Abstract
Practical relevance:
Ciclosporin (CsA) is a systemic immuno-modulatory drug widely used to treat immune-mediated diseases in humans and veterinary species. CsA was registered for use in cats in the USA and Europe in 2011, and is indicated for the treatment of chronic allergic dermatitis at a recommended daily dose of 7 mg/kg PO.
Audience:
This review will be of interest to all veterinarians working with cats, given the wide range of potential applications of CsA and its safety profile. Although the drug is currently only licensed to treat chronic allergic dermatitis in cats, a small number of reports describe its use in non-dermatological conditions.
Evidence base:
This article reviews the mechanism of action, pharmacokinetics, drug interactions, adverse effects and clinical use of CsA, both for the licensed indication and for off-label use in the feline patient. Information presented has been summarised from the existing literature on CsA, with specific interest in studies carried out in cats. For its licensed indication, chronic allergic dermatitis, evidence provided includes randomised, placebo or prednisolone-controlled studies (EBM grade I) and prospective or retrospective open trials.
Discovery and development
Ciclosporin (CsA) is a systemic immuno-modulatory drug licensed for use in humans and veterinary species. It was registered for use in the cat in the USA and Europe in 2011, and is indicated for the treatment of chronic allergic dermatitis.

CsA is a cyclic undecapeptide metabolite of the fungus Tolypocladium inflatum Gams1,2 and its molecular weight is 1203 Daltons. 3 It was discovered in 1971, during a search for new antibiotics produced by fungi. CsA and other metabolites were initially screened for antifungal activity, but their efficacy was poor. Its immunosuppressive, non-cytotoxic activity was discovered and reported in 1976. The first human trial started at the end of the same year.4,5 In the USA, it was approved in 1983 for the prevention of graft rejection following transplantation in human patients, and in 1984 synthetic CsA was produced. 4
CsA is fat soluble, extremely hydrophobic, and is poorly absorbed after oral administration. It has been produced in different formulations over time, with the aim of improving its absorption and bioavailability. The first commercially available CsA formulation, only registered for humans, was a solution containing ethanol and corn oil as excipients (Sandimmune; Novartis Pharma). The CsA formulation currently used in humans, dogs and cats is a microemulsion, which enhances oral absorption of the drug.
In most studies published before 2011, micro-emulsified CsA in soft gelatin capsules, registered for the treatment of atopic dermatitis in dogs, was used in feline patients at a daily dose of one 25 mg capsule/cat. In older studies investigating the use of CsA in feline renal transplant recipients, variable dosages were used, according to the different formulations available at the time. Dosages will be specified in this review, unless microemulsified CsA as the oral solution registered for cats was used at the recommended dosage of 7 mg/kg PO q24h.
Mechanism of action
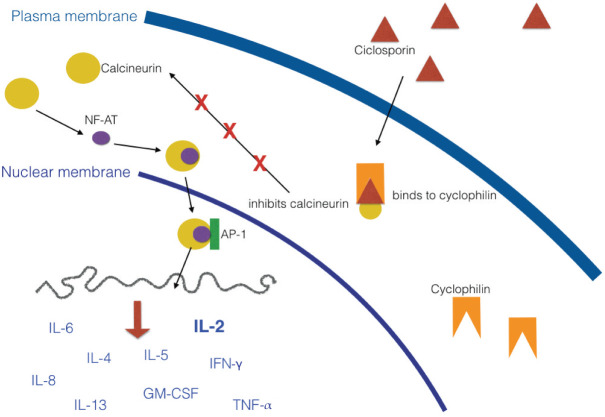
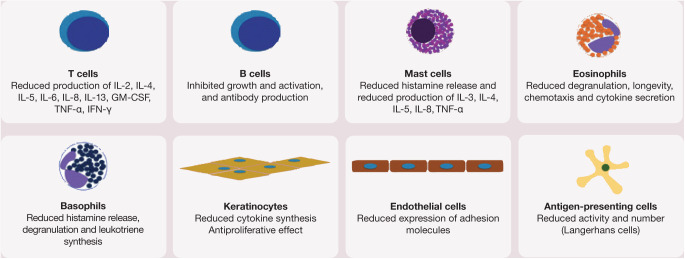
CsA is a systemic drug that exerts its effects mainly on the cell-mediated immune system, while humoral immunity is less affected.1,6 Its mechanism of action is depicted in Figure 1. CsA suppresses the production of interleukin (IL)-2 and the expression of the IL-2 receptor on T lymphocytes, resulting in inhibition of T cell proliferation and activation. It also inhibits the production of IL-4, IL-5, IL-6, IL-8, IL-13, granulocyte-macrophage colony-stimulating factor (GM-CSF), tumour necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ). This leads to a number of different anti-inflammatory effects, including reductions in mast cell degradation, keratinocyte proliferation and cytokine production, as well as decreased tumouricidal and superoxide activity by macrophages. Other effects include reduced expression of intercellular adhesion molecule 1 and leukoctye trafficking in endothelial cells, and inhibition of antigen-presenting cell function (Figure 2).1,6,7
Figure 1.
Mechanism of action of CsA. CsA enters the cytoplasm of T cells and binds to the receptor cyclophilin. The drug–receptor complex secondarily binds to calcineurin, inhibiting its action. In the absence of CsA, calcineurin binds to nuclear factor of activated T cells (NF-AT), which in turn enters the nucleus and binds to activator protein 1 (AP-1), a transcription factor. This complex induces the transcription of cytokine genes by the T cell. IL = interleukin; IFN-γ = interferon gamma; TNF-α = tumour necrosis factor alpha; GM-CSF = granulocyte–macrophage colony-stimulating factor
Figure 2.
