Abstract
Objectives
The aim of this clinical evaluation was to describe the technique and outcomes of negative pressure wound therapy (NPWT) augmented skin grafting in cats.
Methods
Cats with soft tissue and skin defects (n = 6) underwent open wound management. Wounds were initially covered using a NPWT system that was changed to polyurethane foam dressing once infection was controlled and granulation started. Final closure was achieved after establishment of a healthy, fully granulated wound bed by grafting of free full-thickness skin from the lateral abdominal wall. The freshly grafted skin was then treated with an NPWT dressing at a pressure of –125 mmHg for 3 days, with dressing changes performed daily. Percentage graft take, complications, wound bioburden and cosmetic outcome were recorded.
Results
The mean duration of open wound management was 21.4 days (range 3.0–45.0 days), with a mean duration of NPWT of 8.0 days (range 3.0–14.0 days). Five cats received a single graft, while one cat had five grafts transferred to the right hindlimb. In 7/10 grafts, graft take was 100%, in two grafts take was 95% and in one graft take was 80% (mean take rate 97%). Therapy was well tolerated in all patients. The grafted site displayed normal hair regrowth in four cats, sparse hair regrowth in one and no hair growth at all in one patient. Skin sensation was normal in all grafted patients.
Conclusions and relevance
Skin graft augmentation using NPWT in cats is a feasible option that allows graft fixation, even in anatomically demanding areas. Graft take rate reported here is slightly higher than documented in previous reports.
Introduction
The process of free skin grafting in cats has been described for reconstruction of lower limb defects in two studies.1,2 The main indication described in these reports is reconstruction of the lower extremity.1,2 Such injuries are frequently seen after road traffic accidents and include degloving injuries (skin avulsed from subdermal attachments) and, yet more severe, shear injuries, which frequently result in more extensive damage to soft tissue and bony structures. 3 Owing to the massive tissue loss and the frequently high contamination of such injuries, extensive wound care is recommended before reconstruction can be begin. 3 The limited mobility of skin in the lower extremity precludes local advancement techniques, and second-intention healing frequently results in fragile skin and extensive scarring. 3 Alternatives, such as skin expanders and distant direct and indirect flaps, have been described. 3 While distant flap techniques offer a reliable reconstruction method, they require multiple surgical procedures.4,5 Free skin grafts are the simplest reconstruction technique and require only a single surgical procedure, and thus are frequently advocated as the method of choice in cats with skin loss at the extremities. 3 Movement of the graft or accumulations of fluid are to be avoided, as they inhibit the process of plasmatic imbibition, which nourishes the graft during the first 48 h. 3 Augmenting skin grafts with negative pressure wound therapy (NPWT) has proven to increase graft take in humans and dogs by securing the graft to the underlying wound bed and removing accumulating fluid.6–10 In addition, the first published report of NPWT in veterinary medicine in 2007 described the successful treatment of an extensive wound in a cat using NPWT with concurrent skin grafting. 11 However, the exact technique was not described in their report. The purpose of this clinical study is to describe the technique and outcome of negative pressure augmented skin grafting in six cats.
Materials and methods
Six cats that were presented to the clinic with skin defects which required grafting between January 2013 and May 2014 were included in the study (Table 1).
Table 1.
