Abstract
Gabapentin has been widely used in human medicine to control acute and chronic pain. Although the exact mechanism of action has yet to be determined, its use in veterinary medicine is increasing. The clinical use of gabapentin for analgesia in cats has been reported in review articles and one case report. Managing chronic pain, particularly in the feline patient, poses a challenge to veterinary surgeons. This report details the long-term use of gabapentin for musculoskeletal pain or head trauma in three cats. All cats received gabapentin for several months at an average dose of 6.5 mg/kg q12h. Clinical signs suggestive of pain, such as aggression, avoiding human interaction and loss of appetite, were observed to decrease with the administration of gabapentin, used as part of an analgesia regime or as sole medication. Long-term follow-up with the owners of all cats indicated that satisfactory pain management was achieved, administration was easy and no obvious side effects during the period of administration occurred. We conclude that long-term treatment with gabapentin is of potential benefit in controlling pain in cases of head trauma, as well as musculoskeletal disease. It may provide a valuable adjunct for the management of chronic pain in cats and should be investigated further for its clinical use and safety.
Introduction
Gabapentin was developed originally as an antiepileptic medication. 1 It has gained substantial attention for its role in the treatment of chronic neuropathic pain in humans. 1 Gabapentin binds to the N-type voltage calcium channels in the dorsal root ganglion neurons.2,3 Binding to this receptor may result in a reduction in the release of excitatory neurotransmitters, such as glutamate and substance P, 3 and has a modulating effect on gamma-aminobutyric acid receptors. 4 An intracellular effect leading to a delayed expression of voltage-gated calcium channels at the cell surface resulting in a decreased calcium influx has also been postulated. 4 The antinociceptive effects seem to occur primarily after injury and it has been described that gabapentin interacts with N-methyl-D-aspartate receptors (NMDA) involved in the development of central sensitisation. 5 The relative importance of various actions of gabapentin remain to be elucidated. 2
Treating neuropathic pain in cats is a challenge for veterinary surgeons. The choice of drugs available to treat maladaptive pain disorders in cats is limited; cats have a well-recognised limited ability to metabolise drugs by hepatic glucuronidation and administration of medication by the owner can be challenging. 6 Moreover, to diagnose, reliably assess and score pain in these patients is difficult. 6 The following three cases document long-term analgesic management of cats sustaining multiple musculoskeletal injuries or presenting for insidious lameness using gabapentin as part of the pain management strategy.
Case descriptions
Case 1
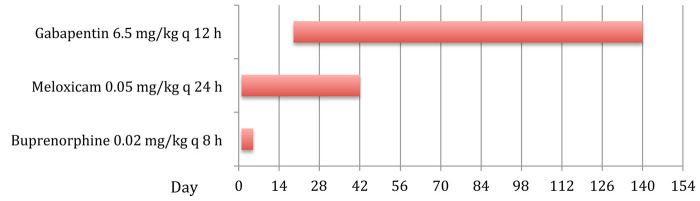
A 3-year-old female neutered domestic shorthair cat suffered multiple skull fractures following a road traffic accident. The fractures were repaired surgically, and the right vertical mandibular ramus and articular process of the temporomandibular joint (TMJ) were removed by the referring veterinary surgeon. An oesophageal feeding tube was placed. Upon presentation at the referral hospital, clinical examination showed malocclusion with lateral displacement of the right mandible. Under sedation the displaced mandible could be reduced to its normal position with a normal range of motion. The cat managed to lick and swallow liquid food and the owners opted for conservative treatment and oesophagostomy tube feeding at home. Pain management consisted of meloxicam (Metacam; Boehringer Ingelheim) 0.05 mg/kg q24h PO and buprenorphine (Vetergesic; Alstoe Ltd for Reckitt Benckiser) 0.02 mg/kg subcutaneously (SC) q8h while hospitalised and a feeding plan was devised. The cat was discharged after 5 days on a continued course of meloxicam 0.05 mg/kg q24h