Abstract
The primary aim of this study was to assess whether human Light’s criteria with the cut-off values previously published for cats are useful and superior to the traditional veterinary classification in diagnosing pathophysiology of fluid formation in cats with pleural effusion. The secondary aim was to assess if the albumin gradient (ALBg) is a reliable criterion for differentiating exudates from transudates in patients with pleural effusion thought to be transudative by clinical criteria but identified as exudative by Light’s criteria. Nineteen client-owned cats with pleural effusion were studied. The aetiology of the pleural effusion was used to establish the pathophysiology of its formation. Parameters measured or calculated undergoing statistical analysis included Light’s criteria, total protein and total nucleated cell count in the pleural effusions, and the ALBg. Based on the pathophysiology of fluid formation there were seven transudates caused by increased hydrostatic pressure and 12 exudates. There was a significant difference in the accuracy of the Light’s criteria in correctly classifying origin of the pleural fluid formation compared with the traditional veterinary classification (84% vs 53%). ALBg values were significantly different between transudates and exudates. One of the three transudates misclassified as exudates by Light’s criteria was correctly identified as a transudate by the ALBg. In conclusion, pleural effusion should be classified as either a transudate or an exudate using Light’s criteria. In cats with pleural effusion thought to be transudative by clinical criteria, but identified as exudative by Light’s criteria, the ALBg may further help in correctly differentiating exudates from transudates.
Introduction
In human medicine, pleural effusions are classified as transudates or exudates. 1 According to Starling’s law, 2 transudates are the effusions resulting from decreased colloid osmotic pressure or increased hydrostatic pressure, while exudates are the effusions derived from an increase in vascular permeability. In human medicine, Light’s criteria (ie, the concurrent use of pleural fluid lactate dehydrogenase concentration [LDHp], pleural fluid/serum lactate dehydrogenase ratio [LDHr] and pleural fluid/serum total protein ratio [TPr]) are considered the gold standard to classify the pathophysiology of pleural effusion formation (Table 1).1,3–6 When a transudate is found, assessing serum total protein or albumin determines if it occurred secondarily to a decrease in colloid osmotic pressure or subsequently to an increase in hydrostatic pressure. The main limitation of Light’s criteria is the misclassification of a few transudates as exudates,1,4,5,7 mostly in patients with congestive heart failure (CHF) after receiving diuretics.7–10 In these patients a serum-effusion albumin gradient (ALBg, ie, serum albumin concentration minus the effusion albumin concentration),1,4,5,7 should be calculated.
Table 1.
Light’s criteria for the differentiation of pleural effusions in humans (and cats)
| Pleural fluid | LDHp | LDHr | TPr |
|---|---|---|---|
| Transudate* | ⩽200 (⩽226) | ⩽0.60 (⩽0.62) | ⩽0.50 (⩽0.56) |
| Exudate † | >200 (>226) | >0.60 (>62) | >0.50 (>0.56) |
Effusions are identified as a transudate if all the conditions are met
Effusions are identified as an exudate if one or more conditions are met
LDHp = pleural fluid lactate dehydrogenase concentration; LDHr = pleural fluid/serum lactate dehydrogenase ratio; TPr = pleural fluid/serum total protein ratio
Pleural effusions in animals were initially classified as transudates or exudates based on pleural effusion: specific gravity, total protein concentration (TPp) and total nucleated cell count (TNCCp). 11 As in these two groups these parameters were often overlapping, Perman introduced the modified transudate. 12 This was defined as having a similar TPp and TNCCp to an exudate but formed secondarily to an increased hydrostatic pressure.12,13 To the best of our knowledge there are no studies to support the veterinary classification scheme and the cut-off values proposed to differentiate transudates, modify transudates and exudates. This results in the questionable adoption of the same cut-off value for different species (ie, dogs and cats) and for a different anatomical localisation of the effusion (ie, pleural and abdominal effusions). 14
A recent study of 20 cats demonstrated that Light’s criteria, with cut-off points for its parameters adapted to the feline species (Table 1), allowed, as in human medicine,1,3–6,15,16 the discrimination of the pathophysiology of pleural effusion (ie, transudates vs exudates) with an accuracy ⩾90%. 17 Using the traditional veterinary classification on the same 20 cats,12–14 the overall accuracy in discriminating the pathophysiological origin of the effusion was only 40%. 17
It can be argued that the high performance in discriminating the pathophysiology of the pleural effusion of Light’s criteria in the study of Zoia et al may be lower if applied in a different population of cats because the cut-off points for the parameters studied were carefully chosen by a receiving operator characteristic (ROC) curve analysis in a small population of cats. 17 Therefore, the primary aim of this study was to assess if, in a different population of cats with pleural effusion, Light’s criteria with the cut-off values previously derived (and the aid of serum total protein for the transudates) 17 were still useful in correctly classifying the pathophysiological mechanism of pleural fluid formation. In addition, it was also assessed whether Light’s criteria were superior in the identification of the pathophysiological mechanism of pleural fluid formation to the traditional veterinary classification scheme. The secondary aim of the study was to assess if the ALBg is a reliable criterion for differentiating exudates from transudates in patients with transudative pleural effusions identified as exudative effusions by Light’s criteria.