Cellular targets of CsA. IL = interleukin; GM-CSF = granulocyte–macrophage colony-stimulating factor; TNF-α = tumour necrosis factor alpha; IFN-γ = interferon gamma
Very few studies have been published specifically addressing the effects of CsA in feline species. In the cat, CsA has been shown to suppress in vitro lymphoblast transformation after stimulation with the mitogens concanavalin A and pokeweed within 7 days of administration, with return to a normal response within 7 days after withdrawal. Large individual variations in CsA serum trough levels were observed in both studies.3,8 In feline peripheral blood mononuclear cells (PBMCs), CsA suppresses the expression of mRNAs for IL-2, IL-4, IL-10, GM-CSF, IFN-γ and TNF-α, and the number of IL-2 secreting lymphocytes, in a dose-dependent manner.9,10 In feline intestinal mucosal biopsies, CsA is able to reduce neutrophil infiltration. 11
Therapeutic monitoring
Due to the extreme variability in absorption and metabolism, monitoring the concentrations of CsA in the blood has been recommended. Knowing the blood concentration may help clinicians adjust the dose, in order to maintain ‘effective’ concentrations. Therapeutic monitoring may also be useful to identify cats at a higher risk of adverse effects or opportunistic infections.15,19 Monitoring is also suggested in patients treated concurrently with imidazoles (see section on drug interactions). CsA concentration should be evaluated in whole blood rather than plasma, because the drug concentrates within blood cells. 20 Ideally, testing should be carried out after 2 weeks of treatment and, where available, high performance liquid chromatography is a better method than immunoassay for evaluating CsA whole blood concentrations. 21
In older studies using CsA twice daily in feline transplant patients, the recommendation was to adjust the dose in order to maintain the 12 h trough blood levels at 300–500 ng/ml. 22 Trough level is defined as the lowest concentration reached by a drug before the next dose is administered, so if CsA is administered once daily, the blood sample should be taken 24 h after the dose. Since trough plasma levels vary widely between tested cats, it has been suggested that blood levels of CsA 2 h after administration (peak concentration) may be more useful for monitoring both therapeutic drug levels and toxicity; however, therapeutic peak concentrations have not been established.15,20
Unfortunately, the correlation between clinical efficacy of CsA and trough blood levels is usually poor. 23 Because CsA concentrates in the skin, reaching higher levels compared with blood,12,14 the current opinion is that when treating skin diseases with CsA, monitoring trough CsA levels is not particularly useful for evaluating the drug’s effectiveness and such monitoring is not considered mandatory, unless adverse effects are observed.
An alternative, although not commercially available, method for assessing the effectiveness of CsA involves pharmacodynamic assays, which evaluate the effect of a drug on its target cells. In human medicine, a large number of immunological markers of the immunosuppressive activity of CsA have been studied and validated for pharmacodynamic monitoring.20,24 In veterinary medicine, a few studies have investigated the expression of specific cytokines by either PBMCs or lesional skin of dogs treated with CsA, revealing decreased expression of IL-2, IL-4 and IFN-γ.25–27 The reduced expression of these biomarkers was very marked with high doses of CsA and still present at the recommended dosage for dogs (5 mg/kg PO q24h), showing that CsA may have immunosuppressive effects even at dosages considered to be low. 28 No studies on pharmacodynamic assays of CsA in the cat have been published yet. However, it is likely that the immunosuppressive effects may be similar to those observed in humans and dogs.
Drug interactions
CsA is metabolised by the cytochrome P450 enzymatic system and any drug able to induce or inhibit these enzymes may respectively increase or decrease CsA metabolism, thus decreasing or increasing CsA blood concentration. CsA is also both a substrate for and an inhibitor of P-glycoprotein, which is an efflux pump able to protect the blood–brain barrier from adverse effects of potentially neurotoxic drugs. CsA shows interactions with many drugs: in humans, 853 drugs are reported. 29 The most commonly reported interactions of CsA with other drugs in cats and humans are summarised in Table 1.
Table 1.
Drugs most commonly reported as interacting with CsA in cats and humans
| Drug | Interaction | References | |
|---|---|---|---|
| Reported in cats | Clarithromycin | Inhibition of P450/CYP3A4 enzymes: decreased CsA metabolism, increased blood CsA concentration | 30 |
| Itraconazole | 31 | ||
| Ketoconazole | 21 | ||
| Reported in humans | Clarithromycin | Inhibition of P450/CYP3A4 enzymes: decreased CsA metabolism, increased blood CsA concentration | 1,6 |
| Erythromycin | |||
| Ketoconazole | |||
| Itraconazole | |||
| Fluconazole | |||
| Miconazole | |||
| Diltiazem | |||
| Verapamil | |||
| Nicardipine | |||
| Methylprednisolone | |||
| Grapefruit juice | |||
| Nafcillin | Upregulation of P450/CYP3A4 enzymes: increased CsA metabolism, decreased blood CsA concentration | ||
| Rifampicin | |||
| Phenobarbitone | |||
| Phenytoin | |||
| Carbamazepine | |||
| Non-steroidal anti-inflammatory drugs | Nephrotoxicity/renal failure | ||
| Aminoglycosides | |||
| Enalapril | |||
| Captopril | |||
| Amphotericin B | |||
| Melphalan | |||
| Cephalosporins | Other interactions, mechanisms unknown | ||
| Chloramphenicol | |||
| Norofloxacin | |||
| Sulfadiazine | |||
| Trimethoprim/sulfadimidine | |||
| Glucocorticoids | |||
| St John’s wort | |||
| Allopurinol | |||
| Digoxin |
Very few studies have been performed to specifically address this issue in feline species. CsA has been shown to interact with both ketoconazole and itraconazole; administration of ketoconazole or itraconazole at 10 mg/kg PO q24h approximately doubles the CsA blood concentration, allowing for a significant reduction of the CsA dose and of the cost of treatment.21,31 The interaction with clarithromycin was studied recently. Administration of this antibiotic significantly increased the oral bioavailability of CsA, reducing the CsA dose required to maintain therapeutic levels by 65%. 30 Robson 32 anecdotally reported that concurrent administration with metoclo-pramide reduced CsA concentrations in 3/8 cats. Co-administration of CsA with macro-cyclic lactones does not seem to be associated with neurological adverse effects in cats. 33

Adverse effects
When using CsA at the recommended daily dose (7 mg/kg PO or, before 2011, 25 mg/cat), the most commonly reported adverse effects have been gastrointestinal (GI).33,35–40 In a study specifically addressing adverse effects in cats treated with CsA, vomiting occurred in 12% of cats and soft stools or diarrhoea in 16% of cats. 38 GI adverse effects are often temporary and many disappear within the first few weeks of treatment. Anecdotally, it has been suggested that metoclopramide 0.3–0.4 mg/kg given orally 20–30 mins before CsA administration, or maropitant 1 mg/kg PO q24h, or freezing the capsule may be used to prevent GI upset.7,32 However, metoclopramide in cats may reduce CsA concentration. 32
Anorexia has been reported in 2% of cats in one study 38 and in 10% of cats in another study. 33 Anorexia may be a serious problem in cats, as it can eventually lead to hepatic lipidosis.6,32,33,38–40 Weight loss has been described in 5–16% of cats.33,38 Some studies reported weight loss at the beginning of CsA therapy, with return to normal weight over the following months after CsA dose reduction.33,39
Gingival hyperplasia, possibly due to the stimulating effect of CsA on fibroblast proliferation consequent to increased transforming growth factor beta production, 41 has been reported rarely in cats.3,6 Recently, systemic CsA has been reported to be a significant risk factor for the development of acute bullous keratopathy in cats. 42
Other reported adverse effects are polyphagia, polydipsia, hyperactivity, 37 hypertrichosis, 43 weight gain, dental tartar and gingivitis, otitis, inflammatory bowel disease, urinary tract infection, cataract, hyperthyroidism and transient inappropriate urination. 38 However, most of these adverse effects are rare and not likely to be directly caused by CsA. 38
In experimental studies using CsA at higher doses (up to five times the normal dose), peripheral lymphadenopathy was reported, 44 and death occurred in one cat with a very high trough CsA concentration and bone marrow hypocellularity. 40 In three cats undergoing renal transplantation and given CsA at 5 mg/kg PO q12h, together with prednisone at 0.25 mg/kg PO q12h, haemolytic uraemic syndrome has been described. 45 CsA was deemed responsible for inducing this syndrome in two of these cases.
