Abstract
Objectives
The objective of this study was to document Cytauxzoon felis infection in domestic cats from southern Illinois.
Methods
Diagnosis of cytauxzoonosis was based upon clinical signs of illness and detection of piroplasms within erythrocytes on peripheral blood smears or schizonts in internal organs consistent with Cytauxzoon infection. Additionally, genomic DNA was extracted from histologic sections of splenic tissue from two cats.
Results
The internal transcribed spacer region-1 (ITS-1) and ITS-2 of the C felis genome were successfully sequenced, confirming infection with the organism.
Conclusions and relevance
Sequence analysis of C felis DNA isolated from histologic lesions in two domestic cats from southern Illinois show either mixed infection or possible heterozygosity (cytosine and thymine) in ITS-2 at the position equivalent to nucleotide 76 (thymine) in the most commonly isolated C felis ITS-2 sequence. Identification of C felis infection in domestic cats from southern Illinois is a critical finding that raises awareness of this often fatal disease process in an area of the USA where, previously, the disease was only anecdotally reported.
Short Communication
There are reports of Cytauxzoon felis infection in domestic cats from Illinois but this is the first scientific publication that documents cytological and histological evidence of cytauxzoonosis in cats in Illinois.1,2 Cytauxzoonosis was first described in four domestic cats from Missouri. 3 Since that report, the disease has been documented in domestic cats from Arkansas, Florida, Georgia, Illinois, Kansas, Kentucky, Louisiana, Missouri, North Carolina, Oklahoma, South Carolina, Tennessee, Texas and Virginia.2,4–15 Cytauxzoonosis has also been reported in domestic cats from Alabama, Mississippi, Ohio and West Virginia. 1 Additionally, C felis infection has been documented in bobcats from North Dakota and Pennsylvania,16,17 though it has not been reported in domestic cats from these two states.
While originally thought of as a universally fatal disease in domestic cats, it is now known that some cats survive infection with C felis.18,19 Cats that survive the acute infection frequently maintain erythroparasitemia for many years after the initial diagnosis. 9 These cats may serve as reservoirs for future infection in other cats.9,20 It is unknown whether differences in disease progression are due to differences in the immune response of the infected cats, genetic differences in C felis isolates, amount of C felis infectious dose, or a combination of these and/or other unknown factors. To date, genetic sequencing of C felis internal transcribed spacer region-1 (ITS-1) and ITS-2 have failed to identify nucleotide variations that are linked to differences in the virulence of the organism. 21
Materials and methods
Case material
To identify cases of cytauxzoonosis in cats from Illinois, electronic medical records from the University of Illinois Veterinary Diagnostic Laboratory (UI-VDL) were searched for the term ‘cytauxzoon’. Twelve cases were identified during a 10 year period (2003–2012). This likely represents only a portion of the cases seen in Illinois: (1) practitioners identify piroplasms in erythrocytes on peripheral blood smears and do not submit the samples to the UI-VDL; and (2) additional search terms were not included so misspellings or abbreviations of ‘cytauxzoon’ may have been missed.
DNA isolation
Blocks of formalin-fixed, paraffin-embedded (FFPE) splenic tissue were available from two cats infected with Cytauxzoon (samples A and B). Genomic DNA was extracted from thick sections of these tissues using a QiaAmp DNA FFPE Tissue Kit (QIAGEN). Quality of DNA extracted from the FFPE tissues was verified by testing for the presence of a feline 28S rRNA amplicon consisting of a 100 base pair (bp) product, which confirmed the presence of feline genomic DNA and absence of inhibitors in the sample. DNA was not extracted from blood smears.
