in vitro,42,44 but its in vivo efficacy is unknown.
Zalcitabine
Zalcitabine (2′,3′-dideoxycytidine, ddC) was previously used to treat HIV infection in humans. 56 It was shown to be active against HIV in 1986, 53 and was approved by the US Food and Drug Administration in 1992. However, it ceased being marketed in 2006. Due to its toxicity, zalcitabine should not be used at concentrations over 5 mg/kg/h by continuous infusion in feline patients. 57
FIV In vitro, antiviral efficacy has been demonstrated against FIV.25 –27,29,31 –33,36,54 A mutant of FIV that is resistant to zalcitabine was selected in cell culture and showed cross-resistance to other antiviral compounds. 54 No in vivo data exist demonstrating efficacy in FIV-infected cats.
FeLV Zalcitabine is effective against FeLV in vitro and has been used in experimental studies to treat FeLV-infected cats.44,57–59 It has a very short half-life and, therefore, has been administered via either an intravenous (IV) bolus or controlled-release subcutaneous (SC) implants. 57 Controlled-release delivery of zalcitabine inhibited de novo FeLV replication and delayed the onset of viraemia after experimental infection; however, when treatment was discontinued an equivalent incidence and level of viraemia was established rapidly. 58 In a study evaluating the prophylactic antiviral activity of zalcitabine against FeLV, the drug was administered by continuous IV infusion for 28 days. Higher doses were extremely toxic, causing death in 8/10 cats. Lower doses caused thrombocytopenia. Only 1/10 cats remained FeLV antigen-negative when receiving the low dose, although the onset of viraemia was delayed for several weeks (EBM grade II). 57
Lamivudine
Lamivudine (2R,cis-4-amino-l-[2-hydroxymethyl-1,3-oxathiolan-5-yl]-[1H]-pyrimidin-2-one, 3TC) is also an approved anti-HIV drug.
FIV Lamivudine is active against FIV in vitro.24,30,38 A combination of zidovudine and lamivudine was shown to have synergistic anti-FIV activities in cell culture. 38 FIV mutants resistant to lamivudine containing a point mutation in the RT gene were selected in vitro and showed cross-resistance to zidovudine. 32 In one in vivo study, cats experimentally infected with FIV were treated with a high dose zidovudine/lamivudine combination. 38 Treatment protected some cats when started before infection, but the zidovudine/lamivudine combination had no anti-FIV activity in chronically infected cats. Severe side effects, including fever, anorexia and marked haematological changes, were observed in some of the cats receiving the high dose dual-drug treatment (EBM II). 38
FeLV No data on the anti-FeLV activity of lamivudine are available.
Nucleotide analogue reverse transcriptase inhibitors
Nucleotide analogue RT inhibitors interact with the catalytic site of the RT and are incorporated into the elongating proviral DNA strand, subsequently causing strand termination.12,15 They compete with the natural nucleotides and thus function as competitive substrate inhibitors. In these respects they are similar to nucleoside RT inhibitors. However, in contrast to nucleoside RT inhibitors, nucleotide RT inhibitors contain a phosphate group and therefore need only two intracellular phosphorylation steps to be converted into their active forms. 12 This circumvents the first (and often rate-limiting) phosphorylation step.15,60
Adefovir
Adefovir (2-[6-amino-9H-purin-9-yl]-ethoxy-methyl-phosphonic acid, PMEA) is active against herpesviruses, hepadnaviruses (hepatitis B) and retroviruses. 61 Adefovir is not licensed as an HIV drug, but is currently approved to treat chronic hepatitis B in an orally available form (bis-POM PMEA).
Adefovir belongs to the acyclic nucleoside phosphonates, in which the alkyl side chain of purines and pyrimidines is linked to a modified phosphate moiety and a C-P phosphonate linkage replaces the normal O5′-P phosphate linkage. 60 This phosphonate bond is non-hydrolysable, which makes it more difficult to cleave off these compounds once they have been incorporated at the 3′-terminal end of the elongating proviral DNA strand. 12
FIV Adefovir inhibits FIV replication in vitro. 62 Several studies have investigated the efficacy of adefovir in cats either experimentally or naturally infected with FIV.10,63 –67 Some of these studies have reported some efficacy, but also severe side effects, mainly in the form of non-regenerative anaemia. More recently, adefovir was used in FIV-infected cats in a 6 week placebo-controlled, double-blind clinical trial; 10 cats received the drug (10 mg/kg SC twice weekly) and 10 cats received placebo. 68 There was no decrease in proviral or viral loads in treated cats and they developed a progressive anaemia, which is a common adverse effect of nucleotide analogues (EBM grade I). 68
FeLV Adefovir is active against FeLV in vitro. 69 In a study in experimentally infected cats, adefovir prevented the development of persistent FeLV viraemia if used early in the infection (EBM grade II); 69 but the drug was not effective in a placebo-controlled, double-blind study in naturally infected cats when used for 3 weeks (EBM grade I). 64
Tenofovir
The antiviral spectrum of tenofovir (2R-1-[6-amino-9H-purin-9-yl]-propan-2-yl-oxy-methyl-phosphonic acid, PMPA) is narrower than that of adefovir, in that it does not extend to herpesviruses, but is confined to hepadnaviruses and retroviruses. 61 Tenofovir is currently the only nucleotide RT inhibitor approved for the treatment of HIV infection; it is marketed as the prodrug tenofovir disoproxil fumarate. 60 Since it was licensed in 2001, it has become one of the most commonly used drugs in HIV therapy.12,17
Non-nucleoside reverse transcriptase inhibitors
Most of the non-nucleoside RT inhibitors are highly specific for HIV. Unlike nucleoside and nucleotide RT inhibitors, which bind to the catalytic site of RT, non-nucleoside RT inhibitors interact with an allosteric site of the enzyme 12 and are not incorporated into the proviral DNA strand. 15 They are classified as non-competitive inhibitors of RT and do not require intracellular activation to inhibit the enzyme.15,16
Non-nucleoside RT inhibitors are a group of structurally diverse compounds that all bind a single site in the HIV RT enzyme. 72 The interaction with the allosteric site, which is located in close proximity to the catalytic site, leads to a number of conformational changes within the RT.72,73 Among other effects, these changes cause a decrease in the interaction between the DNA primer and the polymerase domain of the enzyme and, thus, interfere with virus replication.72,73
The classical non-nucleoside RT inhibitors are not active against FIV and FeLV; however, there is one old drug, suramin, that can be classified as a non-nucleoside RT inhibitor and has been used in veterinary medicine.