Haematological concentrations
In most studies, cats receiving systemic CsA have undergone haematology and biochemistry testing before, during and after the study, in order to identify significant alterations. Relative leukopenia, lymphopenia, neutropenia and eosinopenia, although with values still within normal reference intervals, have all been observed. 39 Biochemistry showed mild increases of total bilirubin, glucose and blood urea nitrogen (BUN) and mild reductions of alanine aminotransferase activity, alkaline phosphatase activity and albumin, but values were also within normal reference intervals.33,39
In experimental studies using CsA at higher doses (up to five times the normal dose), aplastic anaemia, lymphopenia, prolonged activated partial thromboplastin time, reduced total proteins and increased cholesterol, creatinine and BUN were also occasionally reported.40,44
Opportunistic infections
CsA is an immunosuppressive drug, and opportunistic infections may develop in treated patients. The most commonly reported infection in cats is toxoplasmosis (see box).
Feline herpesvirus infection (FHV-1) reactivation has been reported in cats experimentally infected and treated with CsA, methylpred-nisolone acetate or placebo. In most of the cats showing clinical signs of FHV-1 infection, these were mild and self-limiting; however, one cat with a very high CsA blood concentration developed severe clinical manifestations. 51 Conversely, CsA may have antiviral properties: it has been shown to suppress in vitro FIV production and apoptosis in infected cells, as well as feline infectious peritonitis (FIP) coronavirus replication.52,53
Urinary tract infections (UTIs) were investigated in 33 cats treated with either CsA or glucocorticoids for more than 3 months and no association between any therapy and UTI could be demonstrated. 54
Many other infectious diseases in cats receiving CsA have been reported. However, these occurred as single cases and the role of CsA could not be proven with certainty. Moreover, these diseases occurred in cats treated with different dosages of CsA. Reported diseases include Mycobacterium avium complex infection,43,55 severe pneumonia caused by Salmonella species, 56 nasal nodular cryptococ-cosis, 57 dermatophytosis, 58 actinobacillosis, pyelonephritis, retroperitoneal abscess and septicaemia, upper respiratory tract infection, oral abscess, chronic stomatitis, FeLV infection, FIP, fungal pinna dermatitis, septic peritonitis and cholangiohepatitis.23,40,44,59
Malignant neoplasia
The development of neoplasia has been reported in cats treated with CsA. The first report described a cat, immunosuppressed with CsA at 7.5 mg/kg q12h and prednisolone at an initial dosage of 2 mg/kg q24h, which developed FeLV-associated lymphosarcoma 6 months after transplantation surgery. 60 Two retrospective studies on feline transplant patients, treated with combinations of CsA and prednisolone at various dosages, were subsequently published: the first study reported malignant neoplasia in 9/95 (9.5%) cats, while the second reported malignant neoplasia in 11/45 (24%) patients.61,62 Types of malignant tumours included GI or multicentric lymphoma, bronchogenic adenocarcinoma, hepatic adenocarcinoma, gastric or intestinal adenocarcinoma, tonsillar or pharyngeal squamous cell carcinoma, pharyngeal plasmacytoma, transitional cell carcinoma and unspecified malignant round-cell neoplasia.61,62 The authors observed that transplanted cats had more than six times higher odds of developing malignant neoplasia compared with control cats, but the specific role of CsA remains undetermined. 62 Several human studies have also confirmed an increased incidence of post-transplant malignant neoplasia; however, no increased risk could be demonstrated when patients were immunosuppressed with CsA compared with azathioprine.63,64
In cats treated with CsA at the recommended dose for allergic or immune-mediated diseases, malignant neoplasia appears to be a rare occurrence.38,43
Vaccinations
A single study 65 evaluated the effectiveness of vaccinations in cats treated with CsA. Cats were given CsA at a very high dosage (24 mg/kg PO q24h) for 56 days and compared with placebo-treated cats. All cats had been previously vaccinated against calicivirus (FCV), FHV-1, feline parvovirus (FPV), FeLV and rabies, and were given a booster of the same vaccines 4 weeks after initiation of CsA administration. The booster vaccinations were successful in all treatment and placebo group cats. However, a novel vaccination for FIV given at the same time as the boosters did not induce adequate protection in CsA-treated cats. This suggests that booster vaccinations are effective in cats on CsA therapy, but primary immune responses may be impaired. Vaccination with live vaccines is not recommended during CsA therapy. 34
Clinical uses
CsA is licensed only for the treatment of chronic allergic dermatitis in cats, and all other uses mentioned in this review are off-label. The recommended dosing for CsA is shown on the left. In some of the studies using CsA off-label, the drug was administered at a different dose and this will be noted where relevant. CsA can be given to cats either mixed with a small amount of food or directly into the mouth.
At present, recommended pretreatment examinations include complete blood count, biochemistry panel, urinalysis and serology for FeLV and FIV. 7 Serology testing for toxoplasmosis is not essential, but might be helpful as seronegative cats may require more careful management to prevent infection during treatment. Any complicating infection should be treated before commencing CsA administration.
During CsA treatment, physical examination, body weight check, haematology and biochemistry should be performed every 6 months.6,32,44
Chronic allergic dermatitis
Many studies have reported on the effectiveness of CsA in chronic allergic dermatitis (Figure 3), and these are summarised in Table 2. CsA treatment was also used to validate systems for scoring the severity of lesions in allergic cats.71,72
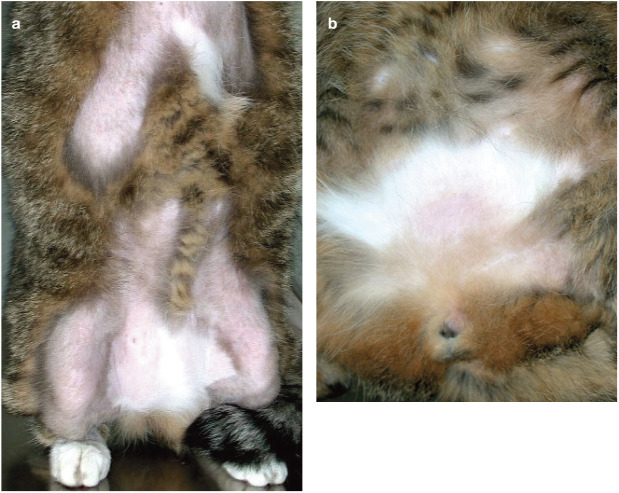
Figure 3.
