Abstract
Objectives
The objective of this study was to investigate serum cystatin C (sCysC) and urinary cystatin C (uCysC) in cats with hyperthyroidism and cats with feline immunodeficiency virus (FIV).
Methods
Thirty cats with FIV, 26 hyperthyroid cats and 28 healthy cats were included. sCysC and uCysC:creatinine (uCysC/uCr) ratio were measured with a human particle-enhanced nephelometric immunoassay, previously validated for feline CysC measurement. Routine renal variables (serum creatinine [sCr], urine specific gravity, urinary protein:creatinine ratio [UPC]) were also measured in the three groups.
Results
Cats with hyperthyroidism had significantly higher sCysC and higher uCysC/uCr ratio, lower sCr and a higher UPC than healthy cats. Cats with FIV infection did not show a significantly higher sCysC concentration but had a significantly higher sCr and UPC than healthy cats. uCysC could be detected in only four of them.
Conclusions and relevance
This study demonstrated that sCysC is increased in cats with hyperthyroidism, in contrast with sCr, but not in cats with FIV. Many hyperthyroid cats, but only four cats with FIV, had an elevated uCysC/uCr ratio. Further studies may reveal if uCysC might be a valuable marker for tubular dysfunction in cats.
Introduction
Cystatin C (CysC) is a low molecular mass protein responsible for the intracellular catabolism of peptides and proteins.1,2 Most of the properties for endogenous glomerular filtrate rate (GFR) markers apply to CysC. 3 Studies in humans and dogs have shown the superiority of serum CysC (sCysC) over serum creatinine (sCr) in the early detection of renal impairment.4–8 Furthermore, urinary CysC (uCysC) was used as a tubular marker in both human and canine studies.9–11 Therefore, interest in investigating CysC as an early renal marker in cats has increased as chronic kidney disease (CKD) is one of the most common diseases in older cats, with a prevalence of up to 30%. 12 Our group has recently validated a human particle-enhanced nephelometric immunoassay (PENIA) for feline CysC measurement and has demonstrated a significant difference in sCysC and uCysC concentration between healthy cats and cats with CKD. 13 We also reported that age, sex and breed do not influence sCysC, in contrast to sCr and serum urea. 14 The obtained reference interval (RI) for sCysC was 0.58–1.95 mg/l. 14
CKD mainly affects aged cats. 15 In this population hyperthyroidism is also a common disease, with a prevalence of up to 11%, 16 and a recent study by our group demonstrated a feline immunodeficiency virus (FIV) seroprevalence of 14% in cats older than 6 years. 17 This means that concurrent CKD and FIV infection or hyperthyroidism might occur. In addition, both hyperthyroidism and FIV appear to have a negative effect on kidney function.18,19
Hyperthyroidism causes an increased metabolism, which is associated with decreased vascular resistance, increased cardiac output, increased renal blood flow, increased GFR, and hypertrophic and hyperplastic tubuli. Untreated hyperthyroidism can induce renal damage or exacerbate existing renal disease. 20 Owing to increased GFR, but also to decreased muscle mass in hyperthyroid patients, sCr decreases, which leads to an overestimation of renal function. 21 The effect of thyroid function on sCysC has been investigated in human medicine. After treatment for hyperthyroidism, sCysC decreased over time. 21 This means GFR is underestimated when considering sCysC levels in untreated hyperthyroid patients, in contrast with sCr.
Human patients with human immunodeficiency virus (HIV) infection have a higher risk of developing CKD.22,23 Therefore, interest in using sCysC as a renal marker in HIV-positive patients has increased, and it has been shown that sCysC is higher in patients with HIV than healthy controls.24,25 There appears to be an association between FIV and kidney abnormalities,18,26 but the effect of FIV on sCysC and uCysC has not been studied.
The objective of this study was to evaluate sCysC and uCysC in cats with hyperthyroidism and in cats naturally infected with FIV.
Materials and methods
Study population
Healthy cats, FIV-positive cats and hyperthyroid cats were prospectively recruited. Cats were considered healthy when there was no disease history and if no clinically relevant abnormalities were detected upon physical examination, complete blood count (CBC), serum biochemistry profile or urinalysis. 12 Healthy cats were excluded when they had received medication, such as non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, antibiotics, β-blocking agents or angiotensin-converting enzyme inhibitors, within 1 month prior to inclusion that might influence renal function.
FIV infection was confirmed with a positive in-house FIV test (Witness FeLV-FIV; Synbiotics). Owing to ethical considerations, FIV-infected cats treated with NSAIDs and/or antibiotics within 1 month prior to inclusion were not excluded. Cats were not included when they showed abnormalities compatible with a concurrent disease on physical examination, CBC, serum biochemistry profile and/or urinalysis.
Cats with hyperthyroidism were included when they demonstrated clinical signs compatible with hyperthyroidism, increased total thyroxine (TT4) concentration and increased thyroidal uptake of pertechnetate (99mTcO4–) on a diagnostic scintigraphic scan. Antithyroid drugs had to be stopped at least 2 weeks before inclusion, as all included hyperthyroid cats were treated with 131I after the sampling, and it has been shown that concurrent administration of antithyroid drugs increases the risk of iatrogenic hypothyroidism and adversely affects the effective half-life of 131I.27,28 Methimazole was stopped for only 3 days in cats previously treated with ⩾10 mg methimazole daily in order to avoid the risk of a thyroid storm by discontinuation of antithyroid drugs for 2 weeks. 29 Cats were excluded if there was evidence of a concurrent disease on physical examination, CBC, serum biochemistry profile and urinalysis.
