Abstract
Objectives
This pilot study aimed to investigate whether and how music and musical genres may influence the depth of anaesthesia, as measured using changes in arterial blood pressure (ABP), including systolic blood pressure (SBP), and heart rate (HR) across three different surgical time points.
Methods
This work focused on a sample of 12 female cats (Felis catus) that were subjected to an elective ovariohysterectomy (OVH), and three different surgical time points were considered (T1, coeliotomy; T2, ligature placement and transection of the ovarian pedicle; and T3, ligature placement and transection of the uterine body). All of the cats were subjected to stimulation with 2 min segments of three music tracks from different genres (pop [PM], classical [CM] and heavy metal [HM]). At the same time, ABP and HR measurements were obtained using a multi-parametric monitor. For statistical analysis, P values <0.05 were considered significant.
Results
For all cats, music exposure induced statistically significant changes in the parameters under study; the same finding was observed for the genre of music. The majority of cats experienced the same variation pattern, with lower values when exposed to CM, intermediate values when exposed to PM and higher values when exposed to HM.
Conclusions and relevance
Our results indicate that the development of sensory processing of acoustic stimuli is maintained by cats under general anaesthesia and reveal the influence of music on the autonomous nervous system, as measured using HR and SBP.
Introduction
Considering the complexity of auditory processing, it appears plausible that music promotes a variable activation of distinct brain areas, producing different physiological responses.1–13Anaesthetic agents exert both direct and indirect effects on cardiovascular function, resulting in a dose-dependent systemic depression that affects autonomic nervous system parameters, such as heart rate (HR) and arterial blood pressure (ABP). These changes may act as indicators of the depth of anaesthesia in the patient. Strong relationships exist between HR and ABP and the depth of anaesthesia.14–16 The aim of this pilot study was to assess whether and how music and specific genres of music influence the depth of anaesthesia, as measured using changes in HR and ABP, including systolic (SBP), across three different surgical time points.
Materials and methods
The study protocol design was the same as that utilised for the paper previously published by Mira et al 17 that studied 12 female domestic breed Felis catus presented for elective ovariohysterectomy surgery. Signed consent forms were obtained from the owners. After achieving an adequate and stable anaesthetic plane, repeated measurements of HR and ABP (ie, SBP) were made at three different surgical time points (STPs): T1, coeliotomy; T2, ligature placement and transection of the ovarian pedicle; and T3, ligature placement and transection of the uterine body. Three different musical genres were considered: pop (PM), ‘Torn’ by Natalie Imbruglia; classical (CM), ‘Adagio for Strings (Opus 11)’ by Samuel Barber; and heavy metal (HM), ‘Thunderstruck’ by AC/DC. For data recording, a multi-parameter electronic monitor was used to assess the physiological parameters, such as HR and ABP (using a non-invasive oscillometric method). For statistical analysis, we used SPSS Statistics for Windows, and a 95% confidence interval was defined as significant, with P values of <0.05.
Results
The elective ovariohysterectomy surgery required a mean time of 35.2 ± 2.3 mins, during which parameters associated with haemodynamic responses, such as HR and ABP (ie, SBP) were recorded. The HR results are shown in Tables 1–3 and Figure 1. The lowest mean HR values for all STPs were recorded with CM stimulation, and the highest HR values were recorded with HM stimulation. The results of Friedman’s test for T1 (due to the presence of a significant outlier value) and repeated-measures ANOVA for the remaining STPs revealed statistically significant differences between the music genres and the CT for all times (P <0.001 for T1, T2 and T3). Wilcoxon’s signed-rank (for T1) and post-hoc Bonferroni (for T2 and T3) tests yielded statistically significant differences during T1 for the comparisons CT/CM (P = 0.002) and HM/CM (P = 0.005), during T2 for the comparisons CT/CM (P = 0.007), PM/HM (P = 0.007) and HM/CM (P = 0.008) and during T3 for the comparisons CT/PM (P = 0.001), CT/CM (P <0.001) and CT/HM (P = 0.012). The SBP results are shown in Tables 1–3 and Figure 2. Most patients exhibited lower SBP values when exposed to CM, intermediate values when exposed to PM and higher values when exposed to HM, at all STPs. Statistically significant differences were observed between the music genres and the control for T2 (P = 0.005) and T3 (P = 0.002). Pairwise comparisons between stimulus conditions (using the post-hoc Bonferroni test) for the data obtained during T1 revealed statistically significant differences for the pairs PM/HM (P = 0.003) and CM/HM (P = 0.018). For T2 and T3, the Wilcoxon’s signed-rank test with the Bonferroni correction revealed statistically significant differences in T2 for the comparison PM/HM (P = 0.002) and in T3 for the comparisons CT/PM (P = 0.002), CT/CM (P = 0.005) and PM/HM (P = 0.002).