PO. At the reassessment visit 2 weeks after the initial presentation the cat was examined and the following tests were performed: (i) food offered in the consult room — the cat showed strong interest in eating, but appeared unable to prehend the food; (ii) when the cat was observed in its own cage it opened its mouth repeatedly, seemed agitated and shook its head; (iii) manipulation of the mandible while the cat was conscious was not possible because of avoidance behaviour when attempting to approach the head. These observations were taken as an indication of pain; however, a component of malocclusion with an avoidance reaction caused by learned behaviour after the accident cannot be excluded. Changes in diet were advised and surgical intervention was planned should the cat not improve. In addition to the meloxicam, gabapentin 6.5 mg/kg (Nova Laboratories) q12h was administered through the feeding tube. The oesophageal feeding tube had been removed by the referring veterinary surgeon and the meloxicam discontinued after about 6 weeks. Four months after discharge the cat was doing well on continued administration of gabapentin as the only analgesic medication provided (Figure 1). The cat was given approximately 6.5 mg/kg of gabapentin every q12h by sprinkling specially formulated 25 mg capsules (Nova Laboratories Ltd UK) over the food. The owner reported no further episodes of pain and the cat was tolerating soft food, despite the presence of a persistently displaced mandible and malocclusion. After this, the cat was lost to follow-up.
Figure 1.
Chronological order and duration of administered analgesic treatment in case 1
Case 2
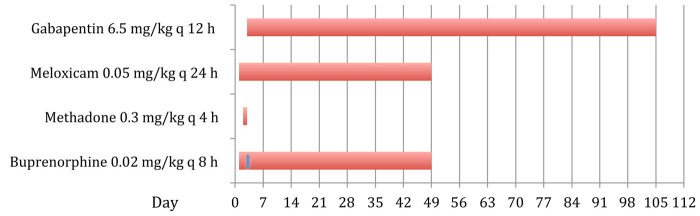
An 8-year-old male neutered Burmese cat was presented with pneumothorax and multiple skull fractures following a road traffic accident 72 h earlier. The referring veterinarian provided emergency treatment, including buprenorphine 0.02 mg/kg intravenously (IV) and an unknown dose of dexamethasone (Dexadreson®; Intervet). On presentation the cat had misalignment of the right mandible to the right with crepitus. Buprenorphine 0.02 mg/kg IV and fluid therapy was continued overnight. A computed tomography (CT) scan under general anaesthesia demonstrated multiple fractures of the mandible and maxilla with small displacement of the fragments and a dislocated TMJ. An oesophageal feeding tube was placed to allow for nutritional support. The mandibular symphyseal fracture was stabilised and a tape muzzle was applied to prevent re-luxation of the TMJ. Owing to partial occlusion of the nasal passages by haemorrhage the tape muzzle was not tolerated. Meloxicam (Metacam; Boehringer Ingelheim) 0.2 mg/kg IV was administered once and continued at 0.05 mg/kg q24h PO. The cat underwent surgery for placement of an external skeletal fixator to stabilise the mouth in a semi-open position. Perioperative analgesia was provided using medetomidine (Domitor; Pfizer), methadone (Physeptone; Martindale Pharmaceuticals), meloxicam and fentanyl (Fentanyl; Martindale Pharmaceuticals). Post-surgical analgesia included 4-hourly methadone 0.3 mg/kg IV q4h and meloxicam 0.05 mg/kg q24h PO. Methadone was substituted with buprenorphine 0.02 mg/kg IV every 8 h after 24 h. The cat was depressed, facing the back wall of the cage and growling when being handled. The administration interval of buprenorphine was changed to q6h, which resolved the ‘growling when handled’. However, the other signs periodically recurred and gabapentin 6.5 mg/kg q12h was added via the feeding tube to provide additional pain relief. The cat improved within 24 h with changes in demeanour, purring when being stroked and interacting with the carer. The buprenorphine was reduced to q8h and stopped after an additional week. The cat was discharged 3 weeks after the surgery on meloxicam 0.05 mg/kg q24h and gabapentin 6.5 mg/kg dispensed as specially formulated 25 mg capsules q12h PO, and the owner was given instructions on how to use the feeding tube. After 5 days inadvertent removal of