Summary of the results of six cats treated with negative pressure wound therapy (NPWT) augmented full-thickness skin grafts
| Cat breed | Sex | Age (years) | Weight (kg) | Wound | Total duration of open wound treatment before grafting (days) | Duration of open wound treatment with NPWT (days) | Graft | Complications during NPWT augmented graft procedure | Protective bandage after NPWT (days) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| DSH | MN | 11.1 | 4.0 | Skin defect after NF, antebrachium | 25 | 5 | 1 full-thickness graft, abdominal wall | None | 3 | 100% take, normal hair growth, normal sensation |
| DSH | MN | 7.4 | 4.7 | Degloving injury, metatarsus | 21 | 7 | 1 full-thickness graft, abdominal wall | None | 10 | 100% take, no hair growth, normal sensation |
| Maine Coon | MN | 2.0 | 5.0 | Crush injury right hindlimb (multiple skin necrosis) | 43 | 10 | 5 full-thickness grafts, abdominal wall | None | 15 | 100% take all grafts, sparse hair growth, normal sensation |
| DSH | FS | 1.1 | 3.5 | Unknown trauma abdominal wall | 3 | 3 | 1 full-thickness graft, abdominal wall | Malodorous secretion at day 3 | None | 80% take, normal hair growth, normal sensation |
| DSH | MN | 10.4 | 5.8 | Fat tissue necrosis, mammary complex five, both sides, unknown origin | 19, grafting was performed owing to low tendency of secondary healing by day 19 | 9 | 1 full-thickness graft, abdominal wall | Minor dressing leakage, could be addressed awake | None | 95% take, normal hair growth, normal sensation |
| DSH | MN | – | – | Fat tissue necrosis after car accident and abdominal wall avulsion mammary complex five, both sides | 14 | 14 | 1 full-thickness graft, abdominal wall | None | None | 95% take, normal hair growth, normal sensation |
DSH = domestic shorthair; MN = male neutered; FS = female spayed; NF = necrotising fasciitis
Open wound management
Wounds were treated while adhering to the basic principles of open wound management. After surgical debridement the wounds were lavaged using 0.1 polyhexanide/betaine surfactant solution (Prontovet; B Braun). In cases where Pseudomonas aeruginosa was detected, the antiseptic was changed to 1% acetic acid (acetic acid diluted in sterile NaCl solution; B Braun). All wounds were initially covered with a NPWT dressing (Greyfoam and ActiVac; KCI Medical) and treated by application of a continuous vacuum of –125 mmHg until clinical signs of infection resolved and a well-vascularised granulation tissue had begun to form. Depending on the amount of granulation, wounds were either covered by a silver-coated foam dressing after NPWT (Acticoat Silver; Smith and Nephews) or directly grafted. Dressing changes were performed under general anaesthetic every 2–3 days. Fluid accumulation, acceptance of the therapy and any complications during therapy were recorded. Multiple bacterial cultures were obtained over the course of the treatment to document the progression of bacterial wound colonisation. All cats received systemic clavulanic acid-potentiated amoxicillin (Synulox, 20 mg/kg q12h; Zoetis) as an initial antibiotic. Systemic antibiotic therapy was adapted or stopped, depending on the condition of the wound, if the results of microbial testing indicated resistance to this antibiotic during the open wound management period. Analgesia was achieved by administration of fentanyl (Fentadon, 25 µg transdermal patch; Albrecht/Durogesic) for the first 10 days of healing.
NPWT augmented skin grafting
Skin grafting was performed if local tissue advancement techniques had failed or were precluded owing to low mobility of the remaining skin around the defect. The grafting procedure was performed once the wound appeared stable and displayed granulation without signs of infection. Full-thickness grafts were harvested from the lateral abdominal wall. The size of the recipient defect was determined by the use of a sterile template and marked at the donor site. The recipient site was then covered using a polyhexanide-moistened gauze. The donor site was closed primary, and the excised graft was freed from all subcutaneous tissue and meshed using a No. 11 scalpel blade. The graft was then transferred to the recipient site, with regard being taken to the direction of hair growth, and secured to the defect with single interrupted sutures (Premilene 3.0; B Braun). The sutured graft was covered with a single layer of non-adherent gauze (Adaptic; Systagenix) and the vacuum dressing was applied on top (Greyfoam; KCI Medical). After sealing of the foam by plastic foil and application of the pressure transducer (Trac Pad; KCI Medical) the vacuum device was connected and a continuous vacuum of –125 mmHg was established (ActiVac; KCI Medical). The vacuum dressing was changed, with the patients awake, once daily for 3 days, and the vitality of the graft, as well as the amount of secretions and acceptance of therapy, were recorded. After removal of the NPWT dressing at day 3, if the location of the grafted site allowed, an additional bandage consisting of a contact layer of non-adherent gauze (Adaptic; Systagenix), cotton (Artiflex; BSN) and an elastic bandage (Peha-haft; Hartmann) was applied to protect the graft until the meshed incisions had healed. Splinting was not performed in any of the patients. Bandage changes were performed every other day with the patients awake. Antibiotic treatment using clavulanic acid-potentiated amoxicillin (20 mg q12h) was continued for 5 days after grafting. Analgesia was achieved by the application of transdermal fentanyl for 4 days post-grafting. Graft take was determined, as previously described,1,2 as percentage of total graft area (Table 1).