Material and methods
Animals
We retrospectively reviewed all consecutive cats that underwent a diagnostic or therapeutic thoracocentesis at the Small Animal Hospital of Glasgow University from January 2005 to December 2007. Inclusion criteria were the ability to collect at presentation a blood and a pleural fluid sample within a maximum of a 2 h period from each other, and the identification of the aetiology of the pleural effusion. Patients that had been given medication prior to sample collection, which could have masked the aetiology of the effusion (eg, corticosteroids or antibiotics) were excluded. Cats that received diuretics before presentation were included in the study.
Diagnostic criteria
For all cats, the diagnosis of the disease causing the pleural effusion was based on history, physical examination, complete blood count, serum biochemistry profile, thoracic radiographs, pleural fluid cytological examination and response to treatment. Further tests, such as serum total thyroxine measurement, feline leukaemia virus and feline immunodeficiency virus status (Speed Duo FeLV/FIV; Vetlab Supplies), feline coronavirus antibody titre (by immunofluorescence, as previously described), 18 abdominal ultrasonography or radiography, pleural fluid culture, and cytological or histological examination of intra-thoracic lesions, were performed as clinically indicated.
Previously published criteria were used to classify the effusions in the following diagnostic groups: 17 (1) congestive heart failure, (2) malignant effusion, (3) pyothorax, (4) chylothorax and (5) pleural effusions secondary to feline infectious peritonitis.
The disease causing the pleural effusion was then used as the gold standard to establish the pathophysiology of the pleural fluid formation. Therefore, following the human classification system, transudates were the effusions secondary to CHF or decreased serum colloid osmotic pressure; exudates were the effusions secondary to neoplasia, pyothorax or other diseases directly involving the pleural surfaces. 15 Following the human literature in this study, chylous effusions were classified as exudates.4,19
Pleural fluid analysis
The pleural effusion collected by means of thoracocentesis was divided and preserved both in plain and K3-EDTA tubes. Centrifugation and separation of the serum and the plain pleural fluid samples were performed within 30 mins and processed within 24 h of collection. From all pleural K3-EDTA tubes an automated cell count and a cytological examination by a board-certified pathologist were performed. The serum and pleural effusion biochemistry were measured by validated feline assays employing an automated wet chemistry analyser (Olympus AU600; Olympus Diagnostica) at the laboratory of the Small Animal Hospital of Glasgow University. Parameters measured or calculated were Light’s criteria (ie, LDHp, LDHr and TPr), TPp, TNCCp and ALBg). To be able to use the previously calculated LDH cut-off values in this study, determination of this enzyme was made using the same reagent kit as used in the previous study (LDH; Olympus Life Science Research Europa), which measures lactate dehydrogenase (LDH) activity with the reaction: Pyruvate + NADH + H + –LDH L-lactate + NAD + . In both studies the reaction was read at a wavelength of 340 nm.
Statistical analysis
For the traditional veterinary classification scheme and for Light’s criteria with the cut-off values previously derived, 17 we evaluated sensitivity, specificity and accuracy to classify correctly the pathophysiological mechanism of pleural fluid formation. In accordance with Perman’s definition, a modified transudate was regarded as an effusion resulting from an increase in hydrostatic pressure.12,13 Previously published cut-off values were used for this classification method (Table 2). 14 A McNemar χ2 test was then used to compare the accuracy in achieving the correct fluid classification between the traditional veterinary method and Light’s criteria.