Sequence analysis
The ITS-1 and ITS-2 regions of the C felis genome were selected for PCR amplification as this region has been sequenced most frequently from cases isolated in numerous different states and in different felids.13,14,16,21–23 Cytauxzoon felis ITS-1 was amplified using two primer pairs previously described: ITS-15C (5’-CGATCGAGT GATCCGGTGAATTA-3’)/ITS-13B (5’-GCTGCGTCCTTC ATCGTTGTG-3’) and nested forward (5’-ATAGAGTAAAC GCTTCCTTCGGC-3’)/reverse (5’-CDCAGAAGTCTGC AAG TCACAATG-3’) primers.13,14 The ITS-2 segment could not be amplified with the primers described by Brown et al, 20 so new ITS-2 forward (5’-AGGTGATTTACAAT CCCTCTATATCTAC-3’) and reverse (5’-TCGATGTATTG AGGAATACTGTGAT-3’) primers were designed based upon the most commonly isolated C felis ITS-2 sequence.13,14 PCR was performed with an annealing temperature of 53°C to amplify genetic segments of both ITS-1 and ITS-2. A negative control lacking DNA was included with all reactions for every primer pair. The PCR products were detected using 1.5% agarose gel electrophoresis and were then purified and sequenced by a commercial laboratory (ACGT Inc, Wheeling, IL, USA). The chromatogram data were visualized using BioEdit Sequence Alignment Editor (Ibis Biosciences) and equivocal data were excluded. Forward and reverse sequences were aligned and combined to identify the longest segment of continuous DNA. The identified sequences were compared with previously archived sequences in GenBank using the Basic Local Alignment Search Tool (BLAST; http://blast.ncbi.nlm.nih.gov/).
Results
Of the 59,536 feline samples that were submitted to the UI-VDL from 2003–2012, 12 (0.02%) were diagnosed with cytauxzoonosis. In 2003 and 2006, there were four cases submitted each year, in 2007 there were two cases, and 2005 and 2012 each had one case. Nine of the 12 cases were submitted during the summer (four each in June and July, and one in August). Three cases were submitted during the fall (one each in September, October and November).
Affected cats varied in age from 9 months to 8 years, with half of the cats aged <2 years of age. Approximately equal numbers of male and female cats were represented. None of the cats were pure bred. All of the cases were fatal and originated from six counties in southern Illinois (Jackson, Johnson, Pulaski, Marion, Union and Williamson).
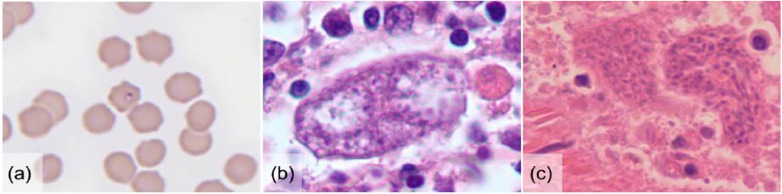
Peripheral blood smears were available for 11/12 cases. Parasitized erythrocytes containing C felis piroplasms were identified in all 11 samples (Figure 1a). Cytopenias were noted on every blood smear diagnosed with C felis. Anemia was documented in 10/11 cases and was characterized as non-regenerative in six cases. Leukopenia was observed in 8/11 blood smears. Thrombocytopenia was reported in 5/11 cases. Additionally, C felis schizonts were identified cytologically in two splenic aspirates (Figure 1b) and histologically in formalin-fixed tissue sections from two cases (Figure 1c). Histologic sections of splenic tissues received in 2005 and 2007 (samples A and B, respectively) were used for DNA extraction and identification of C felis genetic sequences.
Figure 1.
Photomicrographs of three representative cases of Cytauxzoon felis infection in cats from Illinois. (a) C felis piroplasms within erythrocytes on a direct blood smear (Wright-Giemsa stain; × 1000 magnification). (b) Schizonts consistent with C felis in a splenic aspirate (Wright-Giemsa stain; × 500 magnification). (c) C felis schizonts in a histopathologic section of splenic tissue (hematoxylin and eosin stain; × 500 magnification)
High-quality sequence data were retrieved for ITS-1 and ITS-2 from both sample A and B. A 458 bp segment of sequence was amplified from the ITS-1 region of sample B using ITS-15C/13B and nested forward/reverse primers. Only the nested forward/reverse primers were able to yield a high-quality 292 bp sequence from sample A. Both ITS-1 segments were identical to each other and exhibited 100% identity with EU450802, the most commonly reported ITS-1 sequence.13,14,23 Amplification of ITS-2 yielded a 205 bp segment for sample A and a 220 bp segment for sample B. Again, the samples from Illinois were identical to each other, but the chromatograms indicated incorporation of two nucleotides (cytosine and thymine) at the position equivalent to nucleotide 76 in sample EU450804. Interestingly, EU450804 shows incorporation of thymine at this position, while a bobcat sample from Kentucky (HQ383890) contained cytosine at this position. 23 Sequences that we obtained were not submitted to GenBank because they were identical to existing GenBank sequences.