Follow-up
A clinical follow up was performed in all patients at day 10 after grafting. Five cats were additionally presented to the clinic a month after grafting. The sixth cat was not presented again. In addition, owners were consulted by phone 3 months after the grafts had healed in order to estimate the hair growth and sensitivity of the grafted skin.
Recorded data
Numerical data are expressed as mean and range.
Results
Of the six cats, five were domestic shorthairs and one was a Maine Coon. Five cats were neutered males and one was a spayed female. Mean age was 6.0 years (range 1.1–11.1 years) and mean weight was 4.8 kg (range 3.5–5.8 kg). More details for all patients are provided in Table 1. Two wounds were traumatic in origin, while the remaining four were the result of infection. Four patients had no major concurrent injuries and showed only mild abnormalities in complete blood count and routine blood chemistry (mild leukocytosis) during the course of treatment. One patient was initially presented with severe necrotising fasciitis of the antebrachium and displayed a regenerative anaemia (haematocrit [HCT] 21%) at the time of grafting. The sixth patient had sustained a polytrauma, including a traumatic abdominal wall avulsion, fractures of the pubis and ischium, and a sacroileal subluxation, that had been previously treated. The cat proceeded to develop a fat tissue necrosis, including both last mammary complexes, which resulted in major skin loss in this region. At the time of grafting this cat also had a regenerative anaemia (HCT 28.8%) but was otherwise recovering well from its injuries.
Wound management
All cats displayed local signs of infection (swelling, pain, malodorous secretion, necrosis) at initiation of open wound therapy. These symptoms decreased during the first 10 days of treatment (Figure 1). Details regarding the bacterial status of the wounds during treatment are presented in Table 2. Open wound therapy was performed using NPWT initially in all cats (mean duration of open wound therapy until grafting was 21.4 days [range 3.0–43.0 days]) with a mean duration of NPWT therapy of 8.0 days (range 3.0–14.0 days). Fluid production ranged between 10 and 30 ml/day. Therapy was well tolerated in all patients (Figure 2). Five of six cats were initially treated using amoxicillin/clavulanic acid, and, in the light of previous bacterial culture results taken at the time of pelvic fracture repair, one cat was treated with doxycycline. Based on the results of the bacterial culture during open wound management, antibiotics were changed to marbofloxacin in three cats.
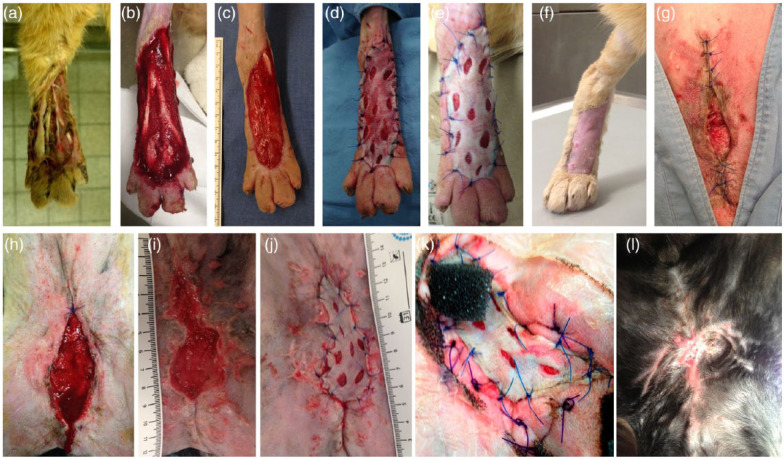
Figure 1.
