Abstract
Overview:
Haemoplasmas are haemotropic bacteria that can induce anaemia in a wide range of mammalian species.
Infection in cats:
Mycoplasma haemofelis is the most pathogenic of the three main feline haemoplasma species known to infect cats. ‘Candidatus Mycoplasma haemominutum’ and ‘Candidatus Mycoplasma turicensis’ are less pathogenic but can result in disease in immunocompromised cats. Male, non-pedigree cats with outdoor access are more likely to be haemoplasma infected, and ‘Candidatus M haemominutum’ is more common in older cats. All three haemoplasma species can be carried asymptomatically.
Transmission:
The natural mode of transmission of haemoplasma infection is not known, but aggressive interactions and vectors are possibilities. Transmission by blood transfusion can occur and all blood donors should be screened for haemoplasma infection.
Diagnosis and treatment:
PCR assays are the preferred diagnostic method for haemoplasma infections. Treatment with doxycycline for 2–4 weeks is usually effective for M haemofelis-associated clinical disease (but this may not clear infection). Little information is currently available on the antibiotic responsiveness of ‘Candidatus M haemominutum’ and ‘Candidatus M turicensis’.

Agent properties
The haemoplasmas are haemotropic bacteria that parasitise red blood cells (Figure 1) and can induce haemolytic anaemia. They are currently classified within the genus Mycoplasma in the Mycoplasmataceae family of bacteria. However, recent work suggests that although the haemoplasmas probably do belong to this family, they may be better placed in their own separate genus. 1 In contrast to many ‘classical’ mycoplasmas, haemoplasmas cannot be cultivated in vitro; their propagation is possible in living animals only.
Figure 1.
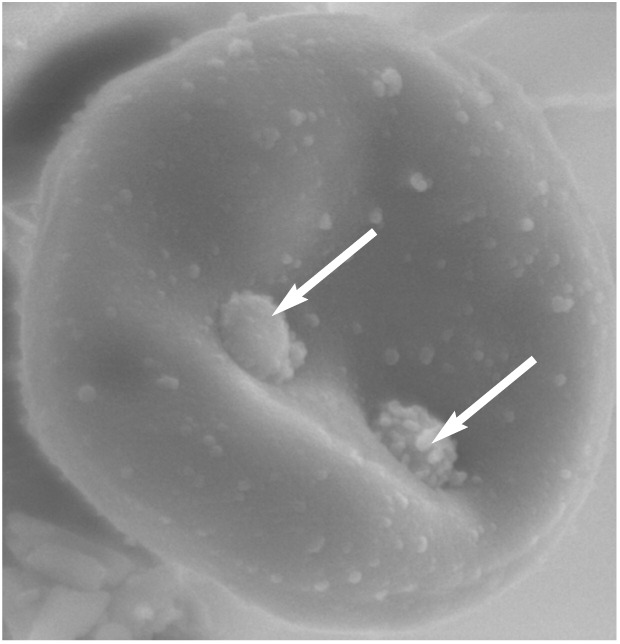
Scanning electron micrograph showing two Mycoplasma haemofelis organisms (arrows) attached to the surface of a feline erythrocyte. Courtesy of Séverine Tasker
The three main haemoplasma species known to infect cats are Mycoplasma haemofelis, ‘Candidatus Mycoplasma haemominutum’ and ‘Candidatus Mycoplasma turicensis’. These mycoplasmas have a worldwide distribution.
Epidemiology
Feline haemoplasma infections are usually more common in male, non-pedigree cats with outdoor access and cat bite abscesses. Infection with ‘Candidatus M haemominutum’ is usually more prevalent in older cats, presumably because cats have an increasing risk of acquiring chronic subclinical infection over their lifetime. Some studies have found an association between haemoplasma infection and feline immunodeficiency virus (FIV) infection,2,3 although others have not. 4 Most studies have failed to show an association between haemoplasma infection and feline leukaemia virus (FeLV) infection.2–4 Overall, variable results have been seen regarding retroviruses as risk factors for haemoplasma infections. Recent epidemiological studies suggest that the host phenotype (eg, aggressive male) may drive some of these associations rather than infections being simple risk factors for each other. 5
In general ‘Candidatus M haemominutum’ is most prevalent in domestic cats (0–46.7% of cats found to be infected in prevalence studies), followed by M haemofelis (0–46.6% of cats) and ‘Candidatus M turicensis’ (0–26.0% of cats). Reported prevalences vary both geographically and also because the populations sampled in different studies are very variable; ie, some test only ill anaemic cats, others sample healthy cats only, some test stray feral cats, whereas others focus on owned cats.