Table 1.
Descriptive statistics of heart rate values (bpm) and systolic arterial pressure (mmHg), according to the music genre and surgical time for the sample studied
| Parameter | n | Statistics | T1 |
T2 |
T3 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PM | CM | HM | PM | CM | HM | PM | CM | HM | |||
| HR | 12 | CI | 35 | 34 | 42 | 43 | 39 | 48 | 35 | 29 | 33 |
| max | 142 | 138 | 145 | 148 | 145 | 156 | 145 | 138 | 146 | ||
| min | 107 | 104 | 103 | 105 | 106 | 108 | 110 | 109 | 113 | ||
| 122.75 | 118.08 | 124.83 | 125.83 | 121.42 | 129.83 | 123.58 | 121.67 | 125.42 | |||
| SD | 12.88 | 10.48 | 13.68 | 13.29 | 11.04 | 14.81 | 11.68 | 9.03 | 10.10 | ||
| SBP | 12 | CI | 81.00 | 86.00 | 84.00 | 98.00 | 63.00 | 98.00 | 86.00 | 66.00 | 78.00 |
| max | 148.00 | 149.00 | 153.00 | 160.00 | 149.00 | 165.00 | 158 | 147.00 | 163.00 | ||
| min | 67.00 | 63.00 | 69.00 | 62.00 | 86.00 | 67.00 | 72.00 | 81.00 | 85.00 | ||
| 109.75 | 107.00 | 115.42 | 117.83 | 112.83 | 122.00 | 105.92 | 106.75 | 115.42 | |||
| SD | 27.77 | 24.05 | 27.88 | 25.04 | 18.49 | 25.13 | 23.95 | 19.03 | 24.13 | ||
Data mean ( ) and dispersion (SD) measures obtained in a 95% confidence interval (CI), presenting the minimum (min) and maximum (max) values in the sample (n) considered
HR = heart rate; SBP = systolic blood pressure; T1 = coeliotomy; T2 = ligature placement and transection of the ovarian pedicle; T3 = ligature placement and transection of the uterine body; CM = classical music; HM = heavy metal music; PM = pop music
Table 2.