Suramin
Suramin (1-[3-benzamido-4-methylbenzamido]naphthalene-4,6,8-trisulfonic acid sym-3′-urea sodium salt), a sulfated naphthylamine and trypan red derivative, is one of the oldest known antimicrobial agents. It is used as a antitrypanosomal agent as well as for the treatment of some (eg, prostatic) tumours. 74 It also has an inhibitory effect on the RT activity of retroviruses and has been used in patients with HIV infection. 75 Suramin inhibits RT by interacting with the template–primer binding site of the enzyme. It competitively binds to the primer binding site and inhibits the template–primer binding that is necessary for DNA prolongation; thus, suramin can be classified as a non-nucleoside RT inhibitor. 76
Suramin is associated with a significant number of severe side effects in humans: nausea and anaphylactic shock as immediate reactions during administration; peripheral neuritis leading to palmar–plantar hyperaesthesia, photophobia, skin reactions, agranulocytosis, haemolytic anaemia and destruction of the adrenal cortex as later side effects.74,75,77 –79 Severe side effects have to be expected in cats as well.
FIV The efficacy of suramin against FIV is unknown.
FeLV Suramin has not been investigated against FeLV in vitro, but has been used to treat FeLV-infected cats, although only a limited number of cats have been evaluated in uncontrolled studies. In one study, serum viral infectivity ceased transiently in two cats with naturally acquired FeLV infection but returned to high levels approximately 14 days after treatment was stopped (EBM grade III). 80 In another study, six anaemic FeLV-infected cats received suramin and, within 4–14 days, erythropoiesis improved. However, progenitor cells remained infected (EBM grade III). 81
Nucleotide synthesis inhibitors
Nucleotide synthesis inhibitors interfere with DNA and RNA synthesis, but not by mimicking nucleosides. They usually have a broad spectrum of activity, but also marked toxicity. Foscarnet and ribavirin have been used in veterinary medicine.
Foscarnet
Foscarnet (phosphonoformic acid, PFA) has a wide spectrum of activity against DNA and RNA viruses, including retroviruses. Foscarnet interferes with exchange of pyrophosphate from deoxynucleoside triphosphate during viral replication by binding to a site on RT or DNA polymerase. 82 This drug has only a short effect; after treatment is stopped, viral replication is reactivated. Foscarnet is mostly administered IV by continuous infusion because of its short half-life, a property that has also been demonstrated in cats. 83 Oral application is possible but can cause irritation of mucous membranes and oral bleeding.
Foscarnet has many side effects in humans as well as in cats, such as nephrotoxicity and myelosuppression. It is also toxic to epithelial cells and mucous membranes, and gastrointestinal side effects and ulcerations of genital epithelium can occur. In addition, it chelates cations, such that hypocalcaemia, hypomagnesaemia and hypokalaemia can develop.84,85
Ribavirin
Ribavirin (1-[β-D-ribofuranosyl]-1H-1,2,4-triazole-3-carboxamide, RTCA) has marked in vitro antiviral activity against a variety of DNA and RNA viruses. 87 Ribavirin has multiple effects on virus replication, such as allowing DNA synthesis to occur, but preventing triphosphate synthesis by inhibiting the enzyme inosine monophosphate dehydrogenase (essential for synthesis of nucleotides); it thus prevents nucleotide production. 87
Systemic application of ribavirin is limited because of side effects. 88 Side effects in cats in several studies (even using low doses) have included haemolysis, which develops as a result of sequestration of the drug in erythrocytes.89,90 In addition, there is a dose-related toxic effect on bone marrow, primarily on megakaryocytes (resulting in thrombocytopenia and haemorrhage) and erythroid precursors (resulting in non-regenerative anaemia). With prolonged treatment or higher doses, the drug suppresses production of neutrophilic granulocytes. Liver toxicity occurs too. An attempt to decrease the toxicity of ribavirin by incorporating it into lecithin-containing liposomes and giving it at lower doses was not successful. 91
Receptor homologues/antagonists
Receptor homologues/antagonists either bind to the virus or to the cellular receptor and thereby inhibit binding of the virus to the cell surface. Most of these drugs are highly selective for HIV and not useful for veterinary medicine. An exception are the bicyclams (eg, plerixafor), which can be used in cats with FIV infection because of similarity between HIV and FIV with respect to chemokine receptor usage.92,93 Chemokine receptors belong to a group of seven transmembrane proteins in which signal transmission is afforded through rapid influx of calcium into the cell. They are essential co-receptors for HIV and FIV in the infection of CD4+ lymphocytes. 94 CXCR4 is the major receptor for FIV infection, but other receptors also have been shown to mediate viral binding. 95 By binding to CXCR4, bicyclams prevent interaction of CXCR4 with other ligands, thereby inhibiting the entry of HIV or FIV into the cell.96 –98
Plerixafor
Plerixafor (1,1′-[1,4-phenylenbismethylene]-bis[1,4,8,11-tetraazacyclotetradecane]-octachloride dehydrate, AMD3100) is the prototype compound among the bicyclams. It is not on the market as an anti-HIV drug, but is used in humans for stem cell mobilisation. 99 Plerixafor is administered to cats at a dose of 0.5 mg/kg SC q12h. Magnesium and calcium levels should be monitored regularly during treatment. 68
FIV Plerixafor is active against FIV in vitro. 97 Its efficacy was investigated in naturally FIV-infected cats in a placebo-controlled, double-blind clinical trial. 68 Treatment with plerixafor resulted in a significant decrease in provirus load; a decrease in serum magnesium levels was also recorded, although this did not have any clinical consequences. No development of resistance of FIV isolates to plerixafor was found during treatment (EBM grade I). 68
FeLV Efficacy against FeLV has not been determined, but plerixafor is likely ineffective against this retrovirus due to the different receptors that FeLV uses for cell entry. 100
Integrase inhibitors
The enzyme integrase catalyses strand transfer (3′-end joining), which inserts both viral DNA ends into a host cell chromosome. The high degree of conservation of integrase-active sites across many retroviruses suggests that FIV and FeLV can be sensitive to integrase inhibitors. 101 The mechanism of action of these drugs is inhibition of integration of the proviral DNA that is produced by reverse transcription of the viral RNA genome. 71
Raltegravir
Raltegravir is used in humans as an anti-HIV compound.