The clustered geographical distribution of infection in some studies supports the role of an arthropod vector in the transmission of haemoplasmas. 6 The cat flea, Ctenocephalides felis, has been implicated in feline haemoplas ma transmission, but only very transient M haemofelis infection has been reported via the haematophagous activity of fleas, and clinical and haematological signs of M haemofelis infection were not induced in the recipient cat in one experimental study. 7 Additionally, a recent study found no evidence of haemo-plasma transmission by fleas in an experiment involving the introduction of fleas into groups of cats housed together. 8
Some observations have suggested that cat fights can result in transmission of the bacteria. Subcutaneous inoculation of ‘Candidatus M turicensis’-containing blood resulted in transmission, whereas the same inoculation method using ‘Candidatus M turicensis’-containing saliva did not. 9 This suggests that haemoplasma transmission by social contact (saliva via mutual grooming, etc) is less likely than transmission by aggressive interaction (blood during a cat bite incident). 9 However, a recent study found evidence of horizontal transmission of ‘Candidatus M haemominutum’, but not M haemofelis, by direct contact between cats in the absence of any apparent significant aggressive interaction or vectors. 8
Blood transfusion is another potential route of transmission, and blood donors should be screened for haemoplasma infection. 10
Pathogenesis
M haemofelis is the most pathogenic feline haemoplasma species. It can result in severe, sometimes fatal, haemolytic anaemia (Figure 2) following acute infection in some cats, although others may develop only mild anaemia. This may be due to differences in host response, or M haemofelis strain variation, but disease can occur in immunocompetent cats. Chronic infection is usually not associated with significant anaemia and carrier cats exist which show no evidence of anaemia. In line with this, some epidemiological studies have not shown associations between anaemia and M haemofelis infection, probably due to the inclusion of chronically infected asymptomatic cats.
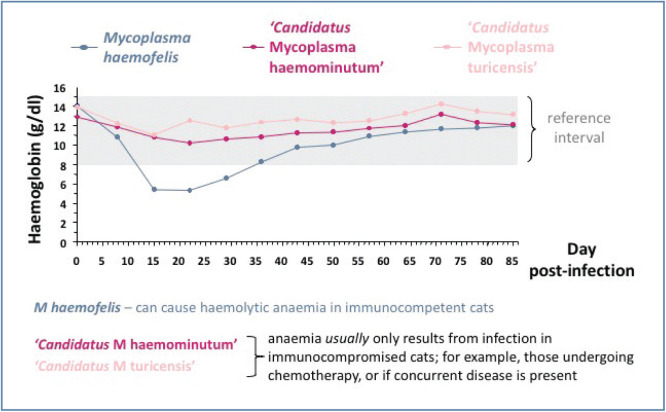
Figure 2.
Graph showing mean haemoglobin values after infection for cats infected with each of the three main feline haemoplasma species. Significant anaemia is only induced in the cats infected with Mycoplasma haemofelis. Although a fall in haemoglobin concentration does occur following infection with both ‘Candidatus Mycoplasma haemominutum’ and ‘Candidatus Mycoplasma turicensis’, anaemia is usually only induced if the infected cat is also immunocompromised. Courtesy of Séverine Tasker
Although ‘Candidatus M haemominutum’ infection can cause erythrocyte parameters (eg, red blood cell count, haemoglobin, haematocrit) to fall (Figure 2), anaemia is not commonly seen following infection unless the cat has concurrent problems; for example, immunosuppression or is undergoing chemotherapy. Many asymptomatic carrier cats of ‘Candidatus M haemominutum’ exist. ‘Candidatus M haemominutum’ has also been associated with the development of myeloproliferative disease in cats with FeLV infection in one experimental study. 11 However, cases of anaemia have been reported in which only ‘Candidatus M haemominutum’ infection was diagnosed and so it appears that in some cases ‘Candidatus M haemominutum’ can cause anaemia in the absence of concurrent disease. 12
‘Candidatus M turicensis’ infection has caused anaemia or a small lowering in erythrocyte parameters in some experimental studies (Figure 2), but generally anaemia is uncommon. Concurrent disease and immunosuppression are both thought to be involved in the pathogenesis of ‘Candidatus M turicensis’ disease, similar to ‘Candidatus M haemominutum’.