Feline atopic syndrome. (a) Generalised self-induced alopecia and (b) 1 month after initiation of CsA therapy
Table 2.
Studies on the use of CsA in feline chronic allergic dermatitis
| Reference | Study type | Disease presentation | Daily dose | Duration | Number of cats | Control | Efficacy |
|---|---|---|---|---|---|---|---|
| 66 | OT | IU, EP, EG | 25 mg/cat | 60 days | 12 | No | EP, EG, not in IU |
| 67 | OT | HD | 5–10 mg/kg | NR | 8 | No | 7/8 cats |
| 35 | PT | P, S-A, EP, MD (no response to flea control and diet) | 25 mg/cat | 30 days | 10 | No | Improvement: 40% P, 57% S-A, 60% erythema, 50% total clinical score (FEGEPESI) Statistically significant decrease of pruritus |
| 36 | OT | EGC, P, PS (no response to flea control and diet) | 5.8–13.3 mg/kg* | 90 days | 23 | No | EGC, P PS: 4/8 cats |
| 68 | REV | EGC | 5–7 mg/kg | NR | NR | No | Disease controlled in 60–75% of cats |
| 37 | RCT | AD | 5 mg/kg | 28 days | 18 | Pred 1 mg/kg | No difference between groups |
| 39 | RCT | HD | 7 mg/kg | 42 days | 33 | Placebo | Improvement: >50% in 70% of cats Result: ‘excellent’ or ‘good’ in 61% of cats |
| 33 | RCT | HD | 7 mg/kg | 12 weeks | 33 | Placebo | 70% of cats maintained on q48h dosing, then 57% of 70% twice weekly |
| 69 | OT | HD | 7 mg/kg | 90 days | 32 | No | 29% of cats maintained on q24h dosing, 26% q48h, 45% q72h |
| 57 | RS | AD | NR | NR | 10 | No | 100% good response |
| 44 | OT | HD | 7 mg/kg | 12 weeks | 191 | No | 31.6% of cats maintained on q24h dosing, 15.5% q48h, 62.9% twice weekly |
| 70 | RCT | HD | 7 mg/kg | 42 days | 144 | Placebo | Improvement in total lesion score, with 65.1% average reduction (9.2% for placebo group) |
Some cats on Sandimmune solution, some on Atoplus capsules
AD = atopic dermatitis; EG = eosinophilic granuloma; EGC = eosinophilic granuloma complex; EP = eosinophilic plaque; HD = hypersensitivity dermatitis; IU = indolent ulcer; MD = miliary dermatitis; NR = not reported; OT = open trial; P = pruritus; Pred = prednisolone; PS = plasmacytic stomatitis; PT = pilot study; RCT = randomised controlled trial; REV = review; RS = retrospective study; S-A = self-induced alopecia; FEGEPESI = feline eosinophilic granuloma, eosinophilic plaque, extension and severity index
Pemphigus complex
The first report of the use of CsA in cats for the treatment of pemphigus foliaceus (PF) (one cat) and pemphigus erythematosus (PE) (one cat) at 15 mg/kg PO q24h was published in 1989. The PF cat showed a partial and temporary response, while the PE cat showed complete resolution maintained with continuation of CsA. 73 A retrospective study in 2012 evaluated CsA in 15 cats with PF, both as an adjuvant and sole drug, in comparison with chlorambucil. All the cats treated with CsA at 5 mg/kg PO q24h were eventually maintained on CsA only and glucocorticoids were either not used from the beginning or discontinued.
In the chlorambucil group, only 1/6 cats could be weaned off glucocorticoids. 43 CsA in feline PF (Figure 4) appears to be a promising drug; however, a controlled, prospective study should be conducted to confirm its effectiveness.
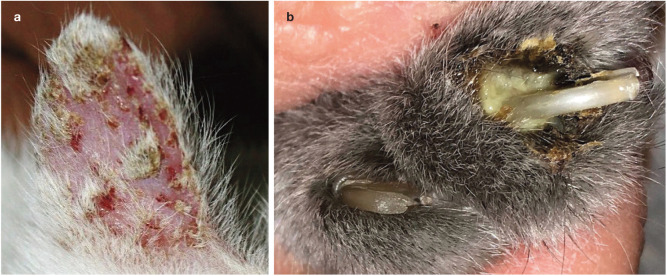
Figure 4.
Feline pemphigus foliaceus. (a) Multifocal crusting covering small erosions on the pinna. (b) Caseous material in the claw fold
Miscellaneous skin diseases
Many different feline dermatological immune-mediated diseases have been treated with CsA off-label and with various doses and protocols. However, this has been described only in single cases or small groups of patients and the results must be viewed with caution. Diseases successfully treated or controlled with CsA include pseudopelade, 58 feline urticaria pigmentosa (Figure 5), 74 idiopathic facial dermatitis of Persian cats, 75 granulomatous folliculitis and furunculosis, sebaceous adenitis, 76 feline lymphocytic mural folliculitis, 77 exfoliative dermatitis not associated with thymoma, 78 an atypical form of feline eosinophilic dermatosis (Figure 6), possibly of genetic origin, 79 and feline plasma cell pododermatitis. 80
Figure 5.
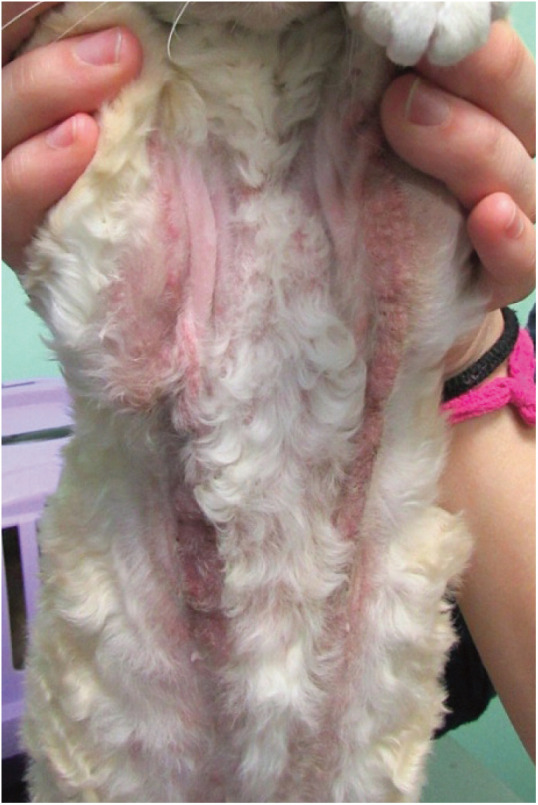
Feline urticaria pigmentosa. Note the linear distribution of the lesions
Figure 6.