Examples of two patients undergoing negative pressure wound therapy (NPWT) augmented skin grafting. (a) Initial situation; (b) wound condition after NPWT treatment (day 7); (c) wound condition at the time of grafting (day 21); (d) appearance of the graft directly after full-thickness grafting; (e) at day 3 after grafting; and (f) at day 10 after grafting in a patient with an extremity wound. Free grafting was performed in the patient with fat tissue necrosis after an unsuccessful attempt at closure by local tissue advancement, and owing to a very slow tendency to heal by secondary intention. (g) Initial situation of a wound after fat tissue necrosis; (h) wound condition after NPWT treatment (day 9); (i) wound condition at the time of grafting (day 19); (j) appearance of the graft directly after full-thickness grafting, (k) at day 3 after grafting; and (l) at day 10 after grafting
Table 2.
Summary of bacterial status of the wounds and antibiotic treatment during therapy
| Cat breed | Sex | Age (years) | Weight (kg) | Initial bacterial status of wound | Bacterial status during open wound management | Antibiotic treatment during open wound management | Bacterial status at time of grafting | Antibiotic treatment after grafting |
|---|---|---|---|---|---|---|---|---|
| DSH | MN | 11.1 | 4.0 |
Streptococcus canis
Clinically infected |
Escherichia coli MR Enterobacter species MR |
Amoxicillin/clavulanic acid changed to marbofloxacin | Staphylococcus epidermidis | Amoxicillin/clavulanic acid |
| DSH | MN | 7.4 | 4.7 |
Staphylococcus aureus
Pasteurella multocida E coli MR Enterococcus faecium MR Clostridium perfringens Clostridium baratii Clostridium sporogenes Clinically infected |
Enterobacter subspecies MR Pseudomonas species MR S aureus |
Amoxicillin/clavulanic acid changed to marbofloxacin | E coli | Amoxicillin/clavulanic acid |
| Maine Coon | MN | 2.0 | 5.0 |
Pseudomonas aeruginosa MR E coli MR Enterococcus casseliflavus MR Clinically infected |
P aeruginosa MR E coli MR Enterococcus faecium MR Staphylococcus felis Signs of clinical infection resolved during therapy |
Amoxicillin/clavulanic acid changed to marbofloxacin Wound lavage with 1% acetic acid |
Negative culture | Amoxicillin/clavulanic acid |
| DSH | FS | 1.1 | 3.5 |
P aeruginosa
E coli Enterococcus species Clinically infected |
Amoxicillin/clavulanic acid No clinical signs of infection |
Amoxicillin/clavulanic acid | P aeruginosa | Amoxicillin/clavulanic acid Local lavage with 1% acetic acid |
| DSH | MN | 10.4 | 5.8 |
S aureus MR Clinically infected |
S aureus MR No clinical signs of infection |
Doxycycline | S aureus | Discontinued |
| DSH | MN | – | – |
Enterococcus species clinically infected |
Negative culture No clinical signs of infection |
Amoxicillin/clavulanic acid | Negative culture | Amoxicillin/clavulanic acid |
DSH = domestic shorthair; MN = male neutered; FS = female spayed; MR = resistance to more than three major antibiotic classes
Figure 2.

Negative pressure wound therapy was well tolerated by all patients
Skin grafting
Skin grafting was uneventful in all cats, five cats were treated with one graft each, while the sixth cat required five separate grafts for defect coverage. The NPWT device was well tolerated and daily dressing changes during the first 3 days after grafting were performed with the patients awake. The grafts showed fast incorporation and granulation. Nine of ten grafts were tightly attached to the underlying tissue and granulation of the meshed incisions had already reached the skin level by day 3 (Figure 1). Fluid accumulation was minor and did not exceed 10 ml during the whole treatment duration in any patient. Vacuum leakage occurred twice in one of the cats owing to the close proximity of the wound to the penis, leaving very little skin for fixation of the NPWT dressing. Leakage was addressed by re-application of the dressing with the patients awake.