Shapiro–Wilk test, repeated measures ANOVA and Friedman’s test results for heart rate (bpm) and systolic arterial pressure (mmHg) values organised according to music genre and surgical time
| Surgical time point | Type of test | Music genre | n | HR |
SBP |
|||
|---|---|---|---|---|---|---|---|---|
| s | P | s | P | |||||
| T1 | Shapiro–Wilk | C | 12 | 0.917 | 0.263 | 0.936 | 0.445 | |
| PM | 12 | 0.902 | 0.171 | 0.915 | 0.246 | |||
| CM | 12 | 0.945 | 0.570 | 0.945 | 0.564 | |||
| HM | 12 | 0.950 | 0.636 | 0.926 | 0.338 | |||
| ANOVA | Sig Mauchly test | – | – | <0.001 | ||||
| P value | – | – | 0.066 | |||||
| Friedman | χ2 | – | 18.630 | – | ||||
| P value | – | <0.001 | – | |||||
| T2 | Shapiro–Wilk | C | 12 | 0.908 | 0.200 | 0.910 | 0.210 | |
| PM | 12 | 0.960 | 0.786 | 0.946 | 0.578 | |||
| CM | 12 | 0.955 | 0.704 | 0.953 | 0.678 | |||
| HM | 12 | 0.952 | 0.673 | 0.942 | 0.530 | |||
| ANOVA | Sig Mauchly test | – | 0.163 | – | ||||
| P value | – | <0.001 | – | |||||
| Friedman | χ2 | – | – | 13.042 | ||||
| P value | – | – | 0.005 | |||||
| T3 | Shapiro–Wilk | C | 12 | 0.986 | 0.997 | 0.839 | 0.027 | |
| PM | 12 | 0.858 | 0.047 | 0.893 | 0.128 | |||
| CM | 12 | 0.857 | 0.045 | 0.915 | 0.247 | |||
| HM | 12 | 0.894 | 0.134 | 0.916 | 0.256 | |||
| ANOVA | Sig Mauchly test | – | 0.393 | – | ||||
| P value | – | <0.001 | – | |||||
| Friedman | χ2 | – | – | 21.700 | ||||
| P value | – | – | <0.001 | |||||
Statistically significant values are shown in bold
s = statistic; Sig = significance; HR = heart rate; SBP = systolic blood pressure; T1 = coeliotomy; T2 = ligature placement and transection of the ovarian pedicule; T3 = ligature placement and transection of the uterine body; CM = classical music; HM = heavy metal music; PM = pop music; C = control
Table 3.
Descriptive statistics with repeated-measures ANOVA test and Wilcoxon’s signed-rank test of heart rate values (bpm) and systolic blood pressure (mmHg), according to music genre and surgical time point
| Surgical time points | Pairs of genres | ANOVA test |
Wilcoxon’s signed-rank test |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR |
SBP |
HR |
SBP |
|||||||
| Md | Sig | Md | Sig | Z | Sig | Z | Sig | |||
| T1 | PM | C | 10.583 | 0.691 | – | – | – | – | ||
| CM | C | 13.333 | 0.195 | – | – | – | – | |||
| PM | – | – | 4.917 | 1.000 | −2.302 | 0.021 | – | – | ||
| HM | C | – | – | −10.583 | 0.691 | −1.140 | 0.254 | – | – | |
| PM | – | – | 2.750 | 1.000 | −2.043 | 0.041 | – | – | ||
| CM | – | – | −5.667 | 0.003 | −2.831 | 0.005 | – | – | ||
| T2 | C | PM | 3.333 | 0.729 | −13.333 | 0.195 | – | – | – | – |
| CM | 7.750 | 0.007 | −2.750 | 1.000 | – | – | – | – | ||
| HM | −0.667 | 1.000 | −8.417 | 0.018 | – | – | – | – | ||