Table 2.
Cut-off values previously published to distinguish pleural effusion in transudate, modified transudate and exudate 14
| Pleural fluid | TPp (g/l) | TNCCp (μl) |
|---|---|---|
| Transudate | <25 | <1500 |
| Modified transudate | 25–75 | 1000–7000 |
| Exudate | >30 | >7000 |
TP = total protein; TNCC = total nucleated cell counts; p = pleural effusion
Normal distribution for ALBg was assessed with the Kolmogorov–Smirnov test, and the median values were compared between transudates and exudates using Mann–Whitney U-tests. An ALBg cut-off value (to maximise both sensitivity and specificity) was calculated using the data from the feline population enrolled in this study using a receiving operator characteristic (ROC) curve analysis and then the sensitivity, specificity and accuracy of this test were calculated.
The statistical significance level was set at 0.05 for all analyses.
Results
Animals and causes of pleural effusions
During the study, pleural effusion samples from 22 consecutive cats were collected; however, three cats with an uncertain diagnosis of the cause of pleural effusion formation were excluded from the analysis. Causes of pleural effusions in the remaining 19 cats and values of the analytes measured and calculated in their pleural effusion and serum are presented in Table 3. Based on the pathophysiology of pleural effusion formation, there were no transudates secondary to decreased colloid osmotic pressure, seven transudates caused by an increase in hydrostatic pressure (all secondary to CHF) and 12 exudates, of which eight were caused by neoplastic diseases, three by chylous effusions and one by a pyothorax. Five of the cats with transudates were male (one entire and four neutered) and two were female (both spayed), with an average age of 8.46 ± 4.12 years (range 1.00–14.00 years). Those with exudates were six males (one entire and five neutered) and six females (one entire and five spayed), with an average age of 10.03 ± 5.70 years (range 2.00–17.00 years). There was no significant difference in age between groups (P = 0.53).
Table 3.
Causes of pleural effusions (PEs) in the 19 cats, and values of the analytes measured and calculated in their PE and serum
| PE |
Serum |
Calculated values |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case no. | Causes of PE | LDHp IU/l | TPp g/l | ALBp g/l | TNCCp × 109/l | LDH IU/l | TP g/l | ALB g/l | LDHr | TPr | ALBg g/l |
| 1* | CHF | 93 | 34 | 16 | 2.75 | 200 | 70 | 31 | 0.465 | 0.48 | 15 |
| 2* | CHF | 100 | 36 | 17 | 1.17 | 5974 | 62 | 26 | 0.016 | 0.58 | 12 |
| 3* | CHF | 131 | 23 | 13 | 0.22 | 378 | 61 | 32 | 0.346 | 0.38 | 19 |
| 4 | CHF | 141 | 18 | 9 | 1.75 | 532 | 74 | 34 | 0.265 | 0.24 | 25 |
| 5* | CHF | 150 | 32 | 18 | 2.61 | 332 | 66 | 34 | 0.451 | 0.48 | 16 |
| 6 | CHF | 166 | 38 | 22 | 4.37 | 197 | 50 | 25 | 0.842 | 0.76 | 3 |
| 7* | CHF | 268 | 24 | 10 | 3.69 | 976 | 71 | 25 | 0.274 | 0.34 | 15 |
| 8 | Neoplastic chylothorax | 243 | 42 | 22 | 13.9 | 439 | 64 | 27 | 0.553 | 0.66 | 5 |
| 9 | Cardiogenic chylothorax | 358 | 26 | 14 | 21.10 | 620 | 60 | 28 | 0.577 | 0.43 | 14 |
| 10 | Carcinoma | 493 | 33 | 16 | 5.08 | 963 | 72 | 31 | 0.511 | 0.46 | 15 |
| 11* | Neoplastic chylothorax | 526 | 43 | 27 | 6.38 | 414 | 65 | 32 | 1.27 | 0.66 | 5 |
| 12 | Carcinoma | 590 | 35 | 18 | 6.10 | 636 | 67 | 29 | 0.927 | 0.52 | 11 |
| 13 | Carcinoma | 743 | 38 | 15 | 12.80 | 614 | 58 | 21 | 1.21 | 0.65 | 6 |
| 14 | Carcinoma | 911 | 39 | 20 | 3.34 | 428 | 66 | 31 | 2.128 | 0.59 | 11 |