An additional soft padded, dry protective bandage was applied in the three cats with extremity wounds (mean duration 9.3 days [range 3–15 days]). In the other cases, the graft was left without a bandage as the location did not allow placement of an effective immobilising bandage. Graft take was 100% in seven grafts in three cats (extremities), 95% in the two cats that had been grafted after fat tissue necrosis and 80% in one graft (mean take rate 97%) at follow-up. Partial graft necrosis occurred owing to lift-off of the graft in the caudal area between the hindlimbs and in the cat that had been grafted 3 days after massive injury, where the pseudomonas infection was not addressed successfully before graft application. This graft transferred to the lateral abdominal wall displayed signs of necrosis onset and a malodorous light-green discharge at day 3 associated with the pseudomonas infection.
According to owner information obtained from the telephone follow-up at 3 months post-graft application, the grafted site displayed normal hair regrowth in four cats, sparse hair regrowth in one and no hair growth at all in one patient (Figure 1). Skin sensation was normal in all grafted patients.
Discussion
Full-thickness skin grafts are easy to harvest and require no specialised equipment. In contrast to split-thickness skin grafts, the donor site is closed after graft removal, resulting in low donor site morbidity and pain. Full-thickness skin grafts include the entire dermis and provide robust wound coverage.1,12 In contrast to split-thickness grafts, full-thickness grafts can display normal hair growth and skin texture, and experience less graft shrinkage and donor site morbidity.12–14 However, the increased thickness of the graft compared with the split-thickness method can impair early graft incorporation. The ‘take rate’ of full-thickness skin grafts in cats has been reported in two studies – performed by Shahar et al (mean take 85%, range 0–100%) 1 and Siegfried et al (mean take rate 94%, range 90–100%) 2 – and seems to be higher than graft take rates reported for the dog. 10 The current finding is attributed to the fact that cat skin is thinner and thus allows more effective incorporation and revascularisation.1,12 In both studies, only grafts to the extremities, where bandaging is easy, were documented.1,2 The take rate reported in this study (97%, range 80–100%) is slightly higher than in previous reports. This is especially interesting with respect to the localisation of the grafts. Three cats described in this study underwent skin grafts to the abdominal wall and to the area of the caudal mammary complexes. Normally, owing to relatively high mobility of the skin in this area, these locations can be easily reconstructed using local tissue transfer 5 . However, in the current cases, the extent of local tissue loss due to massive trauma and infection resulted in limited availability of skin, despite the wound location. In addition, the high amount of movement, especially between the hindlimbs, made the graft locations especially demanding in terms of secure skin graft fixation. This would have been impossible at these locations using a conventional bandage. Early graft vitality is dependent on diffusion of nutrients, a process called plasmatic imbibition.7,10 The graft is connected to the underlying tissue by a fibrin network until a vascular supply is established.2,5,12 Vessel ingrowth starts around day 3 and circulation should be complete within 4–7 days.7,10 Movement – especially shear forces – or fluid accumulation under the graft interfere with revascularisation and are among the main reasons for graft necrosis.1,12 Proper surgical technique, including meticulous haemostasis, meshing of the graft and application of a stabilising dressing can aid in avoidance of these effects, but the application of such dressings is demanding, and improper application resulting in uneven pressure distribution or slipping of bandages can hinder success.1,7 In contrast to conventional bandages, the NPWT dressing can be applied even over demanding areas. It delivers uniform pressure to the graft and allows uniform fixation of the graft on uneven surfaces. Several human case studies indicate that NPWT dressing makes the splinting of affected areas redundant. 14
