Abstract
Objectives
The aims of the study were to assess the possible effects of sex, age and breed on the evolution of pancreatitis, and to understand if low values of serum ionised calcium ([Ca2+i]) can be considered as a prognostic risk factor for determining the clinical course of the disease.
Methods
A sample of 24 cats (n = 24) with pancreatitis was used and grouped according to the disease progress into two groups: (i) non-fatal (NF) for those that recovered and (ii) fatal (F) for those that died. Quantification of [Ca2+i] and feline pancreatic lipase (fPL) was carried out for each patient at two different times: T1 (day of diagnosis) and T2 (day of recovery or death). For statistical analysis, P values <0.05 were considered significant.
Results
At T1, 58.3% of patients presented with hypocalcaemia, 33.3% with normocalcaemia and 8.3% with hypercalcaemia. The [Ca2+i] mean values were higher in the F group than in NF. At T2, 75.0% of patients showed normocalcaemia and 25.0% hypocalcaemia. The mean value of [Ca2+i] for F at T2 was 0.88 ± 0.23 mmol/l, whereas for NF it was 1.10 ± 0.11 mmol/l. There was no sex or age predisposition for disease development, but a breed effect was noted (domestic shorthair cats were more prone to developing pancreatitis).
Conclusions and relevance
The results suggest that hypocalcaemia is common in patients with pancreatitis and that [Ca2+i] may be used as a prognostic risk factor for predicting the clinical course of the disease, with values ⩽1 mmol/l corresponding to a poor prognosis.
Introduction
Pancreatitis is one of the most difficult ante-mortem diagnoses in feline medicine, especially in chronic cases or in patients with non-specific clinical signs. This is associated with the absence of a simple, sensitive and specific diagnostic method,1,2 allowing only the treatment of symptoms in most cases.3,4 Pancreatitis is a serious catabolic disease that leads to a systemic inflammatory response syndrome with multiple organ dysfunctions and even death.2,3,5–8 The exact prevalence of the disease is still unknown, but clinical evidence suggests that overall it is around 0.6%.9,10 In the cat, disease prognosis is directly related to the severity and duration, the extent of pancreatic necrosis, the development of local and systemic complications, and the presence of concomitant diseases. Several systems or indicators have been developed in human medicine to predict the prognosis of patients with pancreatitis, with the purpose of identifying patients at greatest risk as early as possible, allowing the clinician to institute aggressive medical and nutritional therapy earlier.4,6 So far, systemic inflammatory cytokines, such as tumour necrosis factor-α, interleukin-6, acute phase proteins and amyloid-A substance,4,11 have proved to be reliable indicators of patient response to treatment, as well as of disease recurrence. 12 However, despite being adapted for feline medicine, they have not proved useful in clinical practice as they cannot be used in ordinary analyses. In feline pancreatitis laboratory medicine, it is possible to verify that hypocalcaemia is present in 41–49% of patients and is thus one of the most common electrolyte abnormalities; 13 for this reason, it has been suggested as a potential indicator of poor prognosis in these patients. Part of the evaluated hypocalcaemia may be due to extracellular translocation and accumulation of calcium in the intracellular compartment of the soft tissues such as the pancreas, liver and abdominal muscles. 14 Kimmel et al 14 demonstrated that 61% of patients with pancreatitis presented a lower mean serum ionised calcium concentration ([Ca2+i]; 0.07 mmol/l) than normal (1.12 mmol/l). Moreover, concentrations <1 mmol/l were associated with a poor prognosis as 77% of cats with this value died or were euthanased. 14 Of the total calcium, only the ionised calcium fraction is metabolically active, thus presenting a real diagnostic value.15–17 Several mechanisms have been proposed to explain the hypocalcaemia (total and ionised) in pancreatitis: (i) hypoalbuminaemia; (ii) acid–base changes; (iii) hijack of calcium salts in peri-pancreatic fat or local soft tissue; (iv) increase of free fatty acids and calcitonin concentration; and (v) resistance to parathormone effects (which also can be related to secondary hypomagnesaemia).6,13,14,18,19
Profound changes in the structure and function of cell membranes are seen in pancreatitis. Intra-abdominal fat (mesenteric and retroperitoneal) is a very metabolically active tissue that can undergo necrosis by several mechanisms. The lipolytic enzymes released from the inflamed pancreas will activate the macrophages and other inflammatory mediators, thus exacerbating the inflammatory response and promoting self-digestion of the organ and peripancreatic fat. Phospholipase-A2 and proteases attack the adipocyte membranes leading to release and hydrolysis of triglycerides; this produces free fatty acids (mainly unsaturated), which can combine with plasma calcium and precipitate in peripancreatic fat or soft tissue as free fatty acid calcium salts, 20 and promote albumin–calcium binding, reducing the ionised fraction. During pancreatitis other mechanisms participate in calcium regulation; nevertheless, they cannot always maintain it within the normal levels, allowing the establishment of hypocalcaemia. The aims of the study were to assess the possible effects of sex, age and breed on the evolution of pancreatitis, and to understand if low values of [Ca2+i] can be considered as a prognostic risk factor for the clinical course of the disease.