the oesophagostomy tube at home occurred and the cat was hospitalised for 3 weeks. During this time analgesia was provided using gabapentin 6.5 mg/kg every 12 h, meloxicam 0.05 mg/kg q12h via a new feeding tube and buprenorphine 0.02 mg/kg SC q8h (Figure 2). After a CT scan demonstrating adequate callus formation the external skeletal fixator was removed. The cat showed malocclusion, but was able to eat soft food well and the oesophageal tube was removed. The cat was discharged on gabapentin 6.5 mg/kg q12h PO for 2 weeks. After 1 month the cat was revaluated and, despite the malocclusion, showed no signs of oral laceration and was able to eat soft food. The owner reported episodes where the cat was pawing at his mouth and seemed distressed within the last weeks; these signs were thought to be related to intermittent facial pain. Gabapentin was resumed at 6.5 mg/kg q12h PO with instructions to increase the dose to 13 mg/kg q12h PO should the signs not resolve. On further follow-up the owner reported having administered gabapentin 6.5 mg/kg q12h PO intermittently for 2 months following this visit.
Figure 2.
Chronological order and duration of administered analgesic treatment in case 2. Note that during the 24 h administration of methadone postoperatively, the buprenorphine was discontinued (depicted by the blue line on the buprenorphine bar)
Long-term follow-up at 40 months after initial injury revealed the cat to be doing well without receiving any medication. It was eating and drinking normally. Occasional episodes of clinical signs indicative of orofacial pain were observed with a lower severity and frequency, which did not warrant continued gabapentin therapy, according to the owner. The owner reported no side effects and found it easy to administer gabapentin to the cat.
Case 3
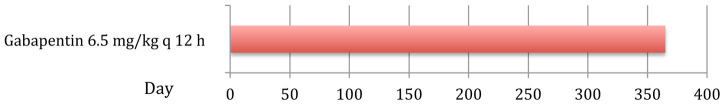
A 9-year-old domestic shorthair cat presented for assessment of a chronic left forelimb lameness of 2 months’ duration. The lameness had been localised to the carpal and elbow region, and was unresponsive to meloxicam. Clinical examination at the referral hospital showed increased weight-bearing on the right forelimb at stance, but subjective gait analysis was hindered by the anxious nature of the cat. Adequate muscle mass and moderate pain of the left carpus and elbow joint were found on palpation. Blood and urine analysis revealed mild elevations of urea and creatinine, with a urine specific gravity of 1.027 and normal urine protein/creatinine ratio. Radiographs performed on the next day were indicative of left carpal joint osteoarthritis, which was supported by the absence of infectious or immune-mediated pathology on synovial fluid analysis. Given the history of azotaemia the meloxicam therapy was discontinued and the cat discharged the next day on gabapentin syrup 40 mg/ml (Nova Laboratories) at a dose of 6.5 mg/kg q12h PO for 4 weeks. A telephone update was obtained after 4 weeks and the owners reported that the clinical signs of lameness had decreased, although they were not completely resolved. Given the positive response it was advised to continue with gabapentin therapy for the next 4–6 weeks and then as required with further episodes of lameness. The owners administered gabapentin syrup (Nova Laboratories) 6.5 mg/kg q12h PO intermittently for 12 months following discharge during episodes of noticeable left forelimb lameness (Figure 3). The owner administered the medication easily and the cat tolerated the drug well, as no obvious side effects were noted. The cat showed no lameness 24 months following discharge and was without medication.
Figure 3.
Chronological order and duration of administered analgesic treatment in case 3
Discussion
The purpose was to report that gabapentin may have a role in the management of chronic pain condition in cats associated with trauma or insidious musculoskeletal pain. In the small number of cases reported here, there were no obvious side effects observed and the owners reported no problems with administration of the drug.