| 15* | Carcinoma | 996 | 46 | 21 | 1.60 | – | 64 | 28 | – | 0.72 | 7 |
| 16* | Carcinoma | 1080 | 44 | 24 | 9.80 | – | 71 | 30 | – | 0.62 | 6 |
| 17* | Lymphoma | 8414 | 50 | 22 | 13.57 | 744 | 53 | 25 | 11.309 | 0.94 | 2 |
| 18 | Lymphoma | 12,090 | 54 | 31 | 17.94 | 6563 | 70 | 36 | 1.849 | 0.77 | 5 |
| 19 | Pyothorax | 62,710 | 40 | 18 | 188.40 | 2052 | 74 | 30 | 30.560 | 0.54 | 12 |
Cats that received diuretics treatment before thoracocentesis
LDH = lactate dehydrogenase; TP = total protein; ALB = albumin; TNCC = total nucleated cell counts; g = gradient; p = pleural effusion; r = ratio; CHF = congestive heart failure
Comparison between the traditional veterinary classification and Light’s criteria
Using the traditional veterinary classification,12–14 1/7 modified transudates (effusion number 3) was misclassified as a pure transudate, and two more effusions (numbers 4 and 7) were not clearly classifiable because of discordant information (ie, intermediate characteristic between a pure transudate and a modified transudate) deriving from TPp and TNCCp (Table 4). Five of the 12 exudates (effusion numbers 10, 11, 12, 14 and 15) were incorrectly classified as modified transudates and one (effusion number 9) was not clearly classifiable because of discordant information (ie, intermediate characteristic between a modified transudate and an exudate) derived from TPp and TNCCp (Table 4).
Table 4.
Comparison between Light’s criteria and the traditional veterinary classification in correctly classifying the pathophysiology of pleural effusion formation in cats
| Case number | Type of effusion according to the gold standard | Type of effusion according to Light’s criteria | Type of effusion according to the traditional veterinary classification |
|---|---|---|---|
| 1* | ↑ HP transudate | ↑ HP transudate | ↑ HP transudate |
| 2* | ↑ HP transudate | Exudate | ↑ HP transudate |
| 3* | ↑ HP transudate | ↑ HP transudate | ↓ COP transudate |
| 4 | ↑ HP transudate | ↑ HP transudate | Not classifiable |
| 5* | ↑ HP transudate | ↑ HP transudate | ↑ HP transudate |
| 6 | ↑ HP transudate | Exudate | ↑ HP transudate |
| 7* | ↑ HP transudate | Exudate | Not classifiable |
| 8 | Exudate | Exudate | Exudate |
| 9 | Exudate | Exudate | Not classifiable |
| 10 | Exudate | Exudate | ↑ HP transudate |
| 11* | Exudate | Exudate | ↑ HP transudate |
| 12 | Exudate | Exudate | ↑ HP transudate |
| 13 | Exudate | Exudate | Exudate |
| 14 | Exudate | Exudate | ↑ HP transudate |
| 15* | Exudate | Exudate | ↑ HP transudate |
| 16* | Exudate | Exudate | Exudate |
| 17* | Exudate | Exudate | Exudate |
| 18 | Exudate | Exudate | Exudate |
| 19 | Exudate | Exudate | Exudate |
Animals that received diuretics treatment before thoracocentesis
↑ HP = increased hydrostatic pressure; ↓ COP = decreased colloid osmotic pressure
Using Light’s criteria with the cut-off values previously derived, 17 3/7 transudates (effusions numbers 3, 6 and 7) were misclassified as exudates. All 12 exudates were correctly classified (Table 4).
Results for misclassified transudates and exudates, sensitivity, specificity and accuracy of the traditional veterinary classification scheme and of Light’s criteria are reported in Table 5. There was a significant difference in the accuracy of the Light’s criteria to classify correctly the origin of the pleural fluid formation compared with the traditionally veterinary classification (84% vs 53%; P = 0.039).
Table 5.