NPWT augmentation has been proven to increase free graft take in numerous experimental studies and clinical trials in human medicine, and in one experimental study in the dog.6–10 Senchenov et al even achieved a take rate of 95% in areas that had undergone radiation therapy in humans (standard non-NPWT take rate was described as 0–70%). 15 There is still some debate regarding the mechanisms by which NPWT increases graft healing. Saaiq et al showed that the pretreatment of the graft bed by vacuum therapy alone increased graft take in humans. 16 There are generally two supposed main effects: the active stimulation of tissue proliferation and revascularisation by mechanotransduction, upregulation of epithelial transcription factors, angiogenesis and microcirculatory flow, and increase of basement membrane integrity; and the prevention of complications such as graft lift, exudate accumulation or shear stress by improved mechanical stabilisation of the graft. 6 Kim and Hong proved histologically that NPWT-augmented grafts showed a tight adhesion of the skin to the underlying tissues by day 3, in contrast to conventional bolstered grafts, where there were still tissue gaps present. 14 Our results mirror this finding as grafts were securely adapted to the underlying tissue by day 3. Becker et al demonstrated faster vessel regeneration in NPWT-augmented grafts, but found that oxygenation of the grafts under NPWT therapy (–125 mmHg) was inferior to controls during the first 3 days. 7
In the study performed by Stanley et al the success rate of free grafts augmented with NPWT in dogs was significantly higher than in control dogs. The authors observed early graft fixation and granulation of the meshed incisions. 10 These findings are similar to the results reported in this study in cats. Apart from the species, there is one major difference between the approach of Stanley et al and the one outlined in this study. Stanley et al used a negative pressure of –65 mmHg, 10 while grafts in this study were treated using –125 mmHg. The majority of studies describing NPWT treatment of skin grafts in human literature use –125 mmHg,6–8,14,17 but lower pressures ranging from –30 to –75 mmHg have also been used successfully.7,14 No study to date has investigated the effects of varying pressure treatment in NPWT. Lower pressures are generally indicated in patients where higher pressures are not well tolerated. 7 None of the cats included in this study displayed signs of discomfort during the NPWT treatment – neither before nor after grafting – and there was thus no reason for pressure reduction.
We stopped NPWT in all cats after 3 days, as all grafts were firmly attached to the underlying surface by this time, and granulation had already achieved skin level. Thus, we had no reason for further NPWT treatment. The dressing changes were well tolerated in all patients and performed awake. In one cat, a malodor and slimy discharge was noted on the last day of NPWT treatment. This was in the patient with the pseudomonas infection. All wounds were cultured positive during the course of wound healing, but despite all wounds being clinically infected at the beginning of treatment, they all displayed healthy granulation over the course of treatment, and could thus be classified as contaminated rather than infected. The usage of systemic antibiotics in open wound care has been controversially discussed.1,2 All of our patients were systemically treated in the beginning, with adjustment of antibiotic treatment based on culture results. This did not prevent bacterial contamination of the wounds, but all patients developed resistant bacteria. NPWT is an effective wound dressing: combined with adequate wound care, lavage and timely dressing changes it is likely that infection could have also been controlled without the use of systemic antibiotics, but we have no control group for comparison. To further elucidate this aspect, further studies examining the effect of NPWT on bacterial colonisation of wounds in dogs and cats are warranted.
One major drawback in this study is the fact that we did not include a control group. We were thus forced to compare our results with previously published data. It would be desirable to design a randomised prospective trial comparing the influence of different wound dressings regarding graft take and practicability to elucidate this matter further. Another drawback is the fact that not all of the cats were evaluated after day 10 and that there was no ‘late’ follow-up, despite owner consultation by telephone.