Two of the three cats received other analgesics during the initial pain management plan, including, but not limited to, buprenorphine and meloxicam. Gabapentin was added at a later stage to provide additional analgesia, therefore using analgesic drugs which act at different levels of the pain pathways. Gabapentin was the sole analgesic administered at home for all cases. In case three gabapentin was chosen as an alternative to non-steroidal anti-inflammatory drugs (NSAIDs) owing to the presence of azotaemia. A NSAID was administered in case two despite an unknown dose of dexamethasone being administered within 48 h of referral. While there is a possible concern of administering a NSAID concurrently with a corticosteroid, there is presently no information on the simultaneous use of dexamethasone and meloxicam in the cat. Nevertheless, the reader is cautioned to use these drugs concurrently or within short succession of each other until further data are available.
Gabapentin was administered for 5 (case 1), 3 (case 2) and one (case 3) month(s) continuously and then intermittently for a further 3, 2 and 12 months, respectively, without clinical adverse effects. Long-term follow-up at 24–36 months by telephone interview showed that gabapentin, both in capsule and syrup formation, was tolerated well by all three cats. There were no obvious side effects in any of the cats, even with administration of the drug up to 1 year following discharge, and the owners reported ease of administration. Although there are currently no scientific reports on long-term efficacy of gabapentin in cats, authors of a recent review paper also comment on its usefulness for the treatment of neuropathic pain in cats. 7
Gabapentin has been increasingly proposed as an analgesic for treating various conditions, ranging from perioperative analgesia to treatment of chronic pain in humans.3,8,9 In veterinary medicine gabapentin has been used in dogs and cats anecdotally to treat chronic and perioperative pain. 9 Gabapentin was found to be analgesic in two cats with multiple injuries, when used as part of a multidrug approach to pain management. 10 Other veterinary studies describe the pharmacokinetics in cats 2 and dogs. 11 In a study using a thermal threshold model, gabapentin at doses of 5–30 mg/kg did not prove to have an acute anti-nociceptive effect in cats. 12 However, this model, may not accurately mirror clinical pain, where, commonly, a combination of inflammatory and neuropathic pain is present. The mechanisms of pain in cases reported here were likely a combination of inflammatory and neuropathic pain, particularly with extensive trauma.13,14 This severe acute pain may have contributed to an up-regulation of pain receptors through central sensitisation. 14
Most commonly, gabapentin is recommended for use in persistent chronic pain or neuropathic pain. 7 Hyperalgesia and allodynia are commonly reported features of neuropathic and chronic pain. 15 It is probable that some of the signs observed were due to allodynia, for example, the presence of food in the mouth evoking a painful reaction in two of the cases reported.
Assessment and diagnosis of pain in cats is challenging, as their signs of pain are different from dogs. 7 Pain scoring systems for cats have only recently been validated for acute pain and chronic pain; however, these tools were not available at the time of treatment of the cats reported here.16 –19 Measuring heart rate, respiration rate, blood pressure, plasma cortisol or β-endorphin values have been shown to not be reliable indicators of pain in cats.6,20 Observation of a cat’s behaviour, as well as interaction with the cat by an experienced veterinary surgeon, is the current standard. 6 This pain assessment strategy has been found most useful in various studies looking at pain assessment in cats.20,21 Other commonly used tools to diagnose pain in cats include inferring from human patients or other species with similar conditions, as well as analgesic trials.6,7 Robertson 6 states that a simple descriptive score, visual analogue score (VAS) or dynamic interactive VAS may be used for pain assessment in cats, in addition to monitoring the response to a test dose of analgesic, particularly if there is uncertainty if the patient is truly painful. These pain assessment strategies were employed successfully in case two where the cat showed behaviours established to be indicative of pain. These included growling when being handled, facing the cage wall, being dull and inappetent, and the improvement of these behaviours with administration of gabapentin. The three cats reported here all had conditions deemed painful in humans or other animals, as well as observed signs suggestive of orofacial or musculoskeletal pain (inappetence, inability to prehend food, pawing at the face, lameness, dullness and staring at the cage wall). In all cats, pain was ongoing for more than 8 days and resolution or improvement of signs appeared to coincide with the administration of gabapentin. Although there were no validated scoring systems available at the time of treatment of the cats reported here, we were in agreement with the findings of other studies6,14,21 and felt that these assessment criteria, including the response to analgesic therapy in all three cases offered a good and reliable method to provide adequate analgesia. In the future the use of a validated pain scoring system would be helpful for evaluation of such animals during hospitalisation, as well as post-discharge, using a multidimensional composite pain scale and a numeric rating scale of different behaviours, respectively.16,22 A method incorporating scoring a number of the observed behaviours has been described as Client Specific Outcome Measures (CSOM) in a study on chronic pain in dogs, 22 and may be also useful in scoring chronic pain in cats. Unfortunately, specific owner-based pain assessment was not systematically carried out in these cases.