Sensitivity, specificity, accuracy, number of misclassified transudate and exudate for Light’s criteria (used in parallel with an ‘or’ rule), calculated using cut-off values previously published in cats 17
| Test | Cut-off values for exudates | Transudates misclassified | Exudates misclassified | Sensitivity (%) | Specificity (%) | Accuracy (%) |
|---|---|---|---|---|---|---|
| Traditional classification scheme | See Table 2 | 3/7 (4/9) | 6/12 (8/11) | 50 (27) | 57 (55) | 53 (40) |
| Light’s criteria | See Table 1 | 3/7 (2/9) | 0/12 (0/11) | 100 (100) | 57 (78) | 84 (90) |
| ALBg (g/l) | ⩽14 | 2/7 (–) | 1/12 (–) | 92 (–) | 71 (–) | 84 (–) |
For ALBg, the cut-off value was calculated in this study by receiving operator characteristic curve analysis. In parentheses are the values obtained in the previous study 17
ALBg = albumin gradient
ALBg
ALBg (median transudates = 15 g/l, range 3–25 g/l; median exudates = 6.5 g/l, range 2–15 g/l) values were significantly different between transudates and exudates (P = 0.017). The optimal cut-off value to maximise both sensitivity and specificity obtained by ROC curve analysis for ALBg was ⩽14 g/l (Figure 1). Three transudates due to increased hydrostatic pressure caused by CHF were misclassified as exudates by Light’s criteria (cases 2, 6 and 7; Table 4). Two of these cats were receiving diuretic treatment (cases 2 and 7; Table 4), and one of these two cats receiving diuretic treatment (case 7) was correctly identified as having transudates by ALBg.
Figure 1.
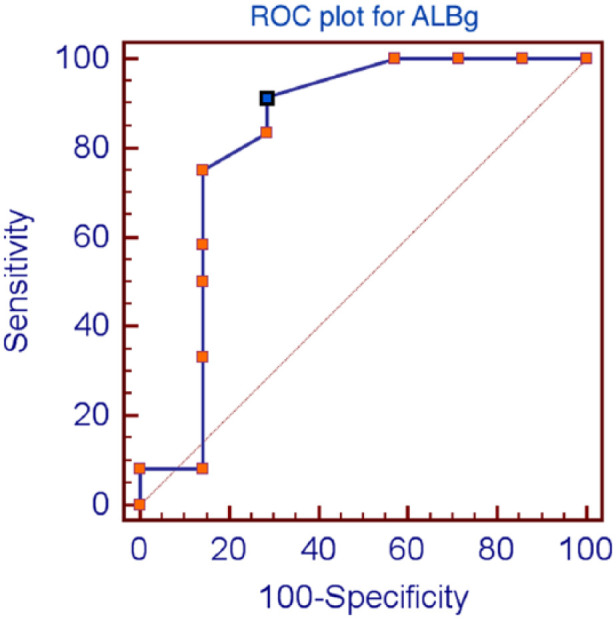
Receiving operator characteristic (ROC) plots of pleural fluid values for albumin gradient (ALBg). The optimum cut-off level (blue dot) was determined by selecting test values that provided the greatest sum of sensitivity and specificity. The optimum cut-off level for ALBg was ⩽14 g/l
Discussion
The results of the present study show that Light’s criteria, with the cut-off values previously derived, 17 are useful and superior to the traditional veterinary classification scheme to classify correctly the pathophysiological mechanism of pleural fluid formation in cats. In addition, the ALBg may be useful in selective cases in the identification of cats with pleural effusion secondary to CHF on diuretic treatment.
As shown in Table 5, the sensitivity, specificity and accuracy of Light’s criteria to discriminate correctly the origin of the pleural fluid formation were superior to the sensitivity, specificity and accuracy of the traditional veterinary classification scheme. Despite this, there was a decrease in specificity (albeit not statistically significant, 78% vs 57%; P = 0.36, one-tailed Fisher’s exact test) in the Light’s criteria in the identification of an exudate compared with what was found in the study of Zoia et al, 17 while the sensitivity in correctly classifying an exudate remained at 100%. This increased misclassification of transudates is in accordance with what has been described in the human literature, where, using the cut-off values originally proposed by Light et al, 3 several subsequent studies failed to reproduce the original specificity of these criteria, misclassifying up to 20–30% of the patients with a transudative effusion.4,6,7,15,20,21 This happened because, in our case and in the study by Light et al, 3 cut-off points were carefully chosen from a relatively small population of patients with a method designed to be close to 100% sensitive in identifying pleural exudates.