In all three groups, cats with isosthenuric urine (ie, urine specific gravity [USG] <1.015) were excluded. Cats with USG <1.035 were only included if concurrent azotaemia (ie, sCr >161.8 µmol/l) was absent. It is known that low USG without concurrent azotaemia does not necessarily reflect impaired kidney function. 30 TT4 was measured in all FIV-positive and healthy cats, and FIV was tested in all hyperthyroid and healthy cats. Healthy cats and FIV-positive cats were excluded if TT4 >45.15 nmol/l. In addition, hyperthyroid and healthy cats that tested FIV-positive were also excluded.
Analytical methods
A standard physical examination was performed in all cats, including thyroid gland palpation in the FIV-infected cats suspected of having hyperthyroidism and all healthy cats older than 6 years. An in-house FIV test (Witness FeLV-FIV) was performed in all cats following the test guidelines.
Five millilitres of blood was taken by jugular venepuncture using a 23 G needle. One millilitre was collected in an EDTA-containing tube for determination of complete blood cell count in all cats (Advia 2120; Siemens). After centrifugation (5 mins at 1931 × g), 1 ml serum was analysed the same day. Serum biochemistry was performed in all cats (Architect C16000; Abbott Max-Planck-Ring). sCr was determined with a modified Jaffe assay with an RI of 64.5–161.8 µmol/l, previously determined by our group according to the American Society of Veterinary Clinical Pathology guidelines.14,31
TT4 was measured in all cats with a chemiluminescent immunoassay (Immulite 2000; Siemens), previously validated in cats (RI 14.19–45.15 nmol/l). 32 In all cats, 10 ml urine was taken by cystocentesis with a 22 G needle. USG was determined with a manual refractometer. Urinalysis consisted of a urinary dipstick test (IQ 200 SPRINT; Instrumentation Laboratory), measurement of the urinary protein:creatinine ratio (UPC) (Iricell Velocity; Instrumentation Laboratory), sediment analysis and bacterial culture (Wask Copan, MLS, Vitek 2 system; BioMerieux). Urine was centrifuged (3 mins at 365 × g) and urinary sediment was analysed within 30 mins, according to Paepe et al. 17 One millilitre of serum and 1 ml urinary supernatant was stored at −72°C until batched analysis.
sCysC and uCysC were determined with the human PENIA (BN Prospec Nephelometric Immunoassay; Siemens). This assay is based on the dispersion of light caused by an immune complex formed by CysC and latex particles coated with polyclonal antihuman CysC antibodies. The human PENIA has recently been validated for feline CysC determination both in serum and urine. 13
Statistical analysis
Analyses were performed with SAS version 9.3 (SAS Institute). The uCysC concentration was below the lower limit of detection (LOD) in several cats, and TT4 concentration was higher than the upper LOD in several hyperthyroid cats. Therefore, non-parametric techniques were used and all measurements for uCysC below the LOD were set at 0.0464 mg/l and all measurements for TT4 above the LOD were set at 193.5 nmol/l. The non-parametric Wilcoxon rank sum test was used to compare sCysC and uCysC/uCr ratio, sCr, UPC and USG between cats with hyperthyroidism or FIV and healthy cats. The Spearman rank correlation coefficients were calculated between sCysC and sCr, between sCysC and uCysC/uCr, and between UPC and uCysC/uCr in the cats with hyperthyroidism, cats with FIV and healthy cats; and between sCysC, sCr and TT4 in the cats with hyperthyroidism and healthy cats. The level of significance was set at 0.05.
Results
Study population
Ninety cats (30 hyperthyroid, 30 FIV-infected and 30 healthy) were evaluated but only 84 were included as four of the hyperthyroid cats and two of the healthy cats were FIV-positive. Their signalment is presented in Table 1. Within 1 month prior to inclusion, antibiotics were administered in 10 FIV cats, NSAIDs in two FIV cats and eight FIV cats had received both. At the time of inclusion, six of them were still receiving antibiotics, one was receiving NSAIDs and one received both. Two of the hyperthyroid cats were not treated with antithyroid drugs before inclusion. In 23 cats, therapy was stopped 2 weeks before inclusion. In one cat, antithyroid therapy was stopped 3 days before inclusion.
Table 1.