Other potential benefits of gabapentin as a long-term analgesic include good bioavailability in cats when given orally, with a reported 88.7% with a half-life of 2.8 h. 2 However, variation can occur and food was implicated to influence uptake of oral gabapentin. 2 Cats have a limited ability for hepatic glucuronidation and thus are more susceptible to toxic side effects. There may also be less active drug available if the parent drug requires metabolising to the active compound by this pathway.
Most commonly observed adverse effects in cats include ataxia and sedation. 7 None of these side effects were observed or reported during hospitalisation or reported by owners in any of the cases.
Oral administration of medication is another challenge in cats in general and especially so in animals with orofacial pain. Although gabapentin was first given via feeding tube in two of the cats, all cats eventually took gabapentin formulated either as a powder or syrup over the food without problems.
Conclusions
Gabapentin was shown to be of conceivable, though unproven, benefit for analgesic management of these cases with orofacial or musculoskeletal problems, which were all characterised by chronic pain. No adverse events or morbidity were observed in these animals during the treatment period, suggesting that gabapentin used at 6–7 mg/kg is a well-tolerated dose, even for more extended administration. Further, owners easily manage administration of this drug. As such, gabapentin may be considered as a long-term treatment for the management of chronic pain in cats. However, more research is needed to confirm and extend these observations, and determine the safety of gabapentin with long-term administration.
Acknowledgments
The authors would like to acknowledge the contribution of Nick Macdonald, who was involved in the primary care of two of the reported cases, the nursing, anaesthesia and imaging staff at the SATH, as well as the referring veterinary surgeons.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors do not have any potential conflicts of interest to declare.
Accepted: 15 November 2012
References
- 1. Wiffen PJ, McQuay HJ, Edwards JE, Moore RA. Gabapentin for acute and chronic pain. Cochrane Database Syst Rev 2005: CD005452. [DOI] [PubMed] [Google Scholar]
- 2. Siao KT, Pypendop BH, Ilkiw JE. Pharmacokinetics of gabapentin in cats. Am J Vet Res 2010; 71: 817–821. [DOI] [PubMed] [Google Scholar]
- 3. Gilron I. Gabapentin and pregabalin for chronic neuropathic and earlypostsurgical pain: current evidence and future directions. Curr Opin Anaesthesiol 2007; 20: 456–472. [DOI] [PubMed] [Google Scholar]
- 4. Wagner AE, Mich PM, Uhrig SR, et al. Clinical evaluation of perioperative administration of gabapentin as an adjunct for postoperative analgesia in dogs undergoing amputation of a forelimb. J Am Vet Med Assoc 2010; 236: 751–756. [DOI] [PubMed] [Google Scholar]
- 5. Pozzi A, Muir III WW, Traverso F. Prevention of central sensitization and pain by N-methyl-D-aspartate receptor antagonists. J Am Vet Med Assoc 2006; 228: 53–60. [DOI] [PubMed] [Google Scholar]
- 6. Robertson SA. Managing pain in feline patients. Vet Clin North Am Small Anim Pract 2008; 38: 1267–1290. [DOI] [PubMed] [Google Scholar]