| PM | C | −3.333 | 0.729 | −4.917 | 1.000 | – | – | – | – | |
| CM | 4.417 | 0.308 | 5.667 | 0.003 | – | – | – | – | ||
| HM | −4.000 | 0.007 | 8.417 | 0.018 | – | – | – | – | ||
| CM | C | −7.750 | 0.007 | – | – | – | – | −0.178 | 0.859 | |
| PM | −4.417 | 0.308 | – | – | – | – | –0.979 | 0.328 | ||
| HM | −8.417 | 0.008 | – | – | – | – | −0.550 | 0.582 | ||
| HM | C | 0.667 | 1.000 | – | – | – | – | −2.125 | 0.034 | |
| PM | 4.000 | 0.007 | – | – | – | – | −3.106 | 0.002 | ||
| CM | 8.417 | 0.008 | – | – | – | – | −2.127 | 0.033 | ||
| T3 | C | PM | 11.250 | 0.001 | – | – | – | – | −3.084 | 0.002 |
| CM | 13.167 | 0.000 | – | – | – | – | −2.827 | 0.005 | ||
| HM | 9.417 | 0.012 | – | – | – | – | −2.121 | 0.034 | ||
| PM | C | −11.250 | 0.001 | – | – | – | – | −0.157 | 0.875 | |
| CM | 1.917 | 1.000 | – | – | – | – | −3.074 | 0.002 | ||
| HM | −1.833 | 1.000 | – | – | – | – | −2.316 | 0.021 | ||
| CM | C | −13.167 | 0.000 | 10.583 | 0.691 | – | – | – | – | |
| PM | −1.917 | 1.000 | 13.333 | 0.195 | – | – | – | – | ||
| HM | −3.750 | 0.143 | 4.917 | 1.000 | – | – | – | – | ||
| HM | C | −9.417 | 0.012 | −10.583 | 0.691 | – | – | – | – | |
| PM | 1.833 | 1.000 | 2.750 | 1.000 | – | – | – | – | ||
| CM | 3.750 | 0.143 | −5.667 | 0.003 | – | – | – | – | ||
Statistically significant values are shown in bold
Md = mean difference; Sig = significance; HR = heart rate; SBP = systolic blood pressure; T1 = coeliotomy; T2 = ligature placement and transection of the ovarian pedicule; T3 = ligature placement and transection of the uterine body; CM = classical music; HM = heavy metal music; PM = pop music; C = control
Figure 1.
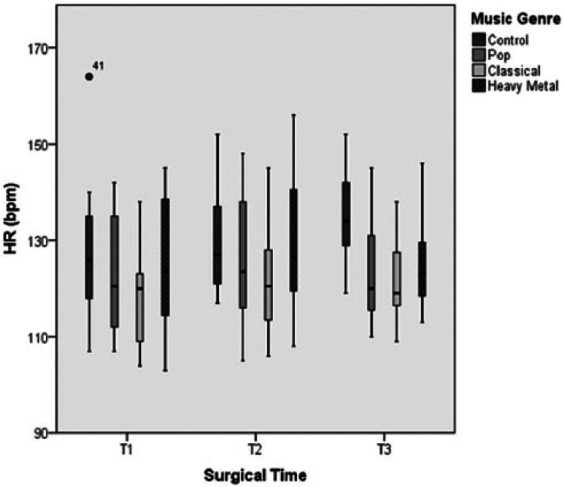
Variation of heart rate (HR) over the three surgical times considered under general anaesthesia and with stimulation of the three different music genres
Figure 2.
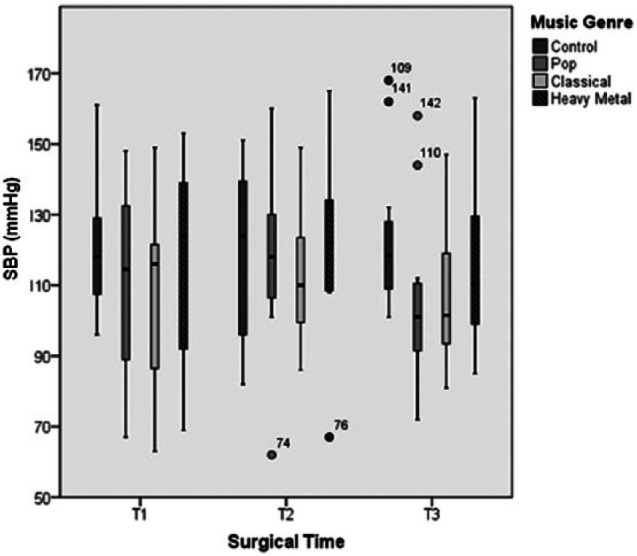
Variation of systolic blood pressure (SBP) over the three surgical times considered under general anaesthesia and with stimulation of the three different music genres
Discussion
The autonomic response to stimuli can be evaluated using physical parameters, such as the haemodynamic responses of HR and ABP, and these parameters, particularly ABP, may act as useful indicators of the depth of anaesthetic during surgery.] 18 For all studied patients, the obtained results support the possibility that music exposure induced statistically significant changes in HR (P <0.005 for T1, T2 and T3) and SBP (P = 0.005 for T2 and T3). According to the study results, the majority of the patients experienced the same pattern variations, with lower values for HR and ABP (ie, SBP) after exposure to CM, intermediate values after exposure to PM and higher values after exposure to HM.