Signalment of all recruited cats
| Cats with FIV (n = 30 ) | Cats with HT (n = 26) | Healthy cats (n = 28) | |
|---|---|---|---|
| Breed | |||
| DSH/DLH | 29 | 25 | 25 |
| Maine Coon | 1 | – | – |
| Norwegian Forest Cat | – | 1 | – |
| Ragdoll | – | – | 1 |
| Peterbald | – | – | 2 |
| Sex | |||
| M | 3 | 4 | 4 |
| MN | 24 | 10 | – |
| F | 1 | 2 | 2 |
| FN | 2 | 10 | 22 |
| Age (years), mean ± SD | 6.7 ± 2.8 | 13.3 ± 2.0 | 10.5 ± 3.7 |
| Body weight (kg), mean ± SD | 4.5 ± 1.0 | 3.8 ± 0.9 | 4.0 ± 0.9 |
FIV = feline immunodeficiency virus; HT = hyperthyroidism; DSH = domestic shorthair; DLH = domestic longhair; M = male; MN = male neutered; F = female; FN = female neutered
Analytical procedures
sCysC, uCysC/uCr, the routine renal variables (sCr, USG, UPC) and TT4 were measured in all cats. Ten hyperthyroid cats had a TT4 concentration >193 nmol/l, the upper measurable concentration limit of the analytical device (Architect C16000).
The descriptive statistics for the variables sCr, USG, UPC, sCysC and uCysC/uCr for the FIV, hyperthyroid and healthy cats are presented in Tables 2 and 3. Several cats had USG <1.035 without concurrent azotaemia: 5/30 FIV-positive cats, 9/26 hyperthyroid cats and 6/28 healthy cats. For the healthy cats, there were no indications of early CKD at the time of inclusion as GFR was normal based on a combined exogenous Cr-iohexol clearance test in four of them, and abdominal ultrasound revealed no renal abnormalities in one cat. More than 2 years after inclusion, USG was >1.035 without concurrent azotaemia in the other healthy cat. For the five FIV-positive cats no follow-up data were available. For the hyperthyroid cats, follow-up data after 131I treatment were only available for three cats, and none of them developed azotaemia when they became euthyroid. Three of the six other cats were treated prior to sampling with antithyroid drugs, and treatment was stopped 2 weeks before sampling. In these three cats, sCr, TT4 and urea remained normal during medical treatment for hyperthyroidism, making concurrent CKD in these cats less likely.
Table 2.
Descriptive statistics for the variables of the included cats with feline immunodeficiency virus (FIV), hyperthyroidism and healthy cats
| Variable | Cats with FIV (n = 30) | Cats with HT (n = 26) | Healthy cats (n =28) |
|---|---|---|---|
| sCr (µmol/l) | 92.4 (67.2–146.7) * | 69.8 (38.0–109.0) * | 99.9 (64.5–142.3) |
| USG | 1.044 (1.020–1.065) | 1.039 (1.015–1.060) | 1.047 (1.020–1.060) |
| UPC | 0.21 (0.07–0.87) * | 0.52 (0.17–1.14) * | 0.18 (0.11–0.37) |
| sCysC (mg/l) | 0.8 (0.3–1.7) | 1.1 (0.4–1.8) * | 0.8 (0.4–1.2) |
| uCysC/uCr (mg/mol) |
<LOD/uCr
*
(<LOD/uCr–118.4) |
21.1
*
(<LOD/uCr–618.5) |
<LOD/uCr |
Values are presented as median (range)
Significantly different compared with healthy cats
HT = hyperthyroidism; sCr = serum creatinine; USG = urine specific gravity; UPC = urinary protein:creatinine ratio; sCysC = serum cystatin C; uCysC/uCr = urinary cystatin C:creatinine ratio; uCr = urinary creatinine; LOD = limit of detection (0.0464 mg/l)
Table 3.
Routine renal parameters of all included cats, with the number of cats having values below, within and above the reference interval
| Variable | Cats with FIV (n = 30) | Cats with HT (n = 26) | Healthy cats (n = 28) |
|---|---|---|---|
| sCr (µmol/l) | |||
| <64.5 | 0 | 1 | 0 |
| 64.5–161.8 | 30 | 25 | 28 |
| >161.8 | 0* | 0* | 0* |
| Serum urea (mmol/l) | |||
| <6.16 | 4 | 3 | 0 |
| 6.16–10.82 | 23 | 17 | 23 |
| >10.82 | 3 | 6 | 5 |
| USG | |||
| <1.015 | 0* | 0* | 0* |
| 1.015–1.035 | 7 | 9 | 5 |
| >1.035 | 23 | 17 | 23 |
| UPC | |||
| <0.2 | 15 | 2 | 16 |
| 0.2–0.4 | 11 | 8 | 12 |
| >0.4 | 4 | 16 | 0* |
Consequence of the inclusion criteria that healthy cats could not have isosthenuric urine (UPC >0.4; sCr had to be <161.8 µmol/l in all cats)
FIV = feline immunodeficiency virus; HT = hyperthyroidism; sCr = serum creatinine; USG = urine specific gravity; UPC = urine protein:creatinine ratio
Cats with hyperthyroidism had significantly higher sCysC (P = 0.01), higher uCysC/uCr ratio (P <0.001), lower sCr (P <0.001) and higher UPC (P <0.001) than healthy cats. The uCysC/uCr ratio was significantly higher in FIV-positive cats compared with healthy cats (P <0.001). However, only four of the FIV-positive cats had a detectable uCysC concentration. One of them had not received NSAIDs or antibiotics prior to sampling. The other three had received both NSAIDs and antibiotics within 2 weeks prior to sampling but did not receive medication at the time of blood collection. In addition, a significantly higher sCr concentration (P <0.001) and a higher UPC (P <0.001), but no significantly higher sCysC concentration, was present compared with the healthy cats (Figures 1 and 2).