Materials and methods
The present study was conducted on a sample of 24 cats (Felis catus) with pancreatitis that met the following inclusion criteria: (i) history, clinical signs and pancreas ultrasonography consistent with a diagnosis of pancreatitis; and (ii) a feline pancreatic lipase (fPL) fPL-SNAP test (IDEXX) result above the reference interval (0–3.5 mg/l). For each patient, a blood sample was collected at two different times: T1 (day of diagnosis) and T2 (day of recovery or death) for [Ca2+i] quantification by the ADVIA 2400 Chemistry System (Siemens; reference interval 1.13–1.38 mmol/l), and for fPL concentration evaluation using a semi-quantitative rapid test enzyme-linked immunosorbent assay fPL SNAP. Patients were grouped according to the clinical course of their disease into two groups: non-fatal (NF) for individuals that recovered and fatal (F) for those that died. All the cats of both groups were submitted at T1 to an ultrasonography examination in order to obtain information about the pancreas, such as whether it was hyperechoic, hypoechoic, of mixed pattern, increased in size or altered in echotexture, as well as to observe signs of inflammation such as reactive mesentery, free liquid, intestinal inflammation and liver/bile inflammation. Statistical analysis was carried out with R for Windows version 2.15.2 (R Development Core Team); Shapiro–Wilk, Wilcoxon, Spearman, Fisher’s exact and ANOVA tests were applied. All P values <0.05 were considered significant.
Results
Sample characterisation
The sample consisted of 50.0% males and 50.0% females. A non-fatal course was recorded in 66.6% of the patients and a fatal course in 33.4%. The patients in the NF group (eight males, eight females) were older than those in the F group (four males and four females), with the youngest individual being 4 years old and the oldest being 16 years old. No statistically significant differences were observed with regard to the age between the two groups (W = 18.00; P = 0.80). The domestic shorthair breed represented 62.5% of the NF patients and 100.0% of the F patients.
Relationship between sample [Ca2+i] values and clinical signs, body condition, laboratory results, ultrasonography and clinical disease course
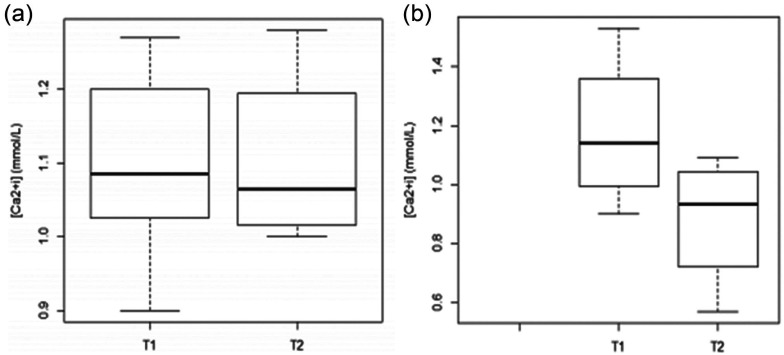
The sample [Ca2+i] mean was 1.08 ± 0.18 mmol/l (normal = 1.13–1.38 mmol/l), with a minimum of 0.57 mmol/l and a maximum of 1.53 mmol/l. At T1, normocalcaemia was recorded in 33.3% of the patients, hypocalcaemia in 58.3% and hypercalcaemia in 8.3%; at T2, normocalcaemia was recorded in 25.0% of patients and hypocalcaemia in the remaining 75.0% (Figure 1). Some patients had other diseases associated with pancreatitis, such as diabetes mellitus (n = 4), feline immunodeficiency virus infection (n = 2) and hepatic lipidosis (n = 2). Other diseases not associated with pancreatitis, such as chronic renal failure (n = 2) and brain tumour (n = 2) were also diagnosed. In descending order, the most common clinical signs recorded were anorexia, prostration, dehydration, vomiting and jaundice. No statistically significant differences were registered between [Ca2+i] values and clinical signs (Table 1). Haematocrit changes (both increased and decreased) were observed in 8.3% of patients. Of the patients, 58.3% presented thrombocytopenia and leukocytosis, and 66.7% had neutrophilia. Creatinine and glucose levels were increased in 41.7% of the patients, alkaline phosphatase (ALP) in 75% and alanine transaminase (ALT) in 58.3%. No statistically significant differences or relationships between [Ca2+i] values and laboratory changes were observed in the two groups (Table 1).