Discussion
This is the first report of cytologic, histologic and genetic evidence of C felis infection in Illinois. Veterinary practitioners need to be aware that cytauxzoonosis occurs in southern Illinois so that they can advise owners about disease prevention, including keeping cats indoors and using tick-control products that are approved in cats. In most cats that are affected by cytauxzoonosis, initial disease signs begin within a few weeks following infection via tick bite by Amblyomma americanum or Dermacentor variabilis, and include fever, anorexia and lethargy. Tachypnea and tachycardia are commonly observed. Within days, clinical signs can progress to severe weakness, icterus, respiratory distress and/or neurologic dullness. Diagnosis can be made by identification of piroplasms within erythrocytes during microscopic examination of a peripheral blood smear. Aggressive treatment, including intensive supportive care and administration of combination therapy with atovaquone and azithromycin, has been shown to limit disease progression in some cats. 19
Sequence analysis of C felis DNA isolated from histologic lesions in two domestic cats from southern Illinois show either mixed infection or possible heterozygosity (cytosine and thymine) in the ITS-2 at the position equivalent to nucleotide 76 (thymine) in the most commonly isolated C felis ITS-2 sequence.13,14 The incorporation of two nucleotides at one position in ITS-2 may indicate a single haploid organism with heterozygosity of the ITS-2 region or multiple organisms with different sequences (as many schizonts were likely sampled within each tissue section). 23
Conclusions
Cytauxzoon felis ITS-1 and ITS-2 genomic segments were amplified from two separate cases of cytauxzoonosis in domestic cats from Illinois. Cytosine at nucleotide 76 in ITS-2 (when compared with GenBank sample EU450804 isolated in Missouri) was also identified in sequence data from organisms isolated in Kentucky. Detailed epidemiologic studies are necessary to determine how C felis was introduced into southern Illinois and to determine if there are infected, asymptomatic felids in Illinois that can serve as reservoirs of the disease.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: Costs for this project were provided by a private donation to University of Illinois research funds.
Accepted: 11 December 2014
References
- 1. Cohn LA, Birkenheuer AJ. Cytauxzoonosis. In: Green CE. (ed). Infectious diseases of the dog and cat. 4th ed. St Louis, MO: Elsevier Sanders, 2012, pp 764–771. [Google Scholar]
- 2. Miller J, Davis CD. Increasing frequency of feline cytauxzoonosis cases diagnosed in western Kentucky from 2001 to 2011. Vet Parasitol 2013; 198: 205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wagner JE. A fatal cytauxzoonosis-like disease in cats. J Am Vet Med Assoc 1976; 168: 585–588. [PubMed] [Google Scholar]
- 4. Bendele RA, Schwartz WL, Jones LP. Cytauxzoonosis-like disease in Texas cats. Southwest Vet 1976; 29: 244–246. [Google Scholar]
- 5. Hauck WN, Snider TG, Lawrence JE. Cytauxzoonosis in a native Louisiana cat. J Am Vet Med Assoc 1982; 180: 1472–1474. [PubMed] [Google Scholar]
- 6. Glenn B, Stair E. Cytauxzoonosis in domestic cats: report of two cases in Oklahoma with a review and discussion of the disease. J Am Vet Med Assoc 1984; 184: 822–825. [PubMed] [Google Scholar]
- 7. Hoover JP, Walker DB, Hedges JD. Cytauxzoonosis in cats: eight cases (1985–1992). J Am Vet Med Assoc 1994; 205: 455–460. [PubMed] [Google Scholar]