Figure 1.
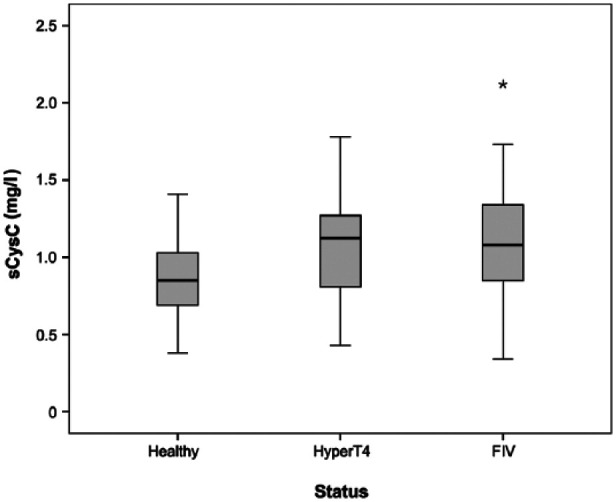
Box plot of serum cystatin C (sCysC) (mg/l) for cats with hyperthyroidism (HyperT4; n = 26), feline immunodeficiency virus (FIV; n = 30) and healthy cats (healthy; n = 28). This displays an outlying value
Figure 2.
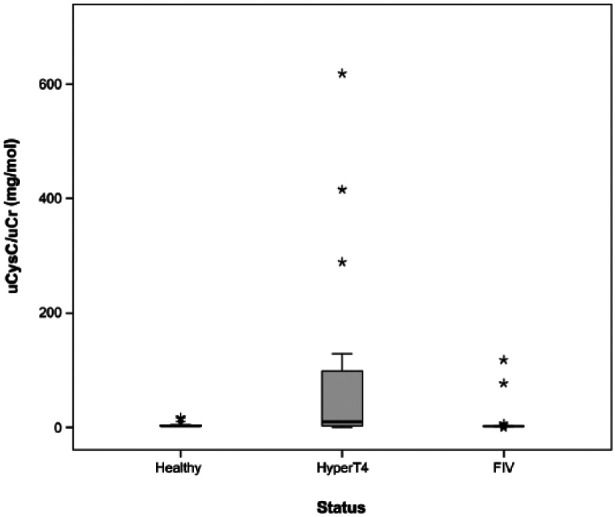
Box plot of the urinary cystatin C/urinary creatinine ratio (uCysC/uCr ratio) for cats with hyperthyroidism (HyperT4; n = 26), feline immunodeficiency virus (FIV; n = 30) and healthy cats (healthy; n = 28)
The correlation coefficients are presented in Table 4. Only in the hyperthyroid cats was a significant correlation was observed between UPC and uCysC/uCr (P <0.001) and between sCysC and uCysC (P = 0.03). A positive but not significant correlation was found between sCysC and TT4 in the hyperthyroid cats. In contrast, sCr was negatively and significantly correlated with TT4 (P = 0.003). In the healthy cats, however, a negative but not significant correlation with TT4 was observed for both sCr and sCysC. No significant correlation between sCysC and sCr was found in the three groups.
Table 4.
Spearman rank correlations for serum cystatin C (sCysC), serum creatinine (sCr), urinary cystatin C (uCysC)/urinary creatinine (uCr), total thyroxine (TT4) and urinary protein:creatinine ratio (UPC) in cats with feline immunodeficiency virus (FIV), hyperthyroid cats and healthy cats
| Variable 1 | Variable 2 | Condition | Correlation coefficient | Pvalue |
|---|---|---|---|---|
| sCysC | sCr | Hyperthyroidism | −0.20 | 0.32 |
| FIV | −0.05 | 0.81 | ||
| Healthy | −0.12 | 0.54 | ||
| sCysC | uCysC/uCr | Hyperthyroidism | 0.42 | 0.03 |
| FIV | 0.07 | 0.73 | ||
| Healthy | 0.26 | 0.18 | ||
| UPC | uCysC/uCr | Hyperthyroidism | 0.71 | <0.001 |
| FIV | 0.27 | 0.15 | ||
| Healthy | 0.16 | 0.42 | ||
| sCysC | TT4 | Hyperthyroidism | 0.24 | 0.33 |
| Healthy | −0.18 | 0.43 | ||
| sCr | TT4 | Hyperthyroidism | −0.55 | 0.003 |
| Healthy | −0.09 | 0.71 |
According to our established RI of (0.58–1.95 mg/l), 14 none of the cats had a sCysC concentration above the upper reference limit. Two cats with FIV, one cat with hyperthyroidism and four healthy cats had a sCysC concentration below the RI. All healthy cats, 26 cats with FIV and eight cats with hyperthyroidism had a uCysC/uCr ratio below the detection limit.
Discussion
Feline sCysC and uCysC were evaluated in cats with hyperthyroidism and in cats with FIV. Cats with hyperthyroidism had a significantly higher sCysC concentration and uCysC/uCr ratio compared with healthy cats. However, cats with FIV only showed a significantly higher uCysC/uCr ratio. However, only a few FIV-infected cats had detectable uCysC.