Figure 1.
Distribution of ordered values of serum ionised calcium ([Ca2+i]; mmol/l) at T1 (day of diagnosis) and T2 (day of recovery or death) in (a) non-fatal and (b) fatal disease course groups
Table 1.
Statistical analysis of association between serum ionised calcium values and clinical signs and laboratory results in non-fatal (NF) and fatal (F) disease course, using the Fisher’s exact and Wilcoxon tests and Spearman correlation coefficient in the sample
| Parameter | NF |
F |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 |
T2 |
T1 |
T2 |
||||||
| Clinical signs | n | P | n | P | n | P | n | P | |
| Abdominal pain | 6 | 0.19 | 6 | 0.46 | 2 | 1.00 | 2 | 1.00 | |
| Vomiting | 6 | 1.00 | 4 | 1.00 | 4 | 0.33 | 4 | 1.00 | |
| Anorexia | 10 | 1.00 | 8 | 1.00 | 8 | 1.00 | 8 | 1.00 | |
| Dehydration | 8 | 0.14 | 4 | 1.00 | 6 | 1.00 | 6 | 1.00 | |
| Dyspnoea | 4 | 0.46 | 4 | 1.00 | 2 | 1.00 | 4 | 1.00 | |
| Jaundice | 4 | 0.46 | 4 | 1.00 | 4 | 1.00 | 6 | 1.00 | |
| Prostration | 10 | 1.00 | 8 | 1.00 | 8 | 1.00 | 4 | 1.00 | |
| Laboratory results | Type of test | P | Type of test | P | Type of test | P | Type of test | P | |
| Haematocrit | Wt | 8.00 | 1.00 | 12.00 | 0.25 | 1.00 | 0.66 | 2.00 | 1.00 |
| r | 81.98 | 0.95 | 122.22 | 0.25 | 2.00 | 0.33 | 18.00 | 0.33 | |
| WBC | Wt | 10.00 | 0.57 | 11.50 | 0.29 | 2.00 | 1.00 | 3.00 | 0.50 |
| r | 102.10 | 0.60 | 129.54 | 0.16 | 14.00 | 0.75 | 12.00 | 0.91 | |
| PLT | Wt | 10.00 | 0.57 | 10.00 | 0.57 | 2.00 | 1.00 | 1.00 | 1.00 |
| r | 111.16 | 0.43 | 93.05 | 0.79 | 12.00 | 0.91 | 6.00 | 0.75 | |
| Neutrophils | Wt | 13.00 | 0.14 | 12.00 | 0.25 | 3.00 | 0.66 | 2.00 | 1.00 |
| r | 117.19 | 0.33 | 141.34 | 0.06 | 18.00 | 0.33 | 6.00 | 0.75 | |
| ALT | Wt | 3.00 | 0.23 | 5.00 | 0.57 | 3.00 | 0.66 | 2.00 | 1.00 |
| r | 59.71 | 0.48 | 82.99 | 0.97 | 18.00 | 0.33 | 6.00 | 0.75 | |
| ALP | Wt | 5.00 | 0.57 | 5.00 | 0.57 | 2.50 | 1.00 | 0.00 | 0.50 |
| r | 65.89 | 0.60 | 77.96 | 0.86 | 16.32 | 0.37 | 0.00 | 0.08 | |
| BUN | Wt | 9.00 | 0.78 | 6.00 | 0.78 | 2.00 | 1.00 | 3.00 | 0.50 |
| r | 93.05 | 0.79 | 51.80 | 0.34 | 14.00 | 0.75 | 12.00 | 0.91 | |
| Creatinine | Wt | 6.00 | 0.78 | 6.50 | 0.88 | 2.00 | 1.00 | 3.00 | 0.50 |
| r | 85.00 | 0.97 | 77.92 | 0.86 | 12.00 | 0.92 | 14.00 | 0.75 | |
| Glucose | Wt | 8.00 | 1.00 | 8.00 | 1.00 | 0.00 | 0.33 | 3.00 | 0.50 |