- 7. Robertson SA, Lascelles BDX. Long-term pain in cats: how much do we know about this important welfare issue? J Feline Med Surg 2010; 12: 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tiippana EM, Hamunen K, Kontinen VK, et al. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety. Anesth Analg 2007; 104: 1545–1556. [DOI] [PubMed] [Google Scholar]
- 9. Lamont LA. Adjunctive analgesic therapy in veterinary medicine. Vet Clin North Am Small Anim Pract 2008; 38: 1187–1203. [DOI] [PubMed] [Google Scholar]
- 10. Vettorato E, Corletto F. Gabapentin as part of multi-modal analgesia in two cats suffering multiple injuries. Vet Anaesth Analg 2011; 38: 518–520. [DOI] [PubMed] [Google Scholar]
- 11. Vollmer KO, von Hodenberg A, Kolle EU. Pharmacokinetics and metabolism of gabapentin in rat, dog and man. Arzneimittelforschung 1986; 36: 830–839. [PubMed] [Google Scholar]
- 12. Pypendop BH, Siao KT, Ilkiw JE. Thermal antinociceptive effect of orally administered gabapentin in healthy cats. Am J Vet Res 2010; 71: 1027–1232. [DOI] [PubMed] [Google Scholar]
- 13. Muir III WW. Mechanisms of pain and their therapeutic implications. J Am Vet Med Assoc 2001; 219: 1346–1356. [DOI] [PubMed] [Google Scholar]
- 14. Wiese AJ MIW, Wittum TE. Characteristics of pain and response to analgesic treatment in dogs and cats examined at a veterinary teaching hospital emergency service. J Am Vet Med Assoc 2005; 226: 2004–2009. [DOI] [PubMed] [Google Scholar]
- 15. Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev 2009; 89: 707–758. [DOI] [PubMed] [Google Scholar]
- 16. Brondani JT, Luna SPL, Podavani CR. Refinement and initial validation of a multidimensional composite scale for use in assessing acute postoperative pain in cats. Am J Vet Res 2011; 72: 174–183. [DOI] [PubMed] [Google Scholar]
- 17. Benito J, et al. Development of a questionnaire to assess chronic pain associated with feline degenerative joint disesae. In: Association of Veterinary Anaesthetists Spring Meeting, 22–23 March 2012, Davos, Switzerland, University of Zürich, pp 22. [Google Scholar]
- 18. Benito J, et al. Owner-assessed indices of quality of life in cats and the relationship to the presence of chronic pain-DJD. In: Association of Veterinary Anaesthetists Spring Meeting, 22–23 March 2012, Davos, Switzerland, University of Zürich, pp 61. [Google Scholar]
- 19. Zamprogno H, Hansen BD, Bondell HD, et al. Item generation and design testing of a questionaire to assess degenerative joint disease-associated pain in cats. Am J Vet Res 2010; 71: 1417–1424. [DOI] [PubMed] [Google Scholar]
- 20. Cambridge AJ, Tobias KM, Newberry RC, et al. Subjective and objective measurements of postoperative pain in cats. J Am Vet Med Assoc 2000; 217: 685–690. [DOI] [PubMed] [Google Scholar]
- 21. Muir WW, Wiese AJ, Wittum TE . Prevalence and characteristics of pain in dogs and cats examined as outpatients at a veterinary teaching hospital. J Am Vet Med Assoc 2004; 224: 1459–1463. [DOI] [PubMed] [Google Scholar]
- 22. Lascelles BDX, Gaynor JS, Smith ES, et al. Amantadine in a multimodal analgesic regimen for alleviation of refractory osteoarthritis pain in dogs. J Vet Intern Med 2008; 22: 53–59. [DOI] [PubMed] [Google Scholar]