Another possible factor that may be responsible for the decrease in specificity of the Light’s criteria in this study compared with the previous one, is the higher number of cats with CHF already on diuretic treatment before sample collection (5/7 vs 3/9). 17 Human medical studies have highlighted that few transudates exhibit a LDHp, LDHr and/or TPr that falls into the exudative range.4,5,7–9 Most of these transudates have been associated with diuretic treatment prior to sample collection, which leads to the removal of the water component from the effusion, resulting in relatively higher TPp and LDHp,7–10 with a consequent increase in LDHr and TPr. These effusions may be correctly classified by calculating the ALBg.1,4,5,7 Following treatment with diuretics, the ALBg could be more accurate than Light’s criteria in classifying a transudate because of the relative increase of non-albuminaemic protein that originates and accumulates in the pleural space. Alternatively, the mathematics of a gradient may be more representative of the protein diffusion than a ratio (ie, TPr or LDHr). 7 Like in humans,4,7,15 as well as in cats with pleural effusion, the ALBg was statistically different between transudates and exudates, and therefore potentially useful in this species as a diagnostic marker. In fact, one of the three misclassified transudates by Light’s criteria in our study was correctly classified by the ALBg. At the time of the pleural fluid sampling the cat was under diuretic treatment.
The results of this study should stimulate further studies in other animal species to assess if the Light’s criteria are of any value other than in humans and cats with pleural effusion. A preliminary study would suggest that in dogs with pleural effusions TPr, LDHr and LDHp may be useful in classifying the pathophysiology of pleural effusion formation. 22
As with the previous study, 17 three major limitations were identified: (1) the small number of cats enrolled, which may have affected the observed sensitivity, specificity and accuracy of this study without changing our major result, that is, Light’s criteria are superior to the traditional veterinary classification; (2) we did not have any cats with transudative effusion secondary to a decrease in colloid osmotic pressure owing to the very low prevalence of this type of effusion in cats (0.04%, ie, one cat of the 245 reported in literature with pleural effusion);17,23–25 and, (3) in cases of chylothorax this classification scheme cannot identify the pathophysiology of pleural fluid formation. In fact, whether or not chylothorax is formed secondarily to an increase in hydrostatic pressure or secondarily to an increase in permeability of the lymphatic vessels, it will, owing to its irritative effect on the pleura, always result in an exudate. 26
Conclusions
Based on the results of this study, pleural effusion should be classified as either a transudate or an exudate using Light’s criteria which, as previously demonstrated, 17 in our opinion are superior to the traditional veterinary scheme. Importantly, if a transudate is found, further fluid analysis, including TNCCp and differential, fluid culture, etc, is not necessary, while measurement of serum total protein will provide additional information on the pathogenesis of the effusion. In the case of an exudate, cytological analysis of the pleural effusion or other tests (eg, fluid culture) may help in its aetiological diagnosis. Finally, in cats on diuretic therapy with pleural effusion thought to be transudative by clinical criteria but identified as exudative by Light’s criteria, ALBg may further help in differentiating exudates from transudates.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Accepted: 26 May 2015
References
- 1. Porcel JM, Light RW. Diagnostic approach to pleural effusion in adults. Am Fam Physician 2006; 73: 1211–1120. [PubMed] [Google Scholar]
- 2. Rose BD, Post TW. Edematous states. In: Rose BD, Post T. (eds). Clinical physiology of acid–base and electrolyte disorders. New York: McGraw-Hill, 2001, pp 478–534. [Google Scholar]