| r | 94.06 | 0.77 | 104.12 | 0.56 | 4.00 | 0.42 | 18.00 | 0.33 | |
T1 = day of diagnosis; T2 = day of recovery or death; WBC = white blood cells; PLT = platelets; ALT = alanine transaminase; ALP = alkaline phosphatase; BUN = blood urea nitrogen; Wt = Wilcoxon test; r = Spearman correlation
Increased pancreas size was observed in 58.3% of patients, and hypoechogenicity of pancreatic parenchyma in 41.6%. It was possible to determine the signs of hepatic inflammation in 75.0% of patients, cholangitis in 41.6% and abdominal free fluid in 50.0%. In the NF group, patients with hyperechoic pancreatic parenchyma showed the highest [Ca2+i] value (1.27 ± 0.23 mmol/l), whereas those with free fluid in the abdominal cavity presented the lowest value (0.99 ± 0.09 mmol/l). In the F group, patients with hyperechoic pancreatic parenchyma presented the highest [Ca2+i] value (1.31 ± 0.31 mmol/l) and those with hypoechoic pancreatic parenchyma and signs of intestinal inflammation had the lowest value (0.90 ± 0.08 mmol/l). No statistically significant differences were determined for [Ca2+i] values and abnormal ultrasound signs (Table 2).
Table 2.
Statistical analysis of association between serum ionised calcium ([Ca2+i]) values and abnormal pancreas ultrasound signs, in non-fatal (NF) and fatal (F) disease course, using Fisher’s exact test at day of diagnosis (T1)
| Parameter | Type | [Ca2+i] (mmol/l) at T1 |
|
|---|---|---|---|
| NF |
F |
||
| P | P | ||
| Abnormal pancreas ultrasonographic signs | Hyperechoic | 0.37 | 1.00 |
| Hyporechoic | 1.00 | 1.00 | |
| Mixed pattern | 1.00 | – | |
| Increased size | 1.00 | 1.00 | |
| Altered echotexture | – | 1.00 | |
| Signs of inflammation | Reactive mesentery | 0.37 | 1.00 |
| Free liquid | 0.19 | 1.00 | |
| Intestinal inflammation | – | 1.00 | |
| Liver/bile inflammation | 0.46 | 1.00 | |
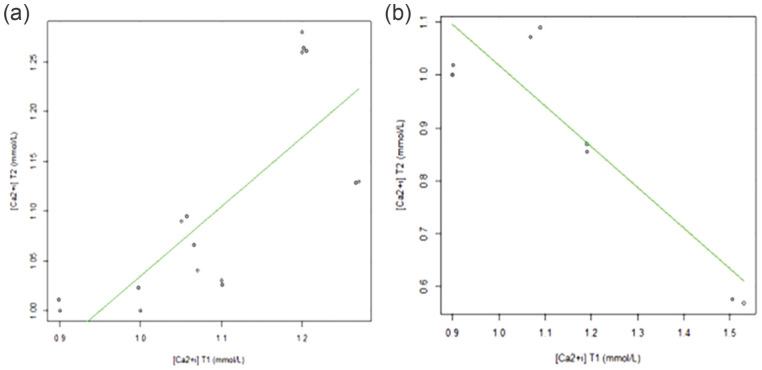
Considering the clinical disease course, the [Ca2+i] concentration was low at T1 in 58.3% of patients and 75.0% of patients at T2. Of the patients with [Ca2+i] ⩽1 mmol/l, 60.0% died. At T1, the [Ca2+i] mean values were higher in the F group (1.18 ± 0.26 mmol/l) but at T2 they decreased, presenting the lowest [Ca2+i] value (0.88 ± 0.23 mmol/l), whereas cats in the NF group maintained the [Ca2+i] value near the values observed at T1. An orderly distribution of [Ca2+i] values at T1 and T2 is shown in Figure 2.