These results suggest that acoustic stimulus sensory processing development is maintained in cats under general anaesthesia and that some effects on autonomic nervous system activity that influence haemodynamic responses, such as HR and ABP, can be modulated by music and by the genre of music to which an individual is exposed. This finding is consistent with several studies carried out in humans and rats19–21 that concluded that exposure to music affects autonomic nervous system activity and regulates cardiovascular function. Music therapy studies suggest that the classical genre is more beneficial for health, the pop genre is more beneficial for motivation and the heavy metal genre promotes anxiety and aggression.21–23 Nevertheless, a discussion of some aspects of the methods used in this pilot study is mandatory. The ABP indicates the extent of tissue perfusion and acts as an indirect indicator of several cardiovascular parameters, including the studied HR. In the present study, ABP was measured using the oscillometric method because it was easily accessed. It is, however, less precise than Doppler which has been shown to correlate well with SBP measurements in both conscious and anaesthetised cats. Still, this method has been reported to provide a consistent measurement of SBP.14,24 Relative to the baseline control data, the majority of the HR or SBP values recorded in the three STPs under music stimulation were lower, reflecting the presence of mechanisms that are associated with a decrease in cardiac output that leads to slower removal of the anaesthetic from the alveoli due to reduced blood flow. As a result, the anaesthetic drug stays in the alveoli longer and the patient consumes less anaesthetic. Therefore, this pilot study presents the possibility of using music as an additional element in individual surgical anaesthetic protocols based on the modulation of autonomic nervous system activity by music, which promotes reduced anaesthetic consumption by stabilising haemodynamic clinical signs, such as HR and SBP. 25
Although the medetomidine–ketamine combination is no longer considered a gold standard anaesthetic protocol for cats, this combination continues to be widely used and represents a suitable combination for anaesthesia in this species.26–28 Ketamine can balance the depressive cardiovascular effects of medetomidine, 27 increasing HR and ABP values within 20 mins of its administration. This combination produces anaesthesia in less than 4 mins, with duration of active surgical anaesthesia between 25 and 50 mins.27,29 Additionally, the authors recognise that opinions may vary regarding the administration of certain premedication drugs, such as atropine and opiates, which can both cause changes in the cardiovascular system. The use of atropine, an anticholinergic medicine with a short activity that may cause tachycardia, increasing myocardial oxygen consumption and the potential for myocardial hypoxaemia development, can be useful in conjunction with opioid or alpha-2 agonist administration to offset the potential bradycardic effects and the drop in cardiac output that are associated with these drugs.29,30,31 The sympathomimetic properties of ketamine counter bradycardia but do not reverse the decrease in cardiac output. Therefore, the use of atropine as a premedication can correct this situation.
In addition, the observed changes in HR and ABP parameters were assumed to occur due to the music genre rather than due to different isoflurane concentrations because across the STPs, a standard 1% isoflurane vaporisation volume was applied for all of the patients. 32 Moreover, as Cullen et al 30 concluded, the haemodynamic responses recorded during the first hour of general anaesthesia are not related to the concentration of isoflurane that is administered to the patient but are directly related to noxious stimuli. In this pilot study, noxious surgical stimulus was suspended by the addition of analgesic components, such as buprenorphine, which prevent sympathetic stimulation and haemodynamic responses. Regardless of the employed protocol, the selection of the appropriate dosing and combination of drugs for each individual will always promote a safer procedure.33,34 During the analysis of the results, the potential effects of the anaesthetics and presurgical drugs used in the protocol were considered with respect to their potential cardiovascular effects, as this study focused on such effects. 34 Because the surgical procedure time exhibited a mean of 35.2 mins and the STPs started 10 mins after drug administration and because the cardiovascular effects of ketamine and atropine occur during the 20 mins following their administration, any increase in HR and ABP values that resulted from the peak effects of ketamine and atropine would be registered during only the T1 STP.