FIV Raltegravir is effective against FIV in vitro. 102 No in vivo studies have been published so far.
FeLV Raltegravir has recently been shown to be highly effective in vitro in several feline cell lines for inhibition of FeLV.71,101 The effective concentration of raltegravir had no effect on cell viability and did not induce apoptosis, suggesting that this might also be an effective and safe drug in vivo. 55 However, raltegravir is partly eliminated as glucuronide, via a metabolic pathway that is not very efficient in cats, and this increases the risk of toxicity due to drug accumulation. 99 A very recent study evaluated efficacy and safety of raltegravir in seven cats with experimentally induced progressive FeLV infection. 103 Raltegravir was administered at 40 mg PO q12h for 6.5 weeks and then at 80 mg PO q12h for 2.5 weeks. No control group was included in the study. The treatment was successful in reducing viraemia in each cat, with up to 1 log10 reduction in viral RNA in 4/7 cats; however, viraemia rebounded in all cats after treatment was stopped. The drug reached sufficient plasma concentrations with both doses. It was found to be safe, with no harmful side effects (EBM grade III). 103
Interferons
Interferons (IFNs) are polypeptide molecules with a variety of biological functions. 104 They play an important role in mediating antiviral and antigrowth responses and in modulating the immune response. 105 They can be divided into two major types – type I and type II IFNs – both of which show antiviral properties. Type I IFNs, including IFN-α, IFN-β and IFN-ω, are produced by virus-infected cells.104,106 Type II IFN, consisting of only IFN-γ, is produced by activated T lymphocytes and natural killer cells in response to their recognition of virus-infected cells. 107
IFNs act in an autocrine or paracrine fashion, 108 inducing an antiviral state in non-infected cells. IFNs bind to specific cell surface receptors and result in the transcription of IFN-stimulated genes. The products of these genes are proteins with potent antiviral properties which interfere with various stages of viral replication. 108 Several studies suggest that retroviral protein synthesis is not affected by IFNs and conclude that the antiviral activity of IFNs is related to interference with later stages of the viral replication cycle, such as virion assembly and release.104,109 Interferons also trigger virus-infected cells to undergo apoptosis by activating the expression of genes that contribute to the process of programmed cell death.107,109 Thereby IFNs prevent the spread of virus from infected cells and aid in the clearance of virus infection. 107
Human IFNs have been manufactured by recombinant DNA technology and are available commercially. Recombinant feline IFN-ω is on the market in Japan, Australia and European countries, licensed for use in cats and dogs.
Human interferon-α
Recombinant human interferon-α (IFN-α) has antiviral and immunomodulatory activity, and is active against many DNA and RNA viruses. 108 There are two common treatment regimens for use of IFN-α in cats: SC injection of high doses (1 x 104 to 1 x 106 IU/kg q24h) or oral application of low doses (1–50 IU/kg q24h). When given parenterally to cats, human IFN-α becomes ineffective after 3–7 weeks due to the development of neutralising antibodies that limit its activity. 46
IFN-α can be given orally for a longer period as no antibodies will develop during oral treatment, and it has been used in this manner to treat FIV and FeLV infections. However, given orally, IFN-α is inactivated by gastric acid and destroyed by trypsin and other proteolytic enzymes in the duodenum. 110 Thus, direct antiviral effects are unlikely after oral application, but it still seems to have immunomodulatory activity. After oral application, IFN-α can bind to mucosal receptors in the oral cavity, stimulating the local lymphoid tissue. This leads to cytokine release on lymphatic cells in the oral or pharyngeal area, triggering a cascade of immunological responses that finally act systemically.111 –113
FIV IFN-α is active against FIV in vitro. 114 Although frequently used in the field for treating FIV-infected cats, no controlled studies have evaluated the effect of high dose parenteral IFN-α in FIV-infected cats.
Use of low dose oral IFN-α in sick cats naturally infected with FIV (50 IU/kg on the oral mucosa q24h for 7 days on alternating weeks for 6 months, followed by a 2 month break, and then repetition of the 6 month treatment) resulted in improvement of clinical signs in a placebo-controlled, double-blind study. 115
FeLV Several studies have been conducted on the use of IFN-α in FeLV-infected cats. In vitro, FeLV replication is inhibited by IFN-α. 116 One study evaluated the therapeutic efficacy of high dose IFN-α (1.6 x 104 to 1.6 x 106 IU/kg SC q24h) in experimentally FeLV-infected cats with persistently high levels of antigenaemia. 46 Treatment resulted in a significant decrease in circulating FeLV antigen beginning 2 weeks after the initiation of therapy (EBM grade II). 46 However, because of anti-IFN-α antibody development, cats became refractory to therapy 3 or 7 weeks after starting treatment. 46 In a placebo-controlled, double-blind study in naturally FeLV-infected cats using a similar high dose regimen, treatment with IFN-α (1 x 105 IU/kg SC q24h for 6 weeks) did not lead to a significant improvement in clinical, laboratory, immunological or virological parameters (EBM grade I). 47
Low dose oral IFN-α was used in a placebo-controlled study in experimentally induced FeLV infection; 0.5 IU/cat (eight cats) or 5 IU/cat (five cats) was given (following experimental challenge) on 7 consecutive days on alternate weeks for a period of 1 month. 111 No difference was found in the development of viraemia between groups; however, treated cats had significantly fewer clinical signs and longer survival times when compared with the placebo group (with a better response in the cats given 0.5 IU/cat) (EBM grade II). 111 Several uncontrolled field studies have also reported a beneficial response in cats when treated with low dose oral IFN-α,116,117 but they only included a limited number of cats and the findings are impossible to interpret without control groups (EBM grade III). In a larger study, the outcome of 69 FeLV-infected cats with clinical signs that were treated with low dose oral IFN-α (30 IU/kg for 7 consecutive days on a 1 week on/1 week off schedule) was compared with historical controls; significantly longer survival times were reported in the treated cats (EBM grade III). 117 In a placebo-controlled study, treatment of sick client-owned FeLV-infected cats with low dose oral IFN-α (30 IU/cat for 7 consecutive days on a 1 week on/1 week off schedule) did not result in a significant difference in FeLV status, survival time, clinical or haematological parameters, or owners’ subjective impression when compared with a placebo group. Thus, this controlled study was not able to demonstrate efficacy (EBM grade I). 118
Feline interferon-ω
Feline interferon-ω (IFN-ω), the corresponding feline interferon, is licensed as a veterinary medicine in Japan, Australia and European countries. Feline IFN-ω is a recombinant product that is produced by baculoviruses containing the feline sequence for this IFN that replicate in silkworms after infection. Results of studies investigating the efficacy of feline IFN-ω against FIV and FeLV are summarised in the box on page 935.