Figure 2.
Distribution of values of serum ionised calcium ([Ca2+i]; mmol/l) at T1 (day of diagnosis) depending on the values of [Ca2+i] at T2 (day of recovery or death) in (a) non-fatal and (b) fatal disease course
Discussion
The results have shown that a low serum [Ca2+i] is a common finding in cats with pancreatitis. The sample proved to be homogeneous with regard to sex (50% males, 50% females) but not to age (range 4–11 years). The domestic shorthair breed was the most represented breed (81.2%). The results are in accordance with the study of Little 21 with regard to the effect of sex on the disease outcome, verifying that there is no sex predisposition (50% males, 50% females). There was a preponderance of domestic shorthairs in both groups (62.5% in the NF group and 100.0% in the F group), which is consistent with published studies that support the observation that this breed is more prone to the disease. It is necessary to take into account that this is the most abundant breed of cats and so it may be easily overestimated. Individuals from the NF group were older (mean age 11.3 years) than those in the F group (mean age 10.5 years). According to Forman 22 and De Cock et al, 23 cats of any age can develop the disease, but, as documented, it is notable that older animals present a higher risk of developing it, and the youngest most readily express the onset of acute pancreatitis associated with high mortality and morbidity, 19 as was reflected by the youngest patient (female aged 4 years) included in the F group.
Anorexia was the first indicator in the list of clinical signs. It is known that anorexia in pancreatitis predisposes to hepatic lipidosis, but it was not possible to confirm whether pancreatitis was the cause or the consequence of this condition, which is in accordance with the study of Kimmel et al. 14 Vomiting was highly expressed in this sample (41.6%), and although very common in humans and dogs with pancreatitis, it is irregular in cats. 7 Abdominal pain was identified in 33.3% of patients, but it is rarely recognised in cats, 7 possibly owing to a systematic lack of physical examination and the difficulty in recognising pain in this species. Contrary to expectations, weight loss and hypothermia were only recorded in 16.6% of the patients, which might be owing to the inconsistency of medical records. Jaundice was seen in 7.0% of the patients, and tends to manifest when pancreatic inflammation causes partial or total common biliary duct blockage.
For blood analysis, leukocytosis was presented in 58.3% (with neutrophilia without a left shift in 66.7%) and polycythemia and anaemia in 8.3% of patients, values that are in accordance with previous studies.19,24 Thrombocytopenia was recorded in 58.3% of cats, which may reflect the serious and systemic nature of the disease, usually associated with the development of intravascular disseminated coagulation. ALP was increased in 75.0% of cats and ALT was increased in 58.3%. Azotaemia was recorded in 75.0% of the patients and hyperglycaemia in 41.7%.
All patients presented abnormal ultrasound signs such as increased organ size (58.3%), hypoechogenicity of pancretic parenchyma (41.6%) and the presence of free fluid in the abdominal cavity (50.0%). A hypoechogenic pancreas pattern was associated with free fluid in the abdominal cavity, related to the acute nature of the disease. Usually it involves multiple organs and is associated with a poorer prognosis owing to an extensive pancreatic necrosis responsible for decreased [Ca2+i]. 7 About 41.6% of the patients were diagnosed with cholangitis, which supports several studies that also found this association, 9 but only 8.3% showed signs of intestinal inflammation. This supports the results of a study in which pancreatitis was documented in 50% of cats with cholangitis;9 however, a higher proportion (39%) of cats with intestinal bowel disease had pancreatitis, 9 perhaps because in that sample the acute presentation was more expressive than the chronic. 25
All patients presented a modified fPL SNAP score above the reference interval (0–3.5 mg/l), ‘suspicious for’ (3.6–5.3 mg/l) or ‘consistent with’ a diagnosis of pancreatitis (>5.4 mg/l).7,10,26 A cautious interpretation of fPL SNAP results in diabetic patients was needed as, according to Forcada et al, 27 these patients have a higher feline pancreatic lipase immunoreactivity (fPLI), 15 leading to a predictive low-value result.