For the hyperthyroid group, routine renal variables, with the exception of USG, significantly differed from healthy cats. The effects of thyroid dysfunction on the kidney can be direct by influencing renal blood flow, and indirect by influencing GFR, tubular secretion and absorption, electrolyte pumps and kidney structure. 19 Owing to increased GFR, but also to decreased muscle mass in patients with hyperthyroidism, sCr decreases, masking possible underlying CKD. 21 Opposite effects are observed in patients with hypothyroidism.33,34 In our study, sCr was, indeed, significantly lower and the UPC significantly higher in the hyperthyroid cats when compared with the control group. Our findings are in accordance with previous studies evaluating kidney function in hyperthyroid cats.35–37
The hyperthyroid cats had a significantly higher sCysC concentration than the healthy cats, in contrast to sCr, which was significantly lower. Based on our established RI for sCysC of 0.58–1.95 mg/l, 14 hyperthyroid cats cannot be distinguished from healthy cats. There was no significant correlation between sCysC and TT4, and an overlap in sCysC concentration between healthy cats and cats with hyperthyroidism was observed (Figure 1). Our observations are comparable with human studies.21,34,38 In humans, GFR is underestimated in hyperthyroid patients when considering sCysC concentration, 39 and the same might be true in cats. The exact mechanism for a higher sCysC concentration in hyperthyroidism is not yet revealed. Nevertheless, several hypotheses have been proposed.21,40,41 Triiodothyronine should increase the level of transforming growth factor-β1 (TGF-β1), which might, in turn, stimulate the secretion of sCysC. 41 This hypothesis appears the most plausible, as stimulation of CysC secretion by TGF-β1 in vascular smooth muscle cells has been demonstrated.42,43
To our knowledge, this is the first study to compare sCysC between healthy cats and cats with hyperthyroidism. In an abstract by Jepson et al, 44 sCysC was measured in 19 hyperthyroid cats before and after treatment with 131I. In contrast with human data, 45 no significant change in sCysC concentration was observed, while a significant change was reported in the GFR and sCr concentration. The authors did not find a correlation between GFR and sCysC in the hyperthyroid cats before treatment. This study by Jepson et al and our present findings suggest that sCysC might not be a reliable GFR marker in cats with hyperthyroidism, which is comparable with the findings in human medicine. 46 However, additional studies with GFR measurement and comparison with healthy cats are warranted.
Hyperthyroid cats had a significantly higher uCysC/uCr ratio than the control group. However, not all hyperthyroid cats had a detectable uCysC concentration. Moreover, the correlation coefficient between UPC and uCysC/uCr ratio was significant (Table 3). The cats without a detectable uCysC concentration did not have proteinuria, while all hyperthyroid cats with a UPC >0.4 did have a detectable uCysC concentration. However, we observed a significant correlation between sCysC and uCysC/uCr, which suggests that uCysC is not independent from sCysC. Further studies will reveal if uCysC is a good marker for proximal tubular damage in hyperthyroid cats. Other tubular markers, such as urinary N-acetyl-β-D-glucosaminidase and urinary retinol-binding protein,35,36 do fulfil the required properties to detect tubular damage in hyperthyroid cats.
Cats with FIV had a significantly higher uCysC/uCr ratio but no significantly higher sCysC concentration. However, only 4/30 cats had detectable uCysC concentration, and three of them had received both NSAIDs and antibiotics within 2 weeks prior to blood collection, which could have influenced the results. However, uCysC could not be detected in all FIV-positive cats that had received medication prior to sampling. Further studies are required to investigate if the presence of uCysC is due to FIV or administration of NSAIDs. In contrast to the hyperthyroid cats, there was no significant correlation between uCysC/uCr ratio and UPC, as not all of the proteinuric cats had a detectable uCysC concentration. None of our cats with FIV had a sCr concentration above the upper reference limit (161.8 µmol/l) of the RI we established in a recent study. 14 Nevertheless, it has been described that cats with FIV can have tubular abnormalities, without concurrent azotaemia, 47 and several studies describe an association between proteinuria and FIV infection.18,48 Our study suggests that tubular dysfunction is absent or not severe enough in most FIV cats to cease tubular catabolism of CysC, leading to the appearance of CysC in the urine. The presence of CysC in the urine of humans with HIV has only been reported in patients receiving a combination antiretroviral therapy and not in non-treated patients with HIV. 49 sCysC was not significantly different between healthy cats and cats with FIV, even although sCr was significantly higher in the FIV cats. A higher prevalence of azotaemia in FIV-infected cats has been described,48,50 but other reports claim no association between FIV and renal azotaemia.18,51 Our results are in contrast with human reports, where a significantly higher concentration of sCysC was observed in HIV-infected patients.25,52 In addition, a correlation of HIV RNA and CD4+ T cell count with a decreased sCysC concentration after therapy has been demonstrated, 25 which is suggestive of an association of HIV replication with the sCysC level, 53 and an underestimation of GFR in non-treated HIV patients. However, in no human report was GFR measured by a gold-standard method, so it is unclear if HIV-associated renal disease also played a role in the higher concentration of sCysC.