Conclusions
The results are very encouraging, and the clinical implications of these data are significant in the context of judging anaesthetic depth based on haemodynamic responses. However, further controlled studies with larger samples are needed based on this pilot study to determine the value of playing music throughout surgical procedures and to explore the effects that this might have.
Acknowledgments
The authors thank CIISA – Interdisciplinary Centre for Research in Animal Health, Faculty of Veterinary Medicine of Lisbon, University of Lisbon, Portugal (FMV/ULisboa), and Anjos of Assis Veterinary Medicine Centre (CMVAA), Barreiro, Portugal.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
The authors do not have any potential conflicts of interest to declare.
Accepted: 6 May 2015
References
- 1. Zaehle T, Wustenberg T, Meyer M, et al. Evidence for rapid auditory perception as the foundation of speech processing: a sparse temporal sampling fMRI study. Eur J Neurosci 2004; 20: 2447–2456. [DOI] [PubMed] [Google Scholar]
- 2. Gangrade A. The effect of music on the production of neurotransmitters, hormones, cytokines, and peptides: a review. Music Med 2012; 4: 40–43. [Google Scholar]
- 3. Strain GM. Deafness in dogs and cats. 1st ed. Oxford: CABI Publishing, 2011. [Google Scholar]
- 4. Zatorre RJ, Belin P. Spectral and temporal processing in human auditory cortex. Cerebral Cortex 2001; 11: 946–953. [DOI] [PubMed] [Google Scholar]
- 5. Okamoto H, Stracke H, Draganova R, et al. Hemispheric asymmetry of auditory evoked fields elicited by spectral versus temporal stimulus change. Cereb Cortex 2009; 19: 2290–2297. [DOI] [PubMed] [Google Scholar]
- 6. Hyde KL, Peretz I, Zatorre RJ. Evidence for the role of the right auditory cortex in fine pitch resolution. Neuropsychologia 2008; 46: 632–639. [DOI] [PubMed] [Google Scholar]
- 7. Perani D, Saccuman MC, Scifo P, et al. Functional specializations for music processing in the human newborn brain. Proc Natl Acad Sci U S A 2010; 107: 4758–4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zatorre RJ. Pitch perception of complex tones and human temporal-lobe function. J Acoust Soc Am 1998; 84: 566–572. [DOI] [PubMed] [Google Scholar]
- 9. Peretz I. Processing of local and global musical information by unilateral brain-damaged patients. Brain 1990; 113: 1185–1205. [DOI] [PubMed] [Google Scholar]
- 10. Mathys C, Loui P, Zheng X, et al. Non-invasive brain stimulation applied to Heschl’s gyrus modulates pitch discrimination. Front Psych 2010; 1: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Belin P, Zilbovicius M, Crozier S, et al. Lateralization of speech and auditory temporal processing. J Cogn Neurosci 1998; 10: 536–540. [DOI] [PubMed] [Google Scholar]
- 12. Menon V, Levitin DJ. The rewards of music listening: response and physiological connectivity of the mesolimbic system. Neuroimage 2005; 28: 175–184. [DOI] [PubMed] [Google Scholar]
- 13. Salimpoor VN, Benovoy M, Larcher K, et al. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci 2011; 14: 257–264. [DOI] [PubMed] [Google Scholar]
- 14. Flaherty D, Musk G. Anaesthetic monitoring equipment for small animals. In: Zollinger RM, Jr, Ellison EC. Zollinger’s atlas of surgical operations. 9th ed. New York: McGraw-Hill Professional, 2010, pp 5–9 [Google Scholar]
- 15. European commission. Hear today, safe tomorrow. http://ec.europa.eu/news/environment/090928_en.htm (2009, accessed on May 20, 2014).