All patients were hospitalised and submitted to a protocol with fluids, analgesia, antiemetics and antibiotics. Hospitalisation ranged between 2 and 11 days for patients that survived, and between 2 and 6 days for those that died. Evolution of the disease was 66.6% NF and 33.3% F. Albumin and total serum protein concentration changes can affect the total calcium concentration, with a positive linear relationship between those parameters. 17 No patient presented hypoalbuminaemia, and no statistically significant differences between NF and F were recorded (S = 18.0, P = 0.33).
Calcium distribution by its ionised, complex and protein-bound fractions is determined by the calcium concentration, the available ligands and the environment pH. 28 The determination of [Ca2+i] should be carried out under anaerobic conditions to ensure that there is a pH increase due to loss of CO2. An acidic pH promotes the dissociation of calcium from protein, increasing the [Ca2+i] in the sample. The alkalinity occurs with the loss of CO2, favouring protein binding and reducing its concentration in the sample. As blood sampling collection was performed under anaerobic conditions and the ionogram was normal (including potassium, which tends to be reduced when moving from the intra- to the extracellular space with the subsequent transfer of hydrogen ions to the intracellular space in an attempt to maintain electrical neutrality and cause a blood pH increase), it is unlikely that the sampled individuals would be in a state of alkalosis.
Several studies report hypocalcaemia (total and ionised) in pancreatitis, and different mechanisms have been proposed for the appearance of this derangement. In this study, hypocalcaemia was a common electrolyte change, present in 58.3% of patients at T1 and in 75.0% at T2, which is in accordance with the literature.6,17,29 Although 62.5% of the NF group presented low [Ca2+i], only 25.0% had concentrations ⩽1 mmol/l, while all patients (100.0%) from the F group presented low [Ca2+i], with 75.0% presenting concentrations ⩽1 mmol/l. Although the value of [Ca2+i] of >1 mmol/l cannot be used to predict if the patient will survive or not, it is appropriate to assign a more cautious and reserved prognosis, and to institute more aggressive medical therapy in patients in which [Ca2+i] is ⩽1 mmol/l.14 At T1, the mean [Ca2+i] was higher in the F group and decreased at T2 for [Ca2+i] ⩽1 mmol/l values, whereas the NF group presented at T2 a mean [Ca2+i] value similar to that registered at T1. A statistically significant high correlation coefficient (r = 0.81) between [Ca2+i] at T1 and T2 was achieved (S = 15.18, P = 0.01) since in the NF group the [Ca2+i] tends to be higher at T2 than at T1, allowing the patient to regain homeostasis during clinical recovery. In the F group, the higher the [Ca2+i] value at T1, the lower the values at T2 were; however, this negative relationship was not statistically significant (S = 18.00, P = 0.33). These patients present a critical clinical condition associated with pain, hypotension, tachycardia, tachypnoea and hypothermia in which the [Ca2+i] requirements are more pronounced.
Conclusions
The present study revealed that despite no clinical signs being recorded associated with hypocalcaemia, patients with pancreatitis presenting a [Ca2+i] ⩽1 mmol/l at T2 were classified into the F group. The [Ca2+i] value of ⩽1 mmol/l should be considered a prognostic risk factor associated with a poorer prognosis; these cases require from the clinician a more intensive and aggressive medical treatment than other patients with pancreatitis in which [Ca2+i] values are >1 mmol/l.