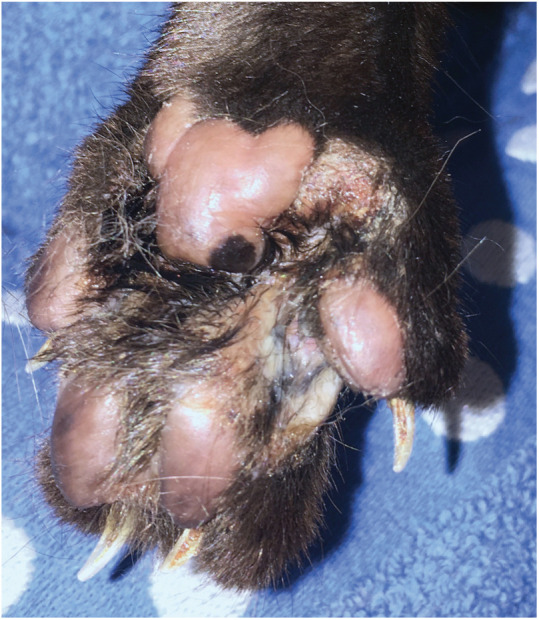
Feline pedal eosinophilic dermatosis
Transplants
CsA has been used to prevent organ rejection in feline transplant patients for more than 25 years.81,82 In an experimental study using the gracilis musculocutaneous flap as an allograft, six cats were treated with CsA (trough whole blood levels 750 ng/ml for 70 days, then 500 ng/ml for 30 days) in combination with prednisolone at 0.5 mg/kg PO q24h, while six other cats were left untreated. In the treated cats, the flaps survived for more than 100 days while the grafts of the untreated cats were rejected within 2 weeks. 60
The first large case series of renal transplants was published in 1997. 23 Immunosuppressive treatment protocols in this series were based on administration of either CsA at 7.5 mg/kg PO q12h or microemulsified CsA at 3 mg/kg PO q12h, always in association with prednisolone at 0.125–0.25 mg/kg PO q12h. Seventy-one percent of the cats survived until discharge from the veterinary hospital. Eight cats died within a year of immunosuppression-related causes such as infectious or neoplastic diseases. In a second case series, cats were treated with CsA at doses able to maintain target whole blood trough levels at 300–500 ng/ml and prednisolone at 0.25 mg/kg PO q12h; 77.5% of cats survived until discharge, with 65% of cats alive 6 months postoperatively. 59
Haematological diseases
Treatment with CsA and prednisolone of severe, non-regenerative anaemia due to pure red blood cell aplasia of autoimmune origin was reported in a young cat in 1998. 83 After this case report, three retrospective studies on pure red blood cell aplasia supported the use of CsA (5–20 mg/kg PO q24h) combined with various immunosuppressive drugs (prednisolone 3–4 mg/kg PO q24h, methylpred-nisolone 2 mg/kg PO q24h, dexamethasone 0.6–0.8 mg/kg q24h or recombinant human erythropoietin 150 U/kg SC three times weekly) to treat this rare condition. Most cats required long-term immunosuppressive treatment to maintain the disease in remission.84–86
Two single case reports described the use of CsA in combination with immunosuppressive drugs at similar dosages to treat idiopathic, possibly immune-mediated, thrombocytopenia and immune-mediated granulocytopenia associated with thymoma.87,88
Feline stomatitis
CsA has been reported to be effective in feline gingivostomatitis. 89 A randomised, double blinded, placebo-controlled clinical trial of CsA in cats with chronic refractory stomatitis confirmed CsA efficacy at 2.5 mg/kg PO q12h, with a mean improvement of 52.7% after 6 weeks in the treated group compared with 12.2% in the placebo group. 90
Feline asthma
Information on the use of CsA to treat feline asthma is scarce and contradictory. In one experimental study, CsA (trough levels between 500 and 850 ng/ml) given to Ascaris suum-experimentally sensitised cats prior to antigen challenge was able to inhibit airway hyper-responsiveness, IL-2 production in PBMCs, increase of eosinophils in broncho-alveolar lavage fluid and airway remodelling. 91 However, a second study showed that CsA administration did not inhibit early phase bronchoconstriction and did not inhibit mast cell degranulation within airway smooth muscle occurring after antigen stimulation in A suum-experimentally sensitised cats. 92 A single case report described the clinical use of CsA at 4 mg/kg PO q24h in a 15-year-old cat suffering from feline asthma and concurrently affected by diabetes mellitus and congestive heart failure. 93
Miscellaneous immune-mediated diseases
One case report described treatment with CsA at 25 mg/cat q24h, associated with dexa-methasone for the first month only, in an FIV-and FeLV-positive cat affected by chronic progressive polyarthritis. 94 Only anecdotal reports are currently available on the use of CsA in the treatment of feline inflammatory bowel disease. 95
Prevention of adverse reactions to drugs
The use of CsA to control acute carrier-induced hypersensitivity reactions due to the chemotherapeutic agent docetaxel has been reported in cats with various neoplastic diseases. 96
Key Points
CsA is a calcineurin inhibitor and very effective in the treatment of allergic dermatitis in cats. It has also been used to manage a variety of immune-mediated diseases in feline species, often with very promising results, although prospective controlled studies are lacking for most diseases.
CsA is safe: the most commonly reported adverse effect is GI upset, which is often temporary and self-resolving. Being an immunosuppressive drug, CsA may increase susceptibility to opportunistic infections and neoplasia; however, this appears to be rare when the drug is used at recommended doses.
Cats should be tested for FIV and FeLV before initiating treatment. Owners should be instructed to keep their cat indoors and not to feed raw meat to prevent toxoplasmosis in seronegative cases.
Routine booster vaccinations can be given to cats on CsA, and have been shown to induce a protective antibody response.
CsA absorption is variable and this is reflected in CsA blood concentrations, which do not correlate with the clinical response, at least in dermatological patients.
CsA blood level determination is not considered mandatory, but it may be useful to identify cats at higher risk of opportunistic infections.
Caution should be used when the concurrent use of CsA with other drugs is required, due to its numerous pharmacological interactions.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- Robson D. Review of the properties and mechanisms of action of cyclosporine with an emphasis on dermatological therapy in dogs, cats and people. Vet Rec 2003; 152: 768–772. [DOI] [PubMed] [Google Scholar]
- Robson DC, Burton GG. Cyclosporin: applications in small animal dermatology. Vet Dermatol 2003; 14: 1–9. [DOI] [PubMed] [Google Scholar]
- Latimer KS, Rakich PM, Purswell BJ. Effects of cyclosporine A administration in cats. Vet Immunol Immunopathol 1986; 11: 161–173. [DOI] [PubMed] [Google Scholar]
- Moore D, Upton H. The cyclosporine story, David Moore’s World of Fungi. http://www.davidmoore.org.uk/Sec04_01.htm (2001, accessed April 19, 2017).