Conclusions
NPWT has proven to be very effective in graft augmentation in humans and dogs, and our results indicate that this also holds true for feline patients. Therapy was well tolerated in all patients, and mean graft take was 97%. It was possible to use the NPWT even in difficult locations. Further studies including control groups are needed to evaluate the true effect on perfusion and graft incorporation under clinical conditions.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Accepted: 5 January 2015
References
- 1. Shahar R, Shamir MH, Brehm DM, et al. Free skin grafting for treatment of distal limb skin defects in cats. J Small Anim Pract 1999; 40: 378–382. [DOI] [PubMed] [Google Scholar]
- 2. Siegfried R, Schmökel H, Rytz U, et al. Treatment of large distal extremity skin wounds with autogenous full-thickness mesh skin grafts in 5 cats. Schweiz Atch Tierheilkd 2004; 146: 277–283. [DOI] [PubMed] [Google Scholar]
- 3. Corr S. Intensive, extensive, expensive: management of distal limb shearing injuries in cats. J Feline Med Surg 2009; 11: 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spodnick GJ, Pavletic MM, Clark GN, et al. Controlled tissue expansion in the distal extremities of dogs. Vet Surg 1993; 22: 436–443. [DOI] [PubMed] [Google Scholar]
- 5. Pavletic MM. Skin flap and skin grafting techniques in small animal surgery. Vet Q 1997; 19 Suppl 1: 24–25. [DOI] [PubMed] [Google Scholar]
- 6. Azzopardi EA, Boyce DE, Dickson WA, et al. Application of topical negative pressure (vacuum-assisted closure) to split thickness skin grafts. Ann Plast Surg 2013; 70: 23–29. [DOI] [PubMed] [Google Scholar]
- 7. Becker ST, Rennekampff HO, Alkatout I, et al. Comparison of vacuum and conventional wound dresings for full thickness skin grafts in the minipig model. Int J Oral Maxillofac Surg 2010; 39: 699–704. [DOI] [PubMed] [Google Scholar]
- 8. Blume PA, Key JJ, Thakor P, et al. Retrospective evaluation of clinical outcomes in subjects with split thickness skin graft: comparing VAC therapy and conventional therapy in foot and ankle reconstructive surgeries. Int Wound J 2010; 7: 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Llanos S, Danilla S, Barraza C, et al. Effectiveness of negative pressure closure in the integration of split thickness skin grafts. A randomized, double masked, contolled trial. Ann Surg 2006; 244: 700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stanley BJ, Pitt KA, Weder CD, et al. Effects of negative pressure wound therapy on healing of free thickness skin grafts in dogs. Vet Surg 2013; 42: 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guille AE, Tseng LW, Orsger RJ. Use of vacuum.assisted closure for management of a large skin wound in a cat. J Am Vet Med Assoc 2007; 11: 1169–1673. [DOI] [PubMed] [Google Scholar]
- 12. Swaim S. The full thickness mesh graft. Vet Med 1986; 81: 524–531. [Google Scholar]
- 13. Barry VM. The effect of graft thickness, donor site and graft bed on graft shrinkage in the hooded rat. Br J Plast Surg 1969; 22: 125–127. [DOI] [PubMed] [Google Scholar]
- 14. Kim EK, Hong JP. Efficacy of negative pressure therapy to enhance take of 1-stage allodermis and a split-thickness graft. Ann Plast Surg 2007; 58: 536–540. [DOI] [PubMed] [Google Scholar]
- 15. Senchenkov A, Petty PM, Knoetgen J, et al. Outcomes of skin graft reconstructions with the use of vacuum assisted closure (VAC®) dressing for irradiated extremity sarcoma defects. World J Surg Oncol 2007; 5: 138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saaiq M, Hameed UD, Khan MI, et al. Vacuum assisted closure therapy as a pretreatment for split thickness skin grafts. J Coll Physicians Surg Pak 2010; 20: 675–679. [PubMed] [Google Scholar]
- 17. Waltzmann JT, Bell DE. Vacuum assisted closure device as a split thickness skin graft bolster in the burn population. J Burn Care Res 2014; 35: e338–e342. [DOI] [PubMed] [Google Scholar]