Future priorities
Unfortunately, the level of efficacy of antiviral chemotherapy is often poor and the duration of treatments used in clinical trials is often inappropriate for infections with such long clinical courses. Additionally, the degree of generalisability between experimental infections in cats kept under laboratory conditions and pet cats infected with field strains is unknown. Therefore, it is very important that more well-designed double-blind, placebo-controlled trials using antivirals in naturally retrovirus-infected cats are undertaken to allow judgement on treatment efficacy and side effects of different antiviral compounds.
Footnotes
Funding: The author received no financial support for the research, authorship and/or publication of this article.
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Hosie MJ, Addie D, Belak S, et al. Feline immunodeficiency. ABCD guidelines on prevention and management. J Feline Med Surg 2009; 11: 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lutz H, Addie D, Belak S, et al. Feline leukaemia. ABCD guidelines on prevention and management. J Feline Med Surg 2009; 11: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sellon RK, Hartmann K. Feline immunodeficiency virus infection. In: Greene CE. (ed). Infectious diseases of the dog and cat. 4th ed. St Louis, MO: Elsevier Saunders, 2012, pp 136–149. [Google Scholar]
- 4. Hartmann K. Antiviral and immunomodulatory chemotherapy. In: Greene CE. (ed). Infectious diseases of the dog and cat. 4th ed. St Louis, MO: Elsevier Saunders, 2012, pp 10–24. [Google Scholar]
- 5. Hartmann K. Feline leukemia virus infection. In: Greene CE. (ed). Infectious diseases of the dog and cat. 4th ed. St Louis, MO: Elsevier Saunders, 2012, pp 108–136. [Google Scholar]
- 6. Gleich SE, Krieger S, Hartmann K. Prevalence of feline immunodeficiency virus and feline leukaemia virus among client-owned cats and risk factors for infection in Germany. J Feline Med Surg 2009; 11: 985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Addie DD, Dennis JM, Toth S, et al. Long-term impact on a closed household of pet cats of natural infection with feline coronavirus, feline leukaemia virus and feline immunodeficiency virus. Vet Rec 2000; 146: 419–424. [DOI] [PubMed] [Google Scholar]
- 8. Levy J, Crawford C, Hartmann K, et al. 2008. American Association of Feline Practitioners’ feline retrovirus management guidelines. J Feline Med Surg 2008; 10: 300–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hartmann K. Clinical aspects of feline immunodeficiency and feline leukemia virus infection. Vet Immunol Immunopathol 2011; 143: 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hartmann K. Clinical aspects of feline retroviruses: a review. Viruses 2012; 4: 2684–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Clercq E. Toward improved anti-HIV chemotherapy: therapeutic strategies for intervention with HIV infections. J Med Chem 1995; 38: 2491–2517. [DOI] [PubMed] [Google Scholar]
- 12. De Clercq E. Anti-HIV drugs: 25 compounds approved within 25 years after the discovery of HIV. Int J Antimicrob Agents 2009; 33: 307–320. [DOI] [PubMed] [Google Scholar]
- 13. Palmisano L, Vella S. A brief history of antiretroviral therapy of HIV infection: success and challenges. Ann Ist Super Sanita 2011; 47: 44–48. [DOI] [PubMed] [Google Scholar]
- 14. Auwerx J, Esnouf R, De Clercq E, et al. Susceptibility of feline immunodeficiency virus/human immunodeficiency virus type 1 reverse transcriptase chimeras to non-nucleoside RT inhibitors. Mol Pharmacol 2004; 65: 244–251. [DOI] [PubMed] [Google Scholar]
- 15. Ravichandran S, Veerasamy R, Raman S, et al. An overview on HIV-1 reverse transcriptase inhibitors. Dig J Nanomater Biostruct 2008; 3: 171–187. [Google Scholar]
- 16. Mohammadi H, Bienzle D. Pharmacological inhibition of feline immunodeficiency virus (FIV). Viruses 2012; 4: 708–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tressler R, Godfrey C. NRTI backbone in HIV treatment: will it remain relevant? Drugs 2012; 72: 2051–2062. [DOI] [PubMed] [Google Scholar]
- 18. De Clercq E. The nucleoside reverse transcriptase inhibitors, nonnucleoside reverse transcriptase inhibitors, and protease inhibitors in the treatment of HIV infections (AIDS). Adv Pharmacol 2013; 67: 317–358. [DOI] [PubMed] [Google Scholar]
- 19. Horwitz JP, Chua J, Noel M. Nucleosides. V. The monomesylates of 1-(2′-deoxy-B-D-lyxofuranosyl)thymine. J Org Chem 1964; 29: 2076–2078. [Google Scholar]
- 20. Mitsuya H, Weinhold KJ, Furman PA, et al. 3′-azido-3′-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci USA 1985; 82: 7096–7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ezzell C. AZT given the green light for clinical treatment of AIDS. Nature 1987; 326: 430. [DOI] [PubMed] [Google Scholar]
- 22. Hartmann K, Donath A, Kraft W. AZT in the treatment of feline immunodeficiency virus infection: part 2. Feline Pract 1995; 23(6): 13–20. [Google Scholar]
- 23. Hartmann K, Donath A, Kraft W. AZT in the treatment of feline immunodeficiency virus infection: part 1. Feline Pract 1995; 23(5): 16–21. [Google Scholar]
- 24. Bisset LR, Lutz H, Boni J, et al. Combined effect of zidovudine (ZDV), lamivudine (3TC) and abacavir (ABC) antiretroviral therapy in suppressing in vitro FIV replication. Antiviral Res 2002; 53: 35–45. [DOI] [PubMed] [Google Scholar]