We did not observe a significant correlation between sCysC and sCr in the three groups. This might have been due to non-renal factors influencing sCr and/or sCysC, such as FIV or hyperthyroidism, hydration status and drugs. 54 Sex and age distributions differed between the groups. In particular, male neutered cats were over-represented in the FIV-infected group, which is similar to other reports.55–57 However, no effect of age or sex on sCysC could be observed in a recent study by our group. The effect of age and sex on uCysC has not yet been evaluated. 14 Besides non-renal factors, other factors might explain the lack of correlation between sCr and sCysC. sCysC could be influenced by early mild kidney dysfunction or might not be a reliable GFR marker. Several healthy cats, cats with hyperthyroidism and FIV-infected cats had USGs <1.035 without concurrent azotaemia. It is hypothesised that the normal range for feline USG is between 1.001 and 1.065, 58 or even 1.080, 59 but no large-scale studies have confirmed this. Because GFR was unavailable in the majority of the included cats, we cannot rule out the possibility that some of these cats may have had early CKD, which might have affected the results of the present study. The GFRs of healthy cats and cats with CKD measured with a gold-standard method need to be correlated with both sCr and sCysC in order to study the sensitivity of sCysC to detect early kidney dysfunction in cats.
Our study is limited by the absence of a gold-standard method to measure GFR. We cannot exclude that early kidney impairment was present in our studied groups. A second limitation was the use of NSAIDs and antibiotics in the cats with FIV prior to inclusion, which might have influenced kidney function.
Conclusions
Cats with hyperthyroidism had a significantly higher sCysC concentration, and most hyperthyroid cats had a higher uCysC/uCr ratio compared with healthy cats. However, based on the RI of sCysC, hyperthyroid cats cannot be distinguished from the healthy cats. Our study suggests that sCysC might not be a reliable GFR marker in hyperthyroid cats. Further studies with GFR measurement are required to reveal the mechanism of a possible increased sCysC in feline hyperthyroidism. Further studies must reveal the mechanism of the increased uCysC/uCr ratio in hyperthyroid cats.
FIV infection did not influence sCysC, in contrast to HIV. Only a few cats had a uCysC/uCr ratio above the detection limit. Further studies are required to show if only a subset of FIV cats have tubular dysfunction or whether only severe tubular dysfunction is detected by measuring uCysC.
Acknowledgments
A special thanks to the institute for promotion and innovation by science and technology in Flanders (IWT) for the financial support of this study. We also thank Mrs E Lecocq and Ms B Weyn for their excellent assistance.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: This work was supported by the institute for promotion and innovation by science and technology in Flanders (IWT) (grant number 111070).
Accepted: 26 May 2015
References
- 1. Abrahamson M. Human cysteine proteinase inhibitors. Isolation, physiological importance, inhibitory mechanism, gene structure and relation to hereditary cerebral hemorrhage. Scand J Clin Lab Invest Suppl 1988; 191: 21–31. [PubMed] [Google Scholar]
- 2. Abrahamson M, Olafsson I, Palsdottir A, et al. Structure and expression of the human cystatin C gene. Biochem J 1990; 268: 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seronie-Vivien S, Delanaye P, Pieroni L, et al. Cystatin C: current position and future prospects. Clin Chem Lab Med 2008; 46: 1664–1686. [DOI] [PubMed] [Google Scholar]
- 4. Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis 2002; 40: 221–226. [DOI] [PubMed] [Google Scholar]
- 5. Roos JF, Doust J, Tett SE, et al. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children-a meta-analysis. Clin Biochem 2007; 40: 383–391. [DOI] [PubMed] [Google Scholar]
- 6. Almy FS, Christopher MM, King DP, et al. Evaluation of cystatin C as an endogenous marker of glomerular filtration rate in dogs. J Vet Intern Med 2002; 16: 45–51. [DOI] [PubMed] [Google Scholar]
- 7. Braun JP, Perxachs A, Péchereau D, et al. Plasma cystatin C in the dog: reference values and variations with renal failure. Comp Clin Pathol 2002; 11: 44–49. [Google Scholar]
- 8. Wehner A, Hartmann K, Hirschberger J. Utility of serum cystatin C as a clinical measure of renal function in dogs. J Am Anim Hosp Assoc 2008; 44: 131–138. [DOI] [PubMed] [Google Scholar]
- 9. Conti M, Moutereau S, Zater M, et al. Urinary cystatin C as a specific marker of tubular dysfunction. Clin Chem Lab Med 2006; 44: 288–291. [DOI] [PubMed] [Google Scholar]