- 16. Meller P. EU wants safe volume settings on portable music players. IDG News Service. Accessed at: http://www.pcworld.com/article/172716/article.html (2009, accessed on May 20, 2014).
- 17. Mira F, Costa A, Mendes E, et al. Influence of music and its genres on respiratory rate and pupil diameter variations in cats under general anaesthesia: contribution to promoting patient safety. J Feline Med Surg 2016; 18: 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Machon R. Anaesthetic monitors in small animal anaesthesia: beyond the numbers. The 3rd Annual Vet Education Online Veterinary Conference–July, Vet Education Pty Practice 2012; 27: 512–521. [Google Scholar]
- 19. Lee G, Mei-Ling C, Gin-You W. Evoked response of heart rate variability using short-duration white noise. Auton Neurosci 2010; 155: 94–97. [DOI] [PubMed] [Google Scholar]
- 20. Roque AL, Valenti VE, Guida HL, et al. The effects of different styles of musical auditory stimulation on cardiac autonomic regulation in healthy women. Noise Health 2013; 15: 281–287. [DOI] [PubMed] [Google Scholar]
- 21. Roque AL, Valenti VE, Guida HL, et al. The effects of auditory stimulation with music on heart rate variability in healthy women. Clinics 2013; 68: 960–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kogan LR, Schoenfeld-Tacher R, Simon AA. Behavioral effects of auditory stimulation on kenneled dogs. J Vet Behav 2012; 7: 268–275. [Google Scholar]
- 23. Cooper M. Milking the music. New Sci 2001; 2297: 12. [Google Scholar]
- 24. Martel E, Egner B, Brown SA, et al. High-definition oscillometry and direct arterial blood pressure measurement. J Feline Med Surg 2013; 15: 1169–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chafin S, Roy M, Gerin W, et al. Music can facilitate blood pressure recovery from stress. Br J Health Psychol 2004; 9: 393–403. [DOI] [PubMed] [Google Scholar]
- 26. Verstegen J, Fargetton X, Ectors F. Medetomidine/ketamine anesthesia in cats. Acta Vet Scand Suppl 1989; 85: 117–123. [PubMed] [Google Scholar]
- 27. Verstegen J, Fargetton X, Donnay I, et al. Comparation of clinical medetomindin/ketamine and xylazine/ketamine combinations for ovariohysterectomy of cats. Vet Rec 1990; 127: 424–426. [PubMed] [Google Scholar]
- 28. Muir WW, Hubbell JAE, Bednarski RM, et al. Handbook of veterinary anesthesia. 4th ed. St Louis, MO: Mosby, 2007. [Google Scholar]
- 29. Fargetton X, Vähä-Vahe T. Medetomidine and atipamezole in small animal practice. Tijdschr Diergeneeskd 1989; 114: 91S–93S. [PubMed] [Google Scholar]
- 30. Cullen DJ. Drugs and anesthetic depth. In: Smith NT, Miller RD, Corbascio AN. (eds). Drug interactions in anesthesia. Philadelphia: Lea & Febiger, 1981, p 287. [Google Scholar]
- 31. Stoelting RK, Longnecker DE, Eger EI, II. Minimum alveolar concentrations in man on awakening from methoxyflurane, halothane, ether and fluroxene anesthesia: MAC awake. Anesthesiology 1970; 33: 5. [DOI] [PubMed] [Google Scholar]
- 32. Zbinden AM, Petersen-Felix S, Thomson DA. Anesthetic depth defined using multiple noxious stimuli during isoflurane/oxygen anesthesia. II. Hemodynamic responses. Anesthesiology 1994; 80: 261. [DOI] [PubMed] [Google Scholar]
- 33. Paddleford RR, Harvey RC. Alpha-2 agonists and antagonists. Vet Clin North Am 1999; 29: 737–745. [DOI] [PubMed] [Google Scholar]
- 34. Kaul HL, Bharti N. Monitoring depth of anaesthesia. Indian J Anaesth 2002; 46: 323–333. [Google Scholar]