- 25. Gobert JM, Remington KM, Zhu YQ, et al. Multiple-drug-resistant mutants of feline immunodeficiency virus selected with 2′,3′-dideoxyinosine alone and in combination with 3′-azido-3′-deoxythymidine. Antimicrob Agents Chemother 1994; 38: 861–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCrackin Stevenson MA, McBroom DG. In vitro characterization of FIV-pPPR, a pathogenic molecular clone of feline immunodeficiency virus, and two drug-resistant pol gene mutants. Am J Vet Res 2001; 62: 588–594. [DOI] [PubMed] [Google Scholar]
- 27. North TW, North GL, Pedersen NC. Feline immunodeficiency virus, a model for reverse transcriptase-targeted chemotherapy for acquired immune deficiency syndrome. Antimicrob Agents Chemother 1989; 33: 915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Remington KM, Chesebro B, Wehrly K, et al. Mutants of feline immunodeficiency virus resistant to 3′-azido-3′-deoxythymidine. J Virol 1991; 65: 308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Remington KM, Zhu YQ, Phillips TR, et al. Rapid phenotypic reversion of zidovudine-resistant feline immunodeficiency virus without loss of drug-resistant reverse transcriptase. J Virol 1994; 68: 632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schwartz AM, McCrackin MA, Schinazi RF, et al. Antiviral efficacy of nine nucleoside reverse transcriptase inhibitors against feline immunodeficiency virus in feline peripheral blood mononuclear cells. Am J Vet Res 2014; 75: 273–281. [DOI] [PubMed] [Google Scholar]
- 31. Smith RA, Remington KM, Lloyd RM, Jr, et al. A novel Met-to-Thr mutation in the YMDD motif of reverse transcriptase from feline immunodeficiency virus confers resistance to oxathiolane nucleosides. J Virol 1997; 71: 2357–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith RA, Remington KM, Preston BD, et al. A novel point mutation at position 156 of reverse transcriptase from feline immunodeficiency virus confers resistance to the combination of (-)-beta-2′,3′-dideoxy-3′-thiacytidine and 3′-azido-3′-deoxythymidine. J Virol 1998; 72: 2335–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smyth NR, McCracken C, Gaskell RM, et al. Susceptibility in cell culture of feline immunodeficiency virus to eighteen antiviral agents. J Antimicrob Chemother 1994; 34: 589–594. [DOI] [PubMed] [Google Scholar]
- 34. Tanabe-Tochikura A, Tochikura TS, Blakeslee JR, Jr, et al. Anti-human immunodeficiency virus (HIV) agents are also potent and selective inhibitors of feline immunodeficiency virus (FIV)-induced cytopathic effect: development of a new method for screening of anti-FIV substances in vitro. Antiviral Res 1992; 19: 161–172. [DOI] [PubMed] [Google Scholar]
- 35. Vahlenkamp TW, De Ronde A, Balzarini J, et al. (R)-9-(2-phosphonylmethoxypropyl)-2,6-diaminopurine is a potent inhibitor of feline immunodeficiency virus infection. Antimicrob Agents Chemother 1995; 39: 746–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu YQ, Remington KM, North TW. Mutants of feline immunodeficiency virus resistant to 2′,3′-dideoxy-2′,3′-didehydrothymidine. Antimicrob Agents Chemother 1996; 40: 1983–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hartmann K. Feline immunodeficiency virus infection: an overview. Vet J 1998; 155: 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arai M, Earl DD, Yamamoto JK. Is AZT/3TC therapy effective against FIV infection or immunopathogenesis? Vet Immunol Immunopathol 2002; 85: 189–204. [DOI] [PubMed] [Google Scholar]
- 39. Greene CE, Watson ADJ. Antiviral drugs. In: Greene CE. (ed). Infectious diseases of the dog and cat. 2nd ed. St Louis, MO: Elsevier Saunders, 1998, pp 6–9. [Google Scholar]
- 40. Hoover EA, Zeidner NS, Mullins JI. Therapy of presymptomatic FeLV-induced immunodeficiency syndrome with AZT in combination with alpha interferon. Ann N Y Acad Sci 1990; 616: 258–269. [DOI] [PubMed] [Google Scholar]
- 41. Mathes LE, Polas PJ, Hayes KA, et al. Pre- and postexposure chemoprophylaxis: evidence that 3′-azido-3′-dideoxythymidine inhibits feline leukemia virus disease by a drug-induced vaccine response. Antimicrob Agents Chemother 1992; 36: 2715–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mukherji E, Au JL, Mathes LE. Differential antiviral activities and intracellular metabolism of 3′-azido-3′-deoxythymidine and 2′,3′-dideoxyinosine in human cells. Antimicrob Agents Chemother 1994; 38: 1573–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tavares L, Roneker C, Johnston K, et al. 3′-Azido-3′-deoxythymidine in feline leukemia virus-infected cats: a model for therapy and prophylaxis of AIDS. Cancer Res 1987; 47: 3190–3194. [PubMed] [Google Scholar]
- 44. Tavares L, Roneker C, Postie L, et al. Testing of nucleoside analogues in cats infected with feline leukemia virus: a model. Intervirology 1989; 30 Suppl 1: 26–35. [DOI] [PubMed] [Google Scholar]
- 45. Zeidner NS, Rose LM, Mathiason-DuBard CK, et al. Zidovudine in combination with alpha interferon and interleukin-2 as prophylactic therapy for FeLV-induced immunodeficiency syndrome (FeLV-FAIDS). J Acquir Immune Defic Syndr 1990; 3: 787–796. [PubMed] [Google Scholar]