- 3. Light RW, MacGregory MI, Luchsinger PC, et al. Pleural effusion: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77: 507–513. [DOI] [PubMed] [Google Scholar]
- 4. Burgess LJ, Martiz FJ, Talljaard JJF. Comparative analysis of the biochemical parameters used to distinguish between pleural transudates and exudates. Chest 1995; 107: 1604–1609. [DOI] [PubMed] [Google Scholar]
- 5. Light RW. Diagnostic principles in pleural disease. Eur Respir J 1997; 10: 476–481. [DOI] [PubMed] [Google Scholar]
- 6. Vives M, Porcel JM, Vicente de Vera M, et al. A study of Light’s criteria and possible modifications for distinguishing exudative from transudative pleural effusions. Chest 1996; 109: 1503–1507. [DOI] [PubMed] [Google Scholar]
- 7. Roth BJ, O’Meara TF, Cragun WH. The serum-effusion albumin gradient in the evaluation of pleural effusions. Chest 1990; 98: 546–549. [DOI] [PubMed] [Google Scholar]
- 8. Chakko SC, Caldwell SH, Sforza PP. Treatment of congestive heart failure. Its effect on pleural fluid chemistry. Chest 1989; 95: 798–802. [DOI] [PubMed] [Google Scholar]
- 9. Chakko SC. Pleural effusion in congestive heart failure. Chest 1990; 98: 521–522. [DOI] [PubMed] [Google Scholar]
- 10. Shinto RA, Light RW. Effects of diuresis on the characteristics of pleural fluid in patients with congestive heart failure. Am J Med 1990; 107: 340–343. [DOI] [PubMed] [Google Scholar]
- 11. Gilmore CH, Munson TO. Abnormal chest fluids including chylothorax. In: Kirk RW. (ed). Current veterinary therapy III small animal practice. 3rd ed. Philadelphia, PA: WB Saunders, 1968, pp 174–177. [Google Scholar]
- 12. Perman P. Transudates and exudates. In: Kaneco JJ, Cornelius CE. (eds). Clinical biochemistry of domestic animals. 2nd ed. New York and London: Academic Press, 1971, pp 255–270. [Google Scholar]
- 13. Perman V, Osborne CA. Pleural effusion. In: Kirk RW. (ed). Current veterinary therapy IV small animal practice. 4th ed. Philadelphia, PA: WB Saunders, 1971, pp 157–160. [Google Scholar]
- 14. Valenciano AC, Arndt TP, Rizzi TE. Effusions: abdominal, thoracic, and pericardial. In: Cowell RL, Valenciano AC. (eds). Cowell and Tyler’s diagnostic cytology and hematology of the dogs and cats. 4th ed. St Louis, MO: Mosby, 2014, pp 244–265. [Google Scholar]
- 15. Romero S, Candela A, Martin C, et al. Evaluation of different criteria for the separation of pleural transudates from exudates. Chest 1993; 104: 399–404. [DOI] [PubMed] [Google Scholar]
- 16. Kirkeby YK, Prydz H. Lactic dehydrogenase activity in pleural and peritoneal effusion. Scand J Clin Lab Invest 1959; 11: 185–189. [Google Scholar]
- 17. Zoia A, Slater LA, Heller J, et al. A new approach to pleural effusion in cats: markers for distinguishing transudates from exudates. J Feline Med Surg 2009; 11: 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Addie DD, Jarrett O. A study of naturally occurring feline coronavirus infections in kittens. Vet Rec 1992; 130: 133–137. [DOI] [PubMed] [Google Scholar]
- 19. Rahman NM, Chapman SJ, Daveis RJ. Pleural effusion: a structured approach to care. Br Med Bull 2005; 72: 31–47. [DOI] [PubMed] [Google Scholar]
- 20. Hamm H, Brohan U, Bohmer R. Cholesterol in pleural effusions. A diagnostic aid. Chest 1987; 92: 296–302. [DOI] [PubMed] [Google Scholar]
- 21. Valdes SL, Pose A, Suarez J, et al. Cholesterol: a useful parameters for distinguishing between pleural exudates and transudates. Chest 1991; 99: 1097–1102. [DOI] [PubMed] [Google Scholar]
- 22. Zoia A, Drigo M, Caldin M. A new approach to pleural effusion in dogs: markers for distinguishing transudates from exudates. J Vet Intern Med 2011; 25: 1505. [Google Scholar]
- 23. Creighton SR, Wilkins RJ. Thoracic effusions in the cat. J Am Anim Hosp Assoc 1975; 11: 66–76. [Google Scholar]
- 24. Davies C, Forrester SD. Pleural effusion in cats (1987 to 1995). J Small Anim Pract 1996; 37: 217–224. [DOI] [PubMed] [Google Scholar]
- 25. Stewart A, Padrid P, Lobingier R. Diagnostic utility of differential cell counts and measurement of LDH, total protein, glucose and pH in the analysis of feline pleural fluid. JVIM, 1990; 4: 119. [Google Scholar]
- 26. Meadow RL, MacWilliams PS. Chylous effusions revisited. Vet Clin Pathol 1994; 23: 54–62. [DOI] [PubMed] [Google Scholar]