Determining the pathogenicity of ‘Candidatus M haemominutum’ and ‘Candidatus M turicensis’ in naturally infected cats can be difficult as coinfections with other haemoplasma species often occur, confounding disease associations.
Carrier cats often have subclinical infections, but reactivation of infection can occur (eg, when a cat has failed to eliminate infection) and may result in clinical disease. 12 A recent study found that cats that had recovered from a previous M haemofelis infection were protected from homologous rechallenge with M haemofelis, confirming the presence of protective immunity, 13 possibly in those that had eliminated the infection. However, another study found that cats that had recovered from previous ‘Candidatus M turicensis’ infection showed more severe and rapid signs of M haemofelis infection than naive cats infected with M haemofelis. 14 Thus more research is required into the relationship between infection with different haemoplasma species and their pathogenesis and immunity.
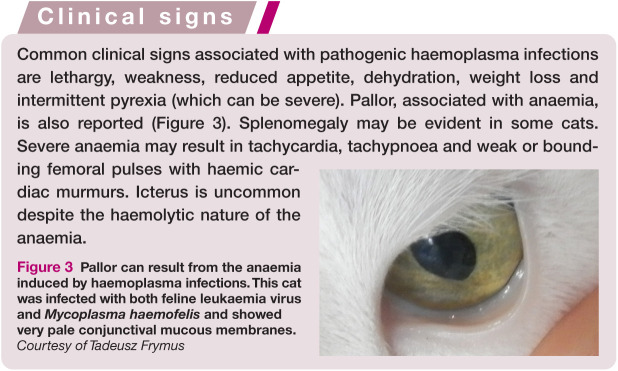
Figure 3.
Pallor can result from the anaemia induced by haemoplasma infections. This cat was infected with both feline leukaemia virus and Mycoplasma haemofelis and showed very pale conjunctival mucous membranes. Courtesy of Tadeusz Frymus
Diagnosis
Pathogenic haemoplasma infections typically cause a regenerative macrocytic hypochromic anaemia, although pronounced reticulocytosis is not always evident. 15 Normoblasts may be present. White blood cell changes may also be seen, including leukopenia, lymphopenia, eosinopenia and monocytosis. Positive Coombs test results can occur, particularly with cold agglutinins, and persistent auto-agglutination has been reported in acute haemoplasmosis, indicating the presence of erythrocyte-bound antibodies. However, in experimental studies 16 these antibodies appear after the development of anaemia. The absence of erythrocyte-bound antibodies at the onset of anaemia could be because the Coombs test is not sensitive enough to detect them in early infection or because erythrocyte-bound antibodies appear as a result of haemoplasma-induced haemolysis, rather than initiating it. Indeed, erythrocyte-bound antibodies disappear with antibiotic and supportive treatment alone, without glucocorticoid treatment.
Hyperbilirubinaemia is seen occasionally, due to haemolysis, and hypoxic liver damage may result in increased activity of alanine aminotransferase. A polyclonal hypergamma-globulinaemia is also sometimes seen.
Cytology of blood smears, stained with Romanowsky-type stains, may reveal haemoplasmas on the surface of erythrocytes but this is known to be very insensitive for diagnosis, and cytology cannot easily differentiate haemoplasma species. The untrained eye may also fail to distinguish stain precipitate and Howell–Jolly bodies from haemoplasmas.
As haemoplasmas do not grow on bacteriological media, in vitro culture is currently not possible.