- 46. Zeidner NS, Myles MH, Mathiason-DuBard CK, et al. Alpha interferon (2b) in combination with zidovudine for the treatment of presymptomatic feline leukemia virus-induced immunodeficiency syndrome. Antimicrob Agents Chemother 1990; 34: 1749–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stuetzer B, Brunner K, Lutz H, et al. A trial with 3′-azido-2′,3′-dideoxythymidine and human interferon-alpha in cats naturally infected with feline leukaemia virus. J Feline Med Surg 2013; 15: 667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. August EM, Marongiu ME, Lin TS, et al. Initial studies on the cellular pharmacology of 3′-deoxythymidin-2′-ene (d4T): a potent and selective inhibitor of human immunodeficiency virus. Biochem Pharmacol 1988; 37: 4419–4422. [DOI] [PubMed] [Google Scholar]
- 49. Baba M, Pauwels R, Herdewijn P, et al. Both 2′,3′-dideoxythymidine and its 2′,3′-unsaturated derivative (2′,3′-dideoxythymidinene) are potent and selective inhibitors of human immunodeficiency virus replication in vitro. Biochem Biophys Res Commun 1987; 142: 128–134. [DOI] [PubMed] [Google Scholar]
- 50. Balzarini J, Kang GJ, Dalal M, et al. The anti-HTLV-III (anti-HIV) and cytotoxic activity of 2′,3′-didehydro-2′,3′-dideoxyribonucleosides: a comparison with their parental 2′,3′-dideoxyribonucleosides. Mol Pharmacol 1987; 32: 162–167. [PubMed] [Google Scholar]
- 51. Lin TS, Schinazi RF, Prusoff WH. Potent and selective in vitro activity of 3′-deoxythymidin-2′-ene (3′-deoxy-2′,3′-didehydrothymidine) against human immunodeficiency virus. Biochem Pharmacol 1987; 36: 2713–2718. [DOI] [PubMed] [Google Scholar]
- 52. Balzarini J, Egberink H, Hartmann K, et al. Antiretrovirus specificity and intracellular metabolism of 2′,3′-didehydro-2′,3′-dideoxythymidine (stavudine) and its 5′-monophosphate triester prodrug So324. Mol Pharmacol 1996; 50: 1207–1213. [PubMed] [Google Scholar]
- 53. Mitsuya H, Broder S. Inhibition of the in vitro infectivity and cytopathic effect of human T-lymphotrophic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV) by 2′,3′-dideoxynucleosides. Proc Natl Acad Sci USA 1986; 83: 1911–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Medlin HK, Zhu YQ, Remington KM, et al. Selection and characterization of a mutant of feline immunodeficiency virus resistant to 2′,3′-dideoxycytidine. Antimicrob Agents Chemother 1996; 40: 953–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhu Y, Antony JM, Martinez JA, et al. Didanosine causes sensory neuropathy in an HIV/AIDS animal model: impaired mitochondrial and neurotrophic factor gene expression. Brain 2007; 130: 2011–2023. [DOI] [PubMed] [Google Scholar]
- 56. Broder S. Progress in targeted therapy against human immunodeficiency virus [corrected]. Crit Care Med 1990; 18: S118–125. [PubMed] [Google Scholar]
- 57. Polas PJ, Swenson CL, Sams R, et al. In vitro and in vivo evidence that the antiviral activity of 2′,3′-dideoxycytidine is target cell dependent in a feline retrovirus animal model. Antimicrob Agents Chemother 1990; 34: 1414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hoover EA, Zeidner NS, Perigo NA, et al. Feline leukemia virus-induced immunodeficiency syndrome in cats as a model for evaluation of antiretroviral therapy. Intervirology 1989; 30 Suppl 1: 12–25. [DOI] [PubMed] [Google Scholar]
- 59. Zeidner NS, Strobel JD, Perigo NA, et al. Treatment of FeLV-induced immunodeficiency syndrome (FeLV-FAIDS) with controlled release capsular implantation of 2′,3′-dideoxycytidine. Antiviral Res 1989; 11: 147–160. [DOI] [PubMed] [Google Scholar]
- 60. Cihlar T, Ray AS. Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after zidovudine. Antiviral Res 2010; 85: 39–58. [DOI] [PubMed] [Google Scholar]
- 61. De Clercq E. Acyclic nucleoside phosphonates: past, present and future. Bridging chemistry to HIV, HBV, HCV, HPV, adeno-, herpes-, and poxvirus infections: the phosphonate bridge. Biochem Pharmacol 2007; 73: 911–922. [DOI] [PubMed] [Google Scholar]
- 62. Balzarini J, Naesens L, Slachmuylders J, et al. 9-(2-Phosphonylmethoxyethyl)adenine (PMEA) effectively inhibits retrovirus replication in vitro and simian immunodeficiency virus infection in rhesus monkeys. Aids 1991; 5: 21–28. [DOI] [PubMed] [Google Scholar]
- 63. Egberink H, Borst M, Niphuis H, et al. Suppression of feline immunodeficiency virus infection in vivo by 9-(2-phosphonomethoxyethyl)adenine. Proc Natl Acad Sci USA 1990; 87: 3087–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hartmann K, Donath A, Beer B, et al. Use of two virustatica (AZT, PMEA) in the treatment of FIV and of FeLV seropositive cats with clinical symptoms. Vet Immunol Immunopathol 1992; 35: 167–175. [DOI] [PubMed] [Google Scholar]
- 65. Hartmann K, Kuffer M, Balzarini J, et al. Efficacy of the acyclic nucleoside phosphonates (S)-9-(3-fluoro-2-phosphonylmethoxypropyl)adenine (FPMPA) and 9-(2-phosphonylmethoxyethyl)adenine (PMEA) against feline immunodeficiency virus. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 17: 120–128. [DOI] [PubMed] [Google Scholar]
- 66. Kuffer M, Balzarini J, Rolinski B, et al. [Comparative investigation of the efficacy of two nucleocapsid analogs in FIV infected cats.] Tierarztl Prax Ausg K Kleintiere Heimtiere 1997; 25: 671–677. [PubMed] [Google Scholar]
- 67. Philpott MS, Ebner JP, Hoover EA. Evaluation of 9-(2-phosphonylmethoxyethyl) adenine therapy for feline immunodeficiency virus using a quantitative polymerase chain reaction. Vet Immunol Immunopathol 1992; 35: 155–166. [DOI] [PubMed] [Google Scholar]
- 68. Hartmann K, Stengel C, Klein D, et al. Efficacy and adverse effects of the antiviral compound plerixafor in feline immunodeficiency virus-infected cats. J Vet Intern Med 2012; 26: 483–490. [DOI] [PubMed] [Google Scholar]