Acknowledgments
We thank CIISA (Interdisciplinary Centre of Research in Animal Health), Faculty of Veterinary Medicine of Lisbon, University of Lisbon, Portugal (FMV-ULisboa); Anjos of Assis Veterinary Medicine Center (CMVAA), Barreiro, Portugal; and to Dr Joana Pontes from FMV-ULisboa, and Dr Alexandra Costa, Dr Eva Mendes and Dr Pedro Azevedo from CMVAA, for helping with some of the pancreas ultrasonography.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Accepted: 20 November 2014
References
- 1. Noort RV. The exocrine pancreas. Proceedings of the European Veterinary Conference Voorjaarsdagen, the Netherlands. 2010 April 22–24; Amsterdam: Voorjaarsdagen, 2010. pp 137–149. [Google Scholar]
- 2. Zoran DL. Feline pancreatitis: diagnosis and treatment connections to cholangitis? Proceedings of the American Board of Veterinary Practitioners Symposium; 2012 Apr; San Antonio, TX, USA. http://www.vin.com/Members/Proceedings/Proceedings.plx?CID=ABVP2012&PID=83 [Google Scholar]
- 3. Marks SL. Feline pancreatitis – a critical appraisal of diagnosis and management. Western Veterinary Conference, 2009. http://www.vin.com/Members/Proceedings/Proceedings.plx?CID=WVC2009&PID=50817&O=VIN
- 4. Simpson KW. An update on pancreatitis in dogs and cats. Proceedings of the Western Veterinary Conference; 2012 Feb 19–13; Las Vegas, NV, USA. http://www.vin.com/Members/Proceedings/Proceedings.plx?CID=WVC2012&PID=86 [Google Scholar]
- 5. Mansfield C. Can we diagnose feline pancreatitis and do we need to? Proceedings of the World Small Animal Veterinary Congress; 2008 August 20–24; Dublin, Ireland. WSAVA, 2008, pp 305–306. [Google Scholar]
- 6. Hernadez J, Pastor J, Simpson K, et al. Principais dificuldades no maneio da pancreatite. Edição especial. France: Veterinary Focus Royal Canin, 2010. [Google Scholar]
- 7. Steiner JM. Acute and chronic feline pancreatitis – diagnostic and therapeutic challenges. Proceedings of the 35th World Small Animal Veterinary Congress; 2010; Geneva, Switzerland. http://www.vin.com/Members/Proceedings/Proceedings.plx?CID=WSAVA2010&PID [Google Scholar]
- 8. Gaschen FP. Feline pancreatitis: a persisting challenge. Proceedings of the International Congress of the Italian Association of Companion Animal Veterinarians; 2011 May 27–29; Rimini, Italy, SCIVAC, 2011, pp 196–197. [Google Scholar]
- 9. Xenoulis P, Steiner J. Feline pancreatitis. Vet Focus 2009; 19: 11–19. [Google Scholar]
- 10. Steiner JM. Diagnosis of pancreatitis. Proceedings of the Atlantic Coast Veterinary Conference; 2011 Oct 10–13; Atlantic City, NJ, USA.http://www.vin.com/Members/Proceedings/Proceedings.plx?CID=ACVC2011&PID=68 [Google Scholar]
- 11. Martinez-Subiela S, Ceron JJ. Evaluation of acute phase protein indexes in dogs with leishmaniasis at diagnosis, during and after short-term treatment. Vet Med 2005; 50: 39–46. [Google Scholar]
- 12. Scherk M. Feline pancreatitis: under-diagnosed and frequently overlooked. International Veterinary Emergency and Critical Care Symposium; 2010 Sep 11–15; San Antonio, TX, USA. http://www.vin.com/Members/Proceedings/Proceedings.plx?CID=IVECCS2010&PID=56943&O=VIN [Google Scholar]
- 13. Galvão JFB, Simpson KW, Birnbaum N. Fluid and electrolyte disturbances in gastrointestinal and pancreatic disease. In: DiBartola SP. (ed). Fluid, electrolyte, and acid–base disorders in small animal practice. 4th ed. St Louis, MO: Elsevier Saunders, 2012, pp 443–462. [Google Scholar]