PCR assays are now the diagnostic method of choice for haemoplasma infection. PCR is far more sensitive and specific than cytology. Quantitative PCR (qPCR) assays (Figure 4) allow quantification of haemoplasma DNA in the sample being analysed, allowing response to treatment to be monitored. It is known that M haemofelis numbers can fluctuate markedly in the blood of some cats for several months following infection; the reason for this is not clear but may be related to antigenic variation. No evidence of significant tissue sequestration of M haemofelis, to explain reduced blood organism numbers, has been found. 17 This is in contrast to ‘Candidatus M turicensis’, where evidence of tissue sequestration was found in PCR-negative cats. 18
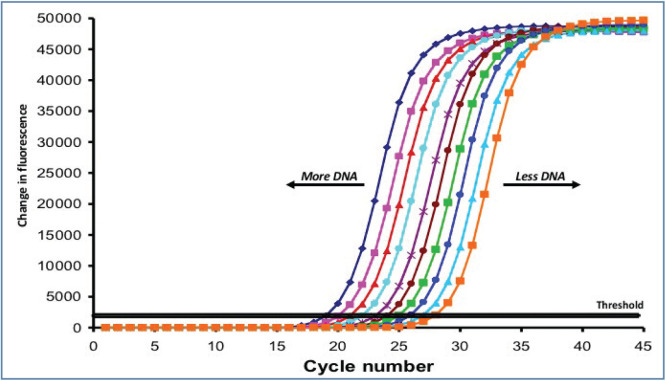
Figure 4.
Quantitative PCR (qPCR) allows the amount of haemoplasma DNA present in a sample to be quantified. Species-specific qPCRs are offered by many diagnostic laboratories. Quantification is performed by measurement of a change in fluorescence in the PCR tube. The more DNA present in the PCR (and thus sample), the earlier a change in fluorescence occurs and reaches the threshold level, as shown in the graph. The cycle number at which each sample reaches the threshold is called the threshold cycle value and this figure may be reported to allow comparison of haemoplasma DNA levels in different samples. Courtesy of Séverine Tasker
Serological tests have been difficult to develop because the inability to culture haemoplasmas in vitro prevents the easy acquisition of significant amounts of haemoplasma proteins for use in serological assays. Such assays are currently only available for use in experimental studies. Based on an M haemofelis DnaK protein, these assays have suggested that antibody levels may differentiate between acute and chronic infection with M haemofelis 19 and have been more sensitive than PCR in detecting haemoplasma exposure (as PCR-negative sero-positive cats have been identified). 20 Issues with cross-reactivity mean that these assays are not yet appropriate for use in field cats.
Treatment
Haemoplasmosis generally has a good prognosis if prompt appropriate treatment is instigated. As haemoplasmas lack a cell wall, [β-lactams (eg, penicillins, cephalosporins) are not effective in the treatment of haemoplasmosis. However, tetracyclines (primarily doxycycline) and fluoroquinolones (eg, marbofloxacin, pradofloxacin) are effective.
Most studies have evaluated the response of M haemofelis to treatment. Doxycycline (10 mg/kg q24h PO or 5 mg/kg q12h PO) is often used as a first-line therapy, typically for 2–4 weeks. Some doxycycline formulations, especially doxycycline hyclate, have been associated with oesophagitis in cats due to their high acidity when they dissolve. Such doxycycline tablets must always be followed by food or water to encourage complete swallowing into the stomach. Other formulations, such as doxycycline monohydrate paste, are far less acidic and take longer to dissolve, and so are associated with fewer side effects. Longer courses of antibiotics are recommended by some to increase the chance of eliminating infection, although these longer treatment courses have not been evaluated for the clearance of infection. One study 21 suggested that pradofloxacin (at two doses; both the standard 5 mg/kg q24h PO, as well as a higher dose of 10 mg/kg q24h PO) may be more effective at clearing M haemofelis than doxycycline. Sometimes dual 22 or sequential therapy with doxycycline and then a fluoroquinolone can be helpful.