- 69. Hoover EA, Ebner JP, Zeidner NS, et al. Early therapy of feline leukemia virus infection (FeLV-FAIDS) with 9-(2-phosphonylmethoxyethyl)adenine (PMEA). Antiviral Res 1991; 16: 77–92. [DOI] [PubMed] [Google Scholar]
- 70. Balzarini J, Vahlenkamp T, Egberink H, et al. Antiretroviral activities of acyclic nucleoside phosphonates [9-(2-phosphonylmethoxyethyl)adenine, 9-(2-phosphonylmethoxyethyl)guanine, (R)-9-(2-phosphonylmethoxypropyl)adenine, and MDL 74,968] in cell cultures and murine sarcoma virus-infected newborn NMRI mice. Antimicrob Agents Chemother 1997; 41: 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Greggs WM, 3rd, Clouser CL, Patterson SE, et al. Discovery of drugs that possess activity against feline leukemia virus. J Gen Virol 2012; 93: 900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xia Q, Radzio J, Anderson KS, et al. Probing nonnucleoside inhibitor-induced active-site distortion in HIV-1 reverse transcriptase by transient kinetic analyses. Protein Sci 2007; 16: 1728–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Das K, Martinez SE, Bauman JD, et al. HIV-1 reverse transcriptase complex with DNA and nevirapine reveals non-nucleoside inhibition mechanism. Nat Struct Mol Biol 2012; 19: 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Garcia-Schurmann JM, Schulze H, Haupt G, et al. Suramin treatment in hormone- and chemotherapy-refractory prostate cancer. Urology 1999; 53: 535–541. [DOI] [PubMed] [Google Scholar]
- 75. Broder S, Yarchoan R, Collins JM, et al. Effects of suramin on HTLV-III/LAV infection presenting as Kaposi’s sarcoma or AIDS-related complex: clinical pharmacology and suppression of virus replication in vivo. Lancet 1985; 2: 627–630. [DOI] [PubMed] [Google Scholar]
- 76. De Clercq E. Suramin: a potent inhibitor of the reverse transcriptase of RNA tumor viruses. Cancer Lett 1979; 8: 9–22. [DOI] [PubMed] [Google Scholar]
- 77. Dorfinger K, Niederle B, Vierhapper H, et al. Suramin and the human adrenocortex: results of experimental and clinical studies. Surgery 1991; 110: 1100–1105. [PubMed] [Google Scholar]
- 78. Kaur M, Reed E, Sartor O, et al. Suramin’s development: what did we learn? Invest New Drugs 2002; 20: 209–219. [DOI] [PubMed] [Google Scholar]
- 79. O’Donnell BP, Dawson NA, Weiss RB, et al. Suramin-induced skin reactions. Arch Dermatol 1992; 128: 75–79. [PubMed] [Google Scholar]
- 80. Cogan DC, Cotter SM, Kitchen LW. Effect of suramin on serum viral replication in feline leukemia virus-infected pet cats. Am J Vet Res 1986; 47: 2230–2232. [PubMed] [Google Scholar]
- 81. Abkowitz JL. Retrovirus-induced feline pure red blood cell aplasia: pathogenesis and response to suramin. Blood 1991; 77: 1442–1451. [PubMed] [Google Scholar]
- 82. Crumpacker CS. Mechanism of action of foscarnet against viral polymerases. Am J Med 1992; 92: 3S–7S. [DOI] [PubMed] [Google Scholar]
- 83. Straw JA, Loo TL, de Vera CC, et al. Pharmacokinetics of potential anti-AIDS agents thiofoscarnet and foscarnet in the cat. J Acquir Immune Defic Syndr 1992; 5: 936–942. [PubMed] [Google Scholar]
- 84. Gerard L, Salmon-Ceron D. Pharmacology and clinical use of foscarnet. Int J Antimicrob Agents 1995; 5: 209–217. [DOI] [PubMed] [Google Scholar]
- 85. Ryrfeldt A, Nordgren T, Lundstrom J. Hypocalcemia induced by foscarnet (Foscavir) infusion in dogs. Fundam Appl Toxicol 1992; 18: 126–130. [DOI] [PubMed] [Google Scholar]
- 86. Swenson CL, Polas PJ, Cheney CM, et al. Prophylactic and therapeutic effects of phosphonoformate against feline leukemia virus in vitro. Am J Vet Res 1991; 52: 2010–2015. [PubMed] [Google Scholar]
- 87. Beaucourt S, Vignuzzi M. Ribavirin: a drug active against many viruses with multiple effects on virus replication and propagation. Molecular basis of ribavirin resistance. Curr Opin Virol 2014; 8: 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lafeuillade A, Hittinger G, Chadapaud S. Increased mitochondrial toxicity with ribavirin in HIV/HCV coinfection. Lancet 2001; 357: 280–281. [DOI] [PubMed] [Google Scholar]
- 89. Povey RC. Effect of orally administered ribavirin on experimental feline calicivirus infection in cats. Am J Vet Res 1978; 39: 1337–1341. [PubMed] [Google Scholar]
- 90. Weiss RC, Cox NR, Boudreaux MK. Toxicologic effects of ribavirin in cats. J Vet Pharmacol Ther 1993; 16: 301–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Weiss RC, Cox NR, Martinez ML. Evaluation of free or liposome-encapsulated ribavirin for antiviral therapy of experimentally induced feline infectious peritonitis. Res Vet Sci 1993; 55: 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rucker J, Edinger AL, Sharron M, et al. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol 1997; 71: 8999–9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Willett BJ, Hosie MJ. The role of the chemokine receptor CXCR4 in infection with feline immunodeficiency virus. Mol Membr Biol 1999; 16: 67–72. [DOI] [PubMed] [Google Scholar]
- 94. Wells TN, Proudfoot AE, Power CA, et al. Chemokine receptors – the new frontier for AIDS research. Chem Biol 1996; 3: 603–609. [DOI] [PubMed] [Google Scholar]
- 95. Willett BJ, Picard L, Hosie MJ, et al. Shared usage of the chemokine receptor CXCR4 by the feline and human immunodeficiency viruses. J Virol 1997; 71: 6407–6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Donzella GA, Schols D, Lin SW, et al. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med 1998; 4: 72–77. [DOI] [PubMed] [Google Scholar]