- 14. Kimmel SE, Washabau RJ, Drobatz KJ. Incidence and prognostic value of low plasma ionized calcium concentration in cats with acute pancreatitis: 46 cases (1996–1998). J Am Vet Med Assoc 2001; 219: 1105–1109. [DOI] [PubMed] [Google Scholar]
- 15. Schenck PA, Chew DJ, Nagode LA, et al. Disorders of calcium: hypercalcaemia and hypocalcaemia. In: DiBartola SP. (ed). Fluid, electrolyte, and acid-base disorders in small animal practice. 4th ed. St Louis, MO: Elsevier Saunders, 2012, pp 120–194. [Google Scholar]
- 16. Willard MD. Pancreatitis: a more common and more difficult diagnosis than we thought. International Veterinary Emergency and Critical Care Symposium; 2010 Sep 11–15; San Antonio, TX, USA. http://www.vin.com/Members/Proceedings/Proceedings.plx?CID=IVECCS2010&PID= [Google Scholar]
- 17. Gründter VS, Weingrill P, Fernandes AL. Aspectos da absorção no metabolismo do cálcio e vitamina D. Rev Bras Reumatol 1997; 37: 143–151. [Google Scholar]
- 18. Matz ME. Laboratory tests for pancreatitis. Proceedings of the North American Veterinary Conference; 2006 Jan 7–11; Orlando, FL, USA. NAVC, pp 468–469. [Google Scholar]
- 19. Washabau RJ. Feline pancreatic disease. In: Ettinger SJ, Feldman ED. (eds). Textbook of veterinary internal medicine: diseases of the dog and the cat. 7th ed. St Louis, MO: Saunders Elsevier, 2010, pp 1704–1709. [Google Scholar]
- 20. Kamaya A, Federle MP, Desser TS. Imaging manifestations of abdominal fat necrosis and its mimics. RadioGraphics 2011; 31: 2021–2034. [DOI] [PubMed] [Google Scholar]
- 21. Little S. Chronic feline pancreatitis: cats are not small dogs. Proceedings of the 36th World Small Animal Veterinary Congress; 2011 Oct 14–17; Jeju, Korea: WSAVA. pp 461–463. [Google Scholar]
- 22. Forman MA. Figure out feline pancreatitis. Proceedings of the American College of Veterinary Medicine; 2012 May 30–Jun 2; New Orleans, LA, USA. http://www.vin.com/Members/Proceedings/Proceedings.plx?CID=ACVIM2012&PID=84397&O=VIN [Google Scholar]
- 23. De Cock HEV, Forman MA, Farver TB, et al. Prevalence and histopathologic characteristics of pancreatitis in cats. Vet Pathol 2007; 44: 39–49. [DOI] [PubMed] [Google Scholar]
- 24. Washabau RJ. Canine pancreatic disease: what’s new in diagnosis and therapy? Proceedings of the 34th World Small Animal Veterinary Congress; 2009 Jul 21–24; São Paulo, Brazil. http://www.vin.com/Members/Proceedings/Proceedings.plx?CID=WSAVA2009&PID [Google Scholar]
- 25. Ferreri JA, Hardam E, Kimmel SE, et al. Clinical differentiation of acute necrotizing from chronic non-suppurative pancreatitis in cats: 63 cases (1996–2001). J Am Vet Med Assoc 2003; 223: 469–474. [DOI] [PubMed] [Google Scholar]
- 26. Forman MA, Marks SL, De Cock HEV, et al. Evaluation of serum feline pancreatic lipase immunoreactivity and helical computed tomography versus conventional testing for the diagnosis of feline pancreatitis. J Vet Intern Med 2004; 18: 807–815. [DOI] [PubMed] [Google Scholar]
- 27. Forcada Y, German AJ, Noble PJM, et al. Determination of serum fPLI concentrations in cats with diabetes mellitus. J Feline Med Surg 2008; 10: 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barber PJ. Investigation of hypercalcaemia and hypocalcaemia. In: Mooney CT, Peterson ME. (eds). BSAVA manual of canine and feline endocrinology. 3rd ed. Gloucester: BSAVA, 2004, pp 26–42. [Google Scholar]
- 29. Zini E, Osto M, Moretti S, et al. Hyperglycemia but not hyperlipidaemia decreases serum amylase and increases neutrophils in the exocrine pancreas of cats. Res Vet Sci 2010; 89: 20–26. [DOI] [PubMed] [Google Scholar]