- 10. Uchida K, Gotoh A. Measurement of cystatin C and creatinine in urine. Clin Chim Acta 2002; 323: 121–128. [DOI] [PubMed] [Google Scholar]
- 11. Monti P, Benchekroun G, Berlato D, et al. Initial evaluation of canine urinary cystatin C as a marker of renal tubular function. J Small Anim Pract 2012; 53: 254–259. [DOI] [PubMed] [Google Scholar]
- 12. Polzin DJ. Chronic kidney disease. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 7th ed. Philadelphia, PA: WB Saunders, 2010, pp 1990–2021. [Google Scholar]
- 13. Ghys LFE, Meyer E, Paepe D, et al. Analytical validation of a human particle-enhanced nephelometric assay for cystatin C measurement in feline serum and urine. Vet Clin Pathol 2014; 43: 226–234. [DOI] [PubMed] [Google Scholar]
- 14. Ghys L, Paepe D, Duchateau L, et al. Biological validation of feline cystatin C: effect of breed, age and sex and establishment of a reference interval. Vet J 2015; 204: 168–173. [DOI] [PubMed] [Google Scholar]
- 15. DiBartola SP, Rutgers HC, Zack PM, et al. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973–1984). J Am Vet Med Assoc 1987; 190: 1196–1202. [PubMed] [Google Scholar]
- 16. Mooney CT. Hyperthryoidism. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 7th ed. Philadelphia, PA: WB Saunders, 2010, pp 1761–1779. [Google Scholar]
- 17. Paepe D, Verjans G, Duchateau L, et al. Routine healthy screening findings in apparently healthy middle-aged and old cats. J Feline Med Surg 2013; 15: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baxter KJ, Levy JK, Edinboro CH, et al. Renal disease in cats infected with feline immunodeficiency virus. J Vet Intern Med 2010; 24: 677–677. [DOI] [PubMed] [Google Scholar]
- 19. den Hollander JG, Wulkan RW, Mantel MJ, et al. Correlation between severity of thyroid dysfunction and renal function. Clin Endocrinol 2005; 62: 423–427. [DOI] [PubMed] [Google Scholar]
- 20. van Hoek I, Daminet S. Interactions between thyroid and kidney function in pathological conditions of these organ systems: a review. Gen Comp Endocrinol 2009; 160: 205–215. [DOI] [PubMed] [Google Scholar]
- 21. Manetti L, Pardini E, Genovesi M, et al. Thyroid function differently affects serum cystatin C and creatinine concentrations. J Endocrinol Invest 2005; 28: 346–349. [DOI] [PubMed] [Google Scholar]
- 22. Gupta SK, Eustace JA, Winston JA, et al. Guidelines for the management of chronic kidney disease in HIV-infected patients: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2005; 40: 1559–1585. [DOI] [PubMed] [Google Scholar]
- 23. Choi A, Scherzer R, Bacchetti P, et al. Cystatin C, albuminuria, and 5-year all-cause mortality in HIV-infected persons. Am J Kidney Dis 2010; 56: 872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Odden MC, Scherzer R, Bacchetti P, et al. Cystatin C level as a marker of kidney function in human immunodeficiency virus infection – the FRAM study. Arch of Intern Med 2007; 167: 2213–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mauss S, Berger F, Kuschak D, et al. Cystatin C as a marker of renal function is affected by HIV replication leading to an underestimation of kidney function in HIV patients. Antivir Ther 2008; 13: 1091–1095. [PubMed] [Google Scholar]
- 26. Miller RJ, Cairns JS, Bridges S, et al. Human immunodeficiency virus and AIDS: insights from animal lentiviruses. J Virol 2000; 74: 7187–7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Broome MR, Turrel JM, Hays MT. Predictive value of tracer studies for I-131 treatment in hyperthyroid cats. Am J Vet Res 1988; 49: 193–197. [PubMed] [Google Scholar]
- 28. Peterson ME, Broome MR. Radioiodine for hyperthyroidism. In: Bonagura JD, Twedt DC. (eds). Current veterinary therapy V. Philadelphia, PA: Saunders Elsevier, 2012, pp 112–121. [Google Scholar]
- 29. Ward CR. Feline thyroid storm. Vet Clin North Am Small Anim Pract 2007; 37: 745–754. [DOI] [PubMed] [Google Scholar]
- 30. Paepe D, Lefebvre HP, Concordet D, et al. Simplified methods for estimating glomerular filtration rate in cats and for detection of cats with low or borderline glomerular filtration rate. J Feline Med Surg 2015; 17: 889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Friedrichs KR, Harr KE, Freeman KP, et al. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol 2012; 41: 441–453. [DOI] [PubMed] [Google Scholar]
- 32. Singh AK, Jiang Y, White T, et al. Validation of nonradioactive chemiluminescent immunoassay methods for the analysis of thyroxine and cortisol in blood samples obtained from dogs, cats, and horses. J Vet Diag Invest 1997; 9: 261–268. [DOI] [PubMed] [Google Scholar]
- 33. Gommeren K, van Hoek I, Lefebvre HP, et al. Effect of thyroxine supplementation on glomerular filtration rate in hypothyroid dogs. J Vet Intern Med 2009; 23: 844–849. [DOI] [PubMed] [Google Scholar]