It has been found that ‘Candidatus M haemominutum’ infection does not necessarily respond to antibiotics similarly to M haemofelis. In one study 23 ‘Candidatus M haemominutum’ organism numbers in the blood fell only temporarily during marbofloxacin (2 mg/kg q24h PO) treatment, with organism numbers returning to pretreatment levels following completion of 4 weeks of treatment.
The response of ‘Candidatus M turicensis’ to antibiotic treatment has not been fully evaluated but doxycycline can be effective. 9
Corticosteroids have been recommended as adjunct treatment for any immune-mediated component of haemoplasma-associated anaemia, although cats usually recover without requiring corticosteroid treatment, as antibiotic and supportive care alone is usually adequate. Supportive care can be important (correction of dehydration with fluid therapy, and blood transfusion if the anaemia is severe).
Prevention
Blood donors should be screened for haemoplasma infection by PCR in order to prevent inadvertent transmission by blood transfusion from asymptomatic carrier cats. There are no vaccines against feline haemoplasmosis. Keeping cats indoors is likely to prevent infection, as outdoor status has been identified as a risk factor (but may be impractical). Although vector transmission has not been proven, preventive flea and tick treatment is probably wise.
Key Points
Mycoplasma haemofelis is the most pathogenic of the three feline haemoplasma species. ‘Candidatus M haemominutum’ and ‘Candidatus M turicensis’ infections are less pathogenic but can result in disease in immunocompromised cats.
Male, non-pedigree cats with outdoor access are more likely to be infected with haemoplasmas.
‘Candidatus M haemominutum’ is more common in older cats.
The natural mode of transmission of haemoplasma infection is not known; aggressive interactions and vectors are possibilities.
Transmission by blood transfusion can occur and all blood donors should be screened for haemoplasma infection.
PCR assays are the preferred diagnostic method for haemoplasma infections.
Asymptomatic carrier cats exist for all feline haemoplasma species.
Treatment with doxycycline for 2–4 weeks is usually effective for M haemofelis-associated clinical disease (but this may not clear infection).
Little information is currently available on the antibiotic responsiveness of ‘Candidatus M haemominutum’ and ‘Candidatus M turicensis’.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article. The ABCD is supported by Boehringer Ingelheim, but is a scientifically independent body and its members receive no stipends from Boehringer Ingelheim.
References
- 1. Hicks CA, Barker EN, Brady C, et al. Non-ribo-somal phylogenetic exploration of Mollicute species: new insights into haemoplasma taxonomy. Infect Genet Evol 2014; 23. 99-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gentilini F, Novacco M, Turba ME, et al. Use of combined conventional and real-time PCR to determine the epidemiology of feline haemoplasma infections in northern Italy. J Feline Med Surg 2009; 11. 277-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Macieira DB, de Menezes RD, Damico CB, et al. Prevalence and risk factors for hemoplasmas in domestic cats naturally infected with feline immunodeficiency virus and/or feline leukemia virus in Rio de Janeiro, Brazil. J Feline Med Surg 2008; 10. 120-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Willi B, Boretti FS, Baumgartner C, et al. Prevalence, risk factor analysis, and follow-up of infections caused by three feline hemoplas-ma species in cats in Switzerland. J Clin Microbiol 2006; 44. 961-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carver S, Beatty JA, Troyer RM, et al. Closing the gap on causal processes of infection risk from cross-sectional data: structural equation models to understand infection and co-infection. Parasit Vectors 2015; 8. 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sykes JE, Drazenovich NL, Ball LM, et al. Use of conventional and real-time polymerase chain reaction to determine the epidemiology of hemoplasma infections in anemic and non-anemic cats. J Vet Intern Med 2007; 21. 685-693. [DOI] [PubMed] [Google Scholar]