- Heusler K, Pletscher A. The controversial early history of cyclosporin. Swiss Med Wkly 2001; 131: 299–302. [DOI] [PubMed] [Google Scholar]
- Kovalik M, Thoday KL, van den broek AH. The use of ciclosporin A in veterinary dermatology. Vet J 2012; 193: 317–325. [DOI] [PubMed] [Google Scholar]
- Whitehouse W, Viviano K. Clinical use of 10 emerging therapies. J Feline Med Surg 2015; 17: 220–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory CR, Taylor NJ, Willits NH, et al. Response to isoantigens and mitogens in the cat: effects of cyclosporin A. Am J Vet Res 1987; 48: 126–130. [PubMed] [Google Scholar]
- Kuga K, Nishifuji K, Iwasaki T. Cyclosporine A inhibits transcription of cytokine genes and decreases the frequencies of IL-2 producing cells in feline mononuclear cells. J Vet Med Sci 2008; 70: 1011–1016. [DOI] [PubMed] [Google Scholar]
- Aronson LR, Stumhofer JS, Drobats KJ, et al. Effect of cyclosporine, dexamethasone, and human CTLA4-Ig on production of cytokines in lymphocytes of clinically normal cats and cats undergoing renal transplantation. Am J Vet Res 2011; 72: 541–549. [DOI] [PubMed] [Google Scholar]
- Kubes P, Hunter J, Granger DN. Effects of cyclosporin A and FK506 on ischemia/reperfu-sion-induced neutrophil infiltration in the cat. Dig Dis Sci 1991; 36: 1469–1472. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Duell EA, Nickoloff BJ, et al. Levels of cyclosporin in epidermis of treated psoriasis patients differentially inhibit growth of keratinocytes cultured in serum free versus serum containing media. J Invest Dermatol 1988; 91: 142–146. [DOI] [PubMed] [Google Scholar]
- Freeman DJ. Pharmacology and pharmacokinetics of cyclosporine. Clin Biochem 1991; 24: 9–14. [DOI] [PubMed] [Google Scholar]
- Steffan J, Maurer M, Rohlfs A. Cyclosporin concentration in the skin following oral administration. Vet Dermatol 2003; 14: 237–267.14617294 [Google Scholar]
- Mehl ML, Kyles AE, Craigmill AL, et al. Disposition of cyclosporine after intravenous and multi-dose oral administration in cats. J Vet Pharmacol Therap 2003; 26: 349–354. [DOI] [PubMed] [Google Scholar]
- Gregory CR, Hietala SK, Pedersen NC, et al. Cyclosporine pharmacokinetics in cats following topical ocular administration. Transplantation 1989; 47: 516–519. [DOI] [PubMed] [Google Scholar]
- Miller R, Schick AE, Boothe DM, et al. Absorption of transdermal and oral cyclosporine in six healthy cats. J Am Anim Hosp Assoc 2014; 50: 36–41. [DOI] [PubMed] [Google Scholar]
- Koch SN, Torres SMF, Diaz S, et al. Subcutaneous administration of ciclosporin for feline allergic skin disease – an open label clinical trial. Vet Dermatol 2016; 27 Suppl 1: 6–121. [DOI] [PubMed] [Google Scholar]
- Lappin MR, VanLare KA, Seewald W, et al. Effect of oral administration of cyclosporine on Toxoplasma gondii infection status of cats. Am J Vet Res 2015; 76: 351–357. [DOI] [PubMed] [Google Scholar]
- Archer TM, Boothe DM, Langston VC, et al. Oral cyclosporine treatment in dogs: a review of the literature. J Vet Intern Med 2014; 28: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnulty JF, Lensmeyer GL. The effects of ketoconazole on the pharmacokinetics of cyclosporine A in cats. Vet Surg 1999; 28: 448–455. [DOI] [PubMed] [Google Scholar]
- Bernsteen L, Gregory CR, Kyles AE, et al. Renal transplantation in cats. Clin Tech Small Anim Pract 2000; 15: 40–45. [DOI] [PubMed] [Google Scholar]
- Mathews KG, Gregory CR. Renal transplants in cats: 66 cases (1987-1996). J Am Vet Med Assoc 1997; 211: 1432–1436. [PubMed] [Google Scholar]
- Bohler T, Nolting J, Kamar N, et al. Validation of immunological biomarkers for the pharma-codynamics monitoring of immunosuppres-sive drugs in humans. Ther Drug Monit 2007; 29: 77–86. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Momoi Y, Iwasaki T. Cyclosporine A inhibits the mRNA expressions of IL-2, IL-4 and IFN-gamma, but not TNF-alpha, in canine mononuclear cells. J Vet Med Sci 2007; 69: 887–892. [DOI] [PubMed] [Google Scholar]
- Tivers MS, Catchpole B, Gregory SP, et al. Interleukin-2 and interferon-gamma mRNA expression in canine anal furunculosis lesions and the effect of ciclosporin therapy. Vet Immunol Immunopathol 2008; 125: 31–36. [DOI] [PubMed] [Google Scholar]
- Fellman CL, Stokes JV, Archer TM, et al. Cyclosporine A affects the in vitro expression of T cell activation-related molecules and cytokines in dogs. Vet Immunol Immunopathol 2011; 140: 175–180. [DOI] [PubMed] [Google Scholar]
- Archer TM, Fellman CL, Stokes JV, et al. Pharmacodynamic monitoring of canine T-cell cytokine responses to oral cyclosporine. J Vet Intern Med 2011; 25: 1391–1397. [DOI] [PubMed] [Google Scholar]
- Drugs.com. Interactions checker. www.drugs.com (accessed October 2, 2017).