- 8. Meier HT, Moore LE. Feline cytauxzoonosis: a case report and literature review. J Am Anim Hosp Assoc 2000; 36: 493–496. [DOI] [PubMed] [Google Scholar]
- 9. Meinkoth JA, Kocan A, Whitworth L, et al. Cats surviving natural infection with Cytauxzoon felis: 18 cases (1997–1998). J Vet Intern Med 2000; 14: 521–525. [DOI] [PubMed] [Google Scholar]
- 10. Birkenheuer AJ, Le JA, Valenzisi AM, et al. Cytauxzoon felis infection in cats in the mid-Atlantic states: 34 cases (1998–2004). J Am Anim Hosp Assoc 2006; 228: 568–571. [DOI] [PubMed] [Google Scholar]
- 11. Jackson CB, Fisher T. Fatal cytauxzoonosis in a Kentucky cat (Felis domesticus). Vet Parasitol 2006; 139: 192–195. [DOI] [PubMed] [Google Scholar]
- 12. Haber MD, Tucker MD, Marr HS, et al. The detection of Cytauxzoon felis in apparently healthy free-roaming cats in the USA. Vet Parasitol 2007; 146: 316–320. [DOI] [PubMed] [Google Scholar]
- 13. Brown HM, Berghaus RD, Latimer KS, et al. Genetic variability of Cytauxzoon felis from 88 infected domestic cats in Arkansas and Georgia. J Vet Diagn Invest 2009; 21: 59–63. [DOI] [PubMed] [Google Scholar]
- 14. Brown HM, Modaresi SM, Cook JL, et al. Genetic variability of archived Cytauxzoon felis histologic specimens from domestic cats in Georgia, 1995–2007. J Vet Diagn Invest 2009; 21: 493–498. [DOI] [PubMed] [Google Scholar]
- 15. Mueller EK, Baum KA, Papeş M, et al. Potential ecological distribution of Cytauxzoon felis in domestic cats in Oklahoma, Missouri, and Arkansas. Vet Parasitol 2013; 192: 104–110. [DOI] [PubMed] [Google Scholar]
- 16. Shock BC, Murphy SM, Patton LL, et al. Distribution and prevalence of Cytauxzoon felis in bobcats (Lynx rufus), the natural reservoir, and other wild felids in thirteen states. Vet Parasitol 2011; 175: 325–330. [DOI] [PubMed] [Google Scholar]
- 17. Birkenheuer AJ, Marr HS, Warren C, et al. Cytauxzoon felis infections are present in bobcats (Lynx rufus) in a region where cytauxzoonosis is not recognized in domestic cats. Vet Parasitol 2008; 153: 126–130. [DOI] [PubMed] [Google Scholar]
- 18. Walker DB, Cowell RL. Survival of a domestic cat with naturally acquired cytauxzoonosis. J Am Vet Med Assoc 1995; 206: 1363–1365. [PubMed] [Google Scholar]
- 19. Cohn LA, Birkenheuer AJ, Brunker JD, et al. Efficacy of atovaquone and azithromycin or imidocarb diproprionate in cats with acute cytauxzoonosis. J Vet Intern Med 2011; 25: 55–60. [DOI] [PubMed] [Google Scholar]
- 20. Brown HM, Latimer KS, Erikson LE, et al. Detection of persistent Cytauxzoon felis infection by polymerase chain reaction in three asymptomatic domestic cats. J Vet Diagn Invest 2008; 20: 485–488. [DOI] [PubMed] [Google Scholar]
- 21. Brown HM, Lockhart LM, Latimer KS, et al. Identification and genetic characterization of Cytauxzoon felis in asymptomatic domestic cats and bobcats. Vet Parasitol 2010; 172: 311–316. [DOI] [PubMed] [Google Scholar]
- 22. Yabsley MJ, Murphy SM, Cunningham MW. Molecular detection and characterization of Cytauxzoon felis and a Babesia species in cougars from Florida. J Wildl Dis 2006; 42: 366–374. [DOI] [PubMed] [Google Scholar]
- 23. Shock BC, Birkenheuer AJ, Patton LL, et al. Variation in the ITS-1 and ITS-2 rRNA genomic regions of Cytauxzoon felis from bobcats and pumas in the eastern United States and comparison with sequences from domestic cats. Vet Parasitol 2012; 190: 29–35. [DOI] [PubMed] [Google Scholar]