- 7. Woods JE, Brewer MM, Hawley JR, et al. Evaluation of experimental transmission of ‘Candidatus Mycoplasma haemominutum’ and Mycoplasma haemofelis by Ctenocephalides felis to cats. Am J Vet Res 2005; 66. 1008-1012. [DOI] [PubMed] [Google Scholar]
- 8. Lappin MR. Feline haemoplasmas are not transmitted by Ctenocephalides felis. 9th Symposium of the CVBD World Forum. Lisbon, Portugal, 2014, pp 44-46. [Google Scholar]
- 9. Museux K, Boretti FS, Willi B, et al. In vivo transmission studies of ‘Candidatus Mycoplasma turicensis’ in the domestic cat. Vet Res 2009; 40. 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pennisi MG, Hartmann K, Addie DD, et al. Blood transfusion in cats. ABCD guidelines for minimising risks of infectious iatrogenic complications. J Feline Med Surg 2015; 17: 588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. George JW, Rideout BA, Griffey SM, et al. Effect of pre-existing FeLV infection or FeLV and feline immunodeficiency virus coinfection on pathogenicity of the small variant of Haemobartonella felis in cats. Am J Vet Res 2002; 63:1172-1178. [DOI] [PubMed] [Google Scholar]
- 12. Weingart C, Tasker S, Kohn B. Infection with haemoplasma species in 22 cats with anaemia. J Feline Med Surg 2016; 18: 129-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hicks CA, Willi B, Riond B, et al. Protective immunity against infection with Mycoplasma haemofelis. Clin Vaccine Immunol 2014; 22:108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baumann J, Novacco M, Willi B, et al. Lack of cross-protection against Mycoplasma haemofelis infection and signs of enhancement in ‘Candidatus Mycoplasma turicensis’-recov-ered cats. Vet Res 2015; 46: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kewish KE, Appleyard GD, Myers SL, et al. Mycoplasma haemofelis and Mycoplasma haemominutum detection by polymerase chain reaction in cats from Saskatchewan and Alberta. Can Vet J 2004; 45: 749-752. [PMC free article] [PubMed] [Google Scholar]
- 16. Tasker S, Peters IR, Papasouliotis K, et al. Description of outcomes of experimental infection with feline haemoplasmas: copy numbers, haematology, Coombs’ testing and blood glucose concentrations. Vet Microbiol 2009; 139: 323-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tasker S, Peters IR, Day MJ, et al. Distribution of feline haemoplasmas in blood and tissue following experimental infection. Microb Pathog 2009; 47: 334-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Novacco M, Riond B, Meli ML, et al. Tissue sequestration of ‘Candidatus Mycoplasma turicensis’. Vet Microbiol 2013; 167: 403-409. [DOI] [PubMed] [Google Scholar]
- 19. Barker EN, Helps CR, Heesom KJ, et al. Detection of humoral response using a recom-binant heat shock protein 70, DnaK, of Mycoplasma haemofelis in experimentally and naturally hemoplasma-infected cats. Clin Vaccine Immunol 2010; 17: 1926-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Novacco M, Boretti FS, Wolf-Jackel GA, et al. Chronic ‘Candidatus Mycoplasma turicensis’ infection. Vet Res 2011; 42: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dowers KL, Tasker S, Radecki SV, et al. Use of pradofloxacin to treat experimentally induced Mycoplasma hemofelis infection in cats. Am J Vet Res 2009; 70: 105-111. [DOI] [PubMed] [Google Scholar]
- 22. Tasker S. Haemotropic mycoplasmas: what’s the real significance in cats? J Feline Med Surg 2010; 12: 369-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tasker S, Caney SMA, Day MJ, et al. Effect of chronic FIV infection, and efficacy of mar-bofloxacin treatment, on ‘Candidatus Myco -plasma haemominutum’ infection. Microbes Infect 2006; 8: 653-661. [DOI] [PubMed] [Google Scholar]
- 24. Maggi RG, Compton SM, Trull CL, et al. Infection with hemotropic Mycoplasma species in patients with or without extensive arthropod or animal contact. J Clin Microbiol 2013; 51: 3237-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steer JA, Tasker S, Barker EN, et al. A novel hemotropic mycoplasma (hemoplasma) in a patient with hemolytic anemia and pyrexia. Clin Infect Dis 2011; 53: e147-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Santos AP, Santos RP, Biondo AW, et al. Hemoplasma infection in HIV-positive patient, Brazil. Emerg Infect Dis 2008; 14: 1922-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]