- 97. Egberink HF, De Clercq E, Van Vliet AL, et al. Bicyclams, selective antagonists of the human chemokine receptor CXCR4, potently inhibit feline immunodeficiency virus replication. J Virol 1999; 73: 6346–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Schols D, Este JA, Henson G, et al. Bicyclams, a class of potent anti-HIV agents, are targeted at the HIV coreceptor fusin/CXCR-4. Antiviral Res 1997; 35: 147–156. [DOI] [PubMed] [Google Scholar]
- 99. Liles WC, Broxmeyer HE, Rodger E, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood 2003; 102: 2728–2730. [DOI] [PubMed] [Google Scholar]
- 100. Katrin Helfer-Hungerbuehler A, Cattori V, Bachler B, et al. Quantification and molecular characterization of the feline leukemia virus A receptor. Infect Genet Evol 2011; 11: 1940–1950. [DOI] [PubMed] [Google Scholar]
- 101. Cattori V, Weibel B, Lutz H. Inhibition of feline leukemia virus replication by the integrase inhibitor Raltegravir. Vet Microbiol 2011; 152: 165–168. [DOI] [PubMed] [Google Scholar]
- 102. Togami H, Shimura K, Okamoto M, et al. Comprehensive in vitro analysis of simian retrovirus type 4 susceptibility to antiretroviral agents. J Virol 2013; 87: 4322–4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Boesch A, Cattori V, Riond B, et al. Evaluation of the effect of short-term treatment with the integrase inhibitor raltegravir (Isentress) on the course of progressive feline leukemia virus infection. Vet Microbiol 2015; 175: 167–178. [DOI] [PubMed] [Google Scholar]
- 104. Domenech A, Miro G, Collado VM, et al. Use of recombinant interferon omega in feline retrovirosis: from theory to practice. Vet Immunol Immunopathol 2011; 143: 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Stark JJ, Dillman RO, Schulof R, et al. Interferon-alpha and chemohormonal therapy for patients with advanced melanoma: final results of a phase I-II study of the Cancer Biotherapy Research Group and the Mid-Atlantic Oncology Program. Cancer 1998; 82: 1677–1681. [DOI] [PubMed] [Google Scholar]
- 106. Gil S, Leal RO, Duarte A, et al. Relevance of feline interferon omega for clinical improvement and reduction of concurrent viral excretion in retrovirus infected cats from a rescue shelter. Res Vet Sci 2013; 94: 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Goodbourn S, Didcock L, Randall RE. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J Gen Virol 2000; 81: 2341–2364. [DOI] [PubMed] [Google Scholar]
- 108. Gerlach N, Gibbert K, Alter C, et al. Anti-retroviral effects of type I IFN subtypes in vivo. Eur J Immunol 2009; 39: 136–146. [DOI] [PubMed] [Google Scholar]
- 109. Gomez-Lucia E, Collado VM, Miro G, et al. Effect of type-I interferon on retroviruses. Viruses 2009; 1: 545–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cantell K, Pyhala L. Circulating interferon in rabbits after administration of human interferon by different routes. J Gen Virol 1973; 20: 97–104. [DOI] [PubMed] [Google Scholar]
- 111. Cummins JM, Beilharz MW, Krakowka S. Oral use of interferon. J Interferon Cytokine Res 1999; 19: 853–857. [DOI] [PubMed] [Google Scholar]
- 112. Koech DK, Obel AO. Efficacy of Kemron (low dose oral natural human interferon alpha) in the management of HIV-1 infection and acquired immune deficiency syndrome (AIDS). East Afr Med J 1990; 67: SS64–70. [PubMed] [Google Scholar]
- 113. Tompkins WA. Immunomodulation and therapeutic effects of the oral use of interferon-alpha: mechanism of action. J Interferon Cytokine Res 1999; 19: 817–828. [DOI] [PubMed] [Google Scholar]
- 114. Tanabe T, Yamamoto JK. Feline immunodeficiency virus lacks sensitivity to the antiviral activity of feline IFN-gamma. J Interferon Cytokine Res 2001; 21: 1039–1046. [DOI] [PubMed] [Google Scholar]
- 115. Pedretti E, Passeri B, Amadori M, et al. Low-dose interferon-alpha treatment for feline immunodeficiency virus infection. Vet Immunol Immunopathol 2006; 109: 245–254. [DOI] [PubMed] [Google Scholar]
- 116. Jameson P, Essex M. Inhibition of feline leukemia virus replication by human leukocyte interferon. Antiviral Res 1983; 3: 115–120. [DOI] [PubMed] [Google Scholar]
- 117. Weiss RC, Cummins JM, Richards AB. Low-dose orally administered alpha interferon treatment for feline leukemia virus infection. J Am Vet Med Assoc 1991; 199: 1477–1481. [PubMed] [Google Scholar]
- 118. McCaw DL, Boon GD, Jergens AE, et al. Immunomodulation therapy for feline leukemia virus infection. J Am Anim Hosp Assoc 2001; 37: 356–363. [DOI] [PubMed] [Google Scholar]
- 119. de Mari K, Maynard L, Sanquer A, et al. Therapeutic effects of recombinant feline interferon-omega on feline leukemia virus (FeLV)-infected and FeLV/feline immunodeficiency virus (FIV)-coinfected symptomatic cats. J Vet Intern Med 2004; 18: 477–482. [DOI] [PubMed] [Google Scholar]
- 120. Gil S, Leal RO, McGahie D, et al. Oral Recombinant Feline Interferon-Omega as an alternative immune modulation therapy in FIV positive cats: clinical and laboratory evaluation. Res Vet Sci 2014; 96: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Leal RO, Gil S, Duarte A, et al. Evaluation of viremia, proviral load and cytokine profile in naturally feline immunodeficiency virus infected cats treated with two different protocols of recombinant feline interferon omega. Res Vet Sci 2015; 99: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Rogers R, Merigan TC, Hardy WD, Jr, et al. Cat interferon inhibits feline leukaemia virus infection in cell culture. Nature New Biol 1972; 237: 270–271. [DOI] [PubMed] [Google Scholar]