- Katayama M, Nishijima N, Okamura Y, et al. Interaction of clarithromycin with cyclo-sporine in cats: pharmacokinetic study and case report. J Feline Med Surg 2012; 14: 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama M, Katayama R, Kamishina H. Effects of multiple oral dosing of itraconazole on the pharmacokinetics of cyclosporine in cats. J Feline Med Surg 2010; 12: 512–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson D. Ciclosporin - 10 years later. Proceedings of the 7th World Congress of Veterinary Dermatology; 2012 July 24-28; Vancouver, Canada, pp 393–407. [Google Scholar]
- Steffan J, Roberts E, Cannon A, et al. Dose tapering for ciclosporin in cats with nonflea-induced hypersensitivity dermatitis. Vet Dermatol 2013; 24: 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atopica for cats (product insert). Greensboro (NC): Novartis Animal Health; 2011. [Google Scholar]
- Noli C, Scarampella F. Prospective open pilot study on the use of ciclosporin for feline allergic skin disease. J Small Anim Pract 2006; 47: 434–438. [DOI] [PubMed] [Google Scholar]
- Vercelli A, Raviri G, Cornegliani L. The use of oral cyclosporin to treat feline dermatoses: a retrospective analysis of 23 cases. Vet Dermatol 2006; 17: 201–206. [DOI] [PubMed] [Google Scholar]
- Wisselink MA, Willemse T. The efficacy of cyclosporine A in cats with presumed atopic dermatitis: a double blind, randomised pred-nisolone-controlled study. Vet J 2009; 180: 55–59. [DOI] [PubMed] [Google Scholar]
- Heinrich NA, McKeever PJ, Eisenschenk MC. Adverse events in 50 cats with allergic dermatitis receiving ciclosporin. Vet Dermatol 2011; 22: 511–520. [DOI] [PubMed] [Google Scholar]
- King S, Favrot C, Messinger L, et al. A randomized double-blinded placebo-controlled study to evaluate an effective ciclosporin dose for the treatment of feline hypersensitivity dermatitis. Vet Dermatol 2012; 23: 440–449. [DOI] [PubMed] [Google Scholar]
- Roberts ES, VanLare KA, Strehlau G, et al. Safety, tolerability and pharmacokinetics of 6-month daily dosing of an oral formulation of cyclosporine (Atopica for cats) in cats. J Vet Pharmacol Therap 2013; 37: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabellini G, Carinci F, Bedani PL, et al. Cyclosporin A and transforming growth factor-beta modify the pattern of extracellular glycosaminoglycans without causing cyto-skeletal changes in human gingival fibroblasts. Transplantation 2002; 73: 1676–1679. [DOI] [PubMed] [Google Scholar]
- Pierce KE, Wilkie DA, Gemensky-Metzler AJ, et al. An association between systemic cyclo-sporine administration and development of acute bullous keratopathy in cats. Vet Ophthalmol 2016; 19 Suppl 1: 77–85. [DOI] [PubMed] [Google Scholar]
- Irwin KE, Beale KM, Fadok VA. Use of modified ciclosporin in the management of feline pemphigus foliaceous: a retrospective analysis. Vet Dermatol 2012; 23: 403–411. [DOI] [PubMed] [Google Scholar]
- Roberts ES, Tapp T, Trimmer A, et al. Clinical efficacy and safety following dose tapering of ciclosporin in cats with hypersensitivity dermatitis. J Feline Med Surg 2015; 18: 898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson LR, Gregory C. Possible hemolytic uremic syndrome in three cats after renal transplantation and cyclosporine therapy. Vet Surg 1999; 28: 135–140. [DOI] [PubMed] [Google Scholar]
- Bernsteen L, Gregory CR, Aronson LR, et al. Acute toxoplasmosis following renal transplantation in three cats and a dog. J Am Vet Med Assoc 1999; 215: 1123–1126. [PubMed] [Google Scholar]
- Last R, Suzuki Y, Manning T, et al. A case of fatal systemic toxoplasmosis in a cat being treated with cyclosporin A for feline atopy. Vet Dermatol 2004; 15: 194–198. [DOI] [PubMed] [Google Scholar]
- Barrs VR, Martin P, Beatty JA. Antemortem diagnosis and treatment of toxoplasmosis in two cats on cyclosporin therapy. Aust Vet J 2006; 85: 30–35. [DOI] [PubMed] [Google Scholar]
- McCabe RC, Luft BT, Remington JS. The effects of cyclosporine on Toxoplasma gondii in vivo and in vitro. Transplantation 1986; 41: 611–615. [DOI] [PubMed] [Google Scholar]
- Silverman JA, Hayes ML, Luft JB, et al. Characterization of anti-Toxoplasma activity of SDZ 215-918, a cyclosporin derivative lacking immunosuppressive and peptidyl-prolyl-isomerase-inhibiting activity: possible role of a P glycoprotein in Toxoplasma physiology. Antimicrob Agents Chemother 1997; 41: 1859–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin MR, Roycroft LM. Effect of ciclosporin and methylprednisolone acetate on cats previously infected with feline herpes-virus 1. J Feline Med Surg 2015; 17: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortola E, Endo Y, Ohno K, et al. The use of two immunosuppressive drugs, cyclosporin A and tacrolimus, to inhibit virus replication and apoptosis in cells infected with feline immunodeficiency virus. Vet Res Comm 1998; 22: 553–563. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Sato Y, Osawa S, et al. Suppression of feline coronavirus replication in vitro by ciclosporin A. Vet Res 2012; 43: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood S Schick A Lewis T II,et al. Frequency of urinary tract infections in feline patients with dermatological disease receiving long-term glucocorticoids and ciclosporin. Proceedings of the 7th World Congress of Veterinary Dermatology; 2012 July 24-28; Vancouver, Canada, p 33. [Google Scholar]
- Griffin A, Newton AL, Aronson LR, et al. Disseminated Mycobacterium avium complex infection following renal transplantation in a cat. J Am Vet Med Assoc 2003; 22: 1097–1101. [DOI] [PubMed] [Google Scholar]
- Callegari C, Palermo G, Greco MF, et al. Pneumonia associated with Salmonella spp. infection in a cat receiving cyclosporine. Schweiz Arch Tierheilkd 2014; 156: 499–503. [DOI] [PubMed] [Google Scholar]
- Ravens PA, Xu BJ, Vogelnest LJ. Feline atopic dermatitis: a retrospective study of 45 cases (2001–2012). Vet Dermatol 2014; 25: 95–102. [DOI] [PubMed] [Google Scholar]
- Olivry T, Power HT, Woo JC, et al. Anti-isthmus autoimmunity in a novel feline acquired alopecia resembling pseudopelade of humans. Vet Dermatol 2000; 11: 261–270. [Google Scholar]
- Schmiedt CW, Holzman G, Schwarz T, et al. Survival, complications, and analysis of risk factors after renal transplantation in cats. Vet Surg 2008; 37: 683–695. [DOI] [PubMed] [Google Scholar]
- Gregory CR, Gourley IM, Ferreira H, et al. Pathologic studies of acute rejection of mismatched feline musculocutaneous flaps. Effect of cyclosporine and prednisolone. Transplantation 1991; 51: 1170–1175. [DOI] [PubMed] [Google Scholar]
- Wooldridge JD, Gregory CR, Mathews KG, et al. The prevalence of malignant neoplasia in feline renal-transplant recipients. Vet Surg 2002; 31: 94–97. [DOI] [PubMed] [Google Scholar]
- Schmiedt CW, Grimes JA, Holzman G, et al. Incidence and risk factors for development of malignant neoplasia after feline renal transplantation and cyclosporine-based immuno-suppression. Vet Comp Oncol 2009; 7: 45–48. [DOI] [PubMed] [Google Scholar]
- Opelz G, Dohler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplantation 2003; 4:222–230. [DOI] [PubMed] [Google Scholar]
- Joss N, Rodger RS, McMillan MA, et al. Randomized study comparing cyclosporine with azathioprine one year after renal transplantation - 15-year outcome data. Transplantation 2007; 83: 582–587. [DOI] [PubMed] [Google Scholar]
- Roberts ES, VanLare KA, Roycroft LM, et al. Effect of high-dose ciclosporin on the immune response to primary and booster vaccination in immunocompetent cats. J Feline Med Surg 2015; 17: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaguere E, Prelaud P. Efficacy of cyclosporin in the treatment of 12 cases of eosinophilic granuloma complex. Vet Dermatol 2000; 11 Suppl 1: 31. [Google Scholar]
- Nakazato A, Ishikawa H, Takizawa Y, et al. Administration of cyclosporin A to cats with allergic dermatitis: improvement of clinical signs and decrease of peripheral eosinophils in an open pilot study. Vet Dermatol 2006; 17: 215. [Google Scholar]
- Bloom PB. Canine and feline eosinophilic skin diseases. Vet Clin Small Anim 2006; 36: 141–160. [DOI] [PubMed] [Google Scholar]
- Noli C, Ortalda C, Galzerano M. The use of cyclosporine in liquid formulation in the treatment of feline allergic diseass [article in Italian]. Veterinaria 2014; 1–8. [Google Scholar]
- Roberts ES, Speranza C, Friberg C. Confirmatory field study for the evaluation of ciclosporin at a target dose of 7.0 mg/kg (3.2 mg/lb) in the control of feline hypersensitivity dermatitides. J Feline Med Surg 2016; 18: 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan J, Olivry T, Forster S, et al. Responsiveness and validity of the SCOR-FAD, an extent and severity scale for feline hypersensitivity dermatitis. Vet Dermatol 2012; 23: 410–418. [DOI] [PubMed] [Google Scholar]
- Noli C, Cena T. Comparison of FEDESI and SCORFAD scoring systems for the evaluation of skin lesions in allergic cats. Vet Dermatol 2015; 26: 481–485. [DOI] [PubMed] [Google Scholar]
- Rosenkrantz WS, Griffin CE, Barr RJ. Clinical evaluation of cyclosporine in animal models with cutaneous immune-mediated disease and epitheliotropic lymphoma. J Am Anim Hosp Assoc 1989; 25: 377–384. [Google Scholar]
- Guaguere E, Fontaine J. Efficacy of cyclosporin in the treatment of feline urticaria pigmentosa: two cases. Vet Dermatol 2004; 15 Suppl 1: 63. [Google Scholar]
- Fontaine J, Heimann M. Idiopathic facial dermatitis of the Persian cat: three cases controlled with cyclosporine. Vet Dermatol 2004; 15 Suppl 1: 64. [Google Scholar]
- Noli C, Toma S. Three cases of immune-mediated adnexal skin disease treated with cyclosporin. Vet Dermatol 2006; 17: 85–92. [DOI] [PubMed] [Google Scholar]
- Lobetti R. Lymphocytic mural folliculitis and pancreatic carcinoma in a cat. J Feline Med Surg 2015; 17: 548–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linek M, Rufenacht S, Brachelente C, et al. Nonthymoma-associated exfoliative dermatitis in 18 cats. Vet Dermatol 2015; 26: 40–47. [DOI] [PubMed] [Google Scholar]
- Pressanti C, Cadiergues MC. Feline familial pedal eosinophilic dermatosis in two litter-mates. JFMS Open Rep 2015; 1; DOI: 10.1177/2055116915579683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noli C. Extra-label use of cyclosporine. Proceedings of the 6th World Congress of Veterinary Dermatology; 2008 Nov 19-22; Hong Kong, China, pp 251–256. [Google Scholar]
- Gregory CR, Gourley IM, Taylor NJ, et al. Preliminary results of clinical renal allograft transplantation in the dog and cat. J Vet Intern Med 1987; 1: 53–60. [DOI] [PubMed] [Google Scholar]
- Gregory CR, Gourley IM, Broaddus TW, et al. Long-term survival of a cat receiving a renal allograft from an unrelated donor. J Vet Intern Med 1990; 4: 1–3. [DOI] [PubMed] [Google Scholar]
- Mischke R. Cyclosporin A therapy in a cat with pure red cell aplasia. Berl Munch Tierarztl Wochenschr 1998; 111: 432–437. [PubMed] [Google Scholar]
- Stokol T, Blue JT. Pure red cell aplasia in cats: 9 cases (1989-1997). J Am Vet Med Assoc 1999; 214: 75–79. [PubMed] [Google Scholar]
- Viviano KR, Webb JL. Clinical use of cyclosporine as an adjunctive therapy in the management of feline idiopathic pure red cell aplasia. J Feline Med Surg 2011; 13: 885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black V, Adamantos S, Barfield D, et al. Feline non-regenerative immune-mediated anaemia: features and outcome in 15 cases. J Feline Med Surg 2016; 18: 597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon CL, Scott MA, Selting KA, et al. Idiopathic thrombocytopenic purpura in a cat. J Am Anim Hosp Assoc 1999; 35: 464–470. [DOI] [PubMed] [Google Scholar]
- Fidel JL, Pargass IS, Dark MJ, et al. Granulocytopenia associated with thymoma in a domestic shorthaired cat. J Am Anim Hosp Assoc 2008; 44: 210–217. [DOI] [PubMed] [Google Scholar]
- Lyon KF. Gingivostomatitis. Vet Clin Small Anim 2005; 35: 891–911. [DOI] [PubMed] [Google Scholar]
- Lommer MJ. Efficacy of cyclosporine for chronic, refractory stomatitis in cats: a randomized, placebo-controlled, double-blinded clinical study. J Vet Dentistry 2013; 30: 8–17. [DOI] [PubMed] [Google Scholar]
- Padrid PA, Cozzi P, Leff AR. Cyclosporine A inhibits airway reactivity and remodeling after chronic antigen challenge in cats. Am J Resp Crit Care Med 1996; 154: 1812–1818. [DOI] [PubMed] [Google Scholar]
- Mitchell RW, Cozzi P, Ndukwu IM, et al. Differential effects of cyclosporine A after acute antigen challenge in sensitized cats in vivo and ex vivo. Brit J Pharmacol 1998; 123: 1198–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafe LA, Leach SB. Treatment of feline asthma with ciclosporin in a cat with diabetes mellitus and congestive heart failure. J Feline Med Surg 2015; 17: 1073–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oohashi E, Yamada K, Oohashi M, et al. Chronic progressive polyarthritis in a female cat. Vet Med Sci 2010; 72: 511–514. [DOI] [PubMed] [Google Scholar]
- Jergens AE. Feline idiopathic inflammatory bowel disease. What we know and what remains to be unraveled. J Feline Med Surg 2012; 14: 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee MC, Rassnick KM, Bailey DB, et al. Phase I and pharmacokinetic evaluation of the combination of orally administered docetaxel and cyclosporin A in tumor-bearing cats. J Vet Intern Med 2006; 20: 1370–1375. [DOI] [PubMed] [Google Scholar]