- 34. Kreisman SH, Hennessey JV. Consistent reversible elevations of serum creatinine levels in severe hypothyroidism. Arch Intern Med 1999; 159: 79–82. [DOI] [PubMed] [Google Scholar]
- 35. van Hoek I, Meyer E, Duchateau L, et al. Retinol-binding protein in serum and urine of hyperthyroid cats before and after treatment with radioiodine. J Vet Intern Med 2009; 23: 1031–1037. [DOI] [PubMed] [Google Scholar]
- 36. Lapointe C, Belanger MC, Dunn M, et al. N-acetyl-beta-D-glucosaminidase index as an early biomarker for chronic kidney disease in cats with hyperthyroidism. J Vet Intern Med 2008; 22: 1103–1110. [DOI] [PubMed] [Google Scholar]
- 37. Feeney DA, Jessen CR, Weichselbaum RC. Paired pre- and post-treatment serum biochemical parameters and thyroxine concentrations in a cohort of ninety seven radioiodine-treated hyperthyroid cats. Int J Appl Res Vet Med 2011; 9: 40–51. [Google Scholar]
- 38. Jayagopal V, Keevil BG, Atkin SL, et al. Paradoxical changes in cystatin C and serum creatinine in patients with hypo- and hyperthyroidism. Clin Chem 2003; 49: 680–681. [DOI] [PubMed] [Google Scholar]
- 39. Schmitt R, Bachmann S. Impact of thyroid dysfunction on serum cystatin C. Kidney Int 2003; 64: 1139–1140. [DOI] [PubMed] [Google Scholar]
- 40. Hoek FJ, Kwakkel GJ, Bakker O. Thyroid hormone appears not to influence cystatin C synthesis. Clin Chim Acta 2005; 355: S187–S188. [Google Scholar]
- 41. Kotajima N, Yanagawa Y, Aoki T, et al. Influence of thyroid hormones and transforming growth factor-beta1 on cystatin C concentrations. J Int Med Res 2010; 38: 1365–1373. [DOI] [PubMed] [Google Scholar]
- 42. Liu JA, Sukhova GK, Sun JS, et al. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol 2004; 24: 1359–1366. [DOI] [PubMed] [Google Scholar]
- 43. Shi GP, Sukhova GK, Grubb A, et al. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J Clin Invest 1999; 104: 1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jepson RE, Slater L, Nash S, et al. Evaluation of Cystatin C as a marker of GFR in hyperthyroid cats. J Vet Intern Med 2006; 20: 740. [Google Scholar]
- 45. Fricker M, Wiesli P, Brandle M, et al. Impact of thyroid dysfunction on serum cystatin C. Kidney Int 2003; 63: 1944–1947. [DOI] [PubMed] [Google Scholar]
- 46. Goede DL, Wiesli P, Brandle M, et al. Effects of thyroxine replacement on serum creatinine and cystatin C in patients with primary and central hypothyroidism. Swiss Med Wkly 2009; 139: 339–344. [DOI] [PubMed] [Google Scholar]
- 47. Poli A, Abramo F, Taccini E, et al. Renal involvement in feline immunodeficiency virus infection: a clinicopathological study. Nephron 1993; 64: 282–288. [DOI] [PubMed] [Google Scholar]
- 48. Avila A, Reche A, Kogika MM, et al. Occurrence of chronic kidney disease in cats naturally infected with immunodeficiency virus. J Vet Intern Med 2010; 24: 760–761. [Google Scholar]
- 49. Jaafar A, Seronie-Vivien S, Malard L, et al. Urinary cystatin C can improve the renal safety follow-up of tenofovir-treated patients. Aids 2009; 23: 257–259. [DOI] [PubMed] [Google Scholar]
- 50. Thomas JB, Robinson WF, Chadwick BJ, et al. Association of renal-disease indicators with feline immunodeficiency virus infection. J Am Anim Hosp Assoc 1993; 29: 320–326. [Google Scholar]
- 51. Poli A, Tozon N, Guidi G, et al. Renal alterations in feline immunodeficiency virus (FIV)-infected cats: a natural model of lentivirus-induced renal disease changes. Viruses 2012; 4: 1372–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Overton ET, Patel P, Mondy K, et al. Cystatin C and baseline renal function among HIV-infected persons in the SUN study. Aids Res Human Retrovir 2012; 28: 148–155. [DOI] [PubMed] [Google Scholar]
- 53. Gagneux-Brunon A, Mariat C, Delanaye P. Cystatin C in HIV-infected patients: promising but not yet ready for prime time. Nephrol Dial Transplant 2012; 27: 1305–1313. [DOI] [PubMed] [Google Scholar]
- 54. Braun JP, Lefebvre HP. Kidney function and damage. In: Kaneko JJ, Harvey JW, Bruss ML. (eds). Clinical biochemistry of domestic animals. 6th ed. London: Elsevier, 2008, pp 485–528. [Google Scholar]
- 55. Courchamp F, Yoccoz NG, Artois M, et al. At-risk individuals in feline immunodeficiency virus epidemiology: evidence from a multivariate approach in a natural population of domestic cats (Felis catus). Epidemiol Infect 1998; 121: 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fleming EJ, McCaw DL, Smith JA, et al. Clinical, hematologic, and survival data from cats infected with feline immunodeficiency virus: 42 cases (1983–1988). J Am Vet Med Assoc 1991; 199: 913–916. [PubMed] [Google Scholar]
- 57. Taffin E, Paepe D, Goris N, et al. Antiviral treatment of feline immunodeficiency virus-infected cats with (R)-9-(2-hosphonylmethoxypropyl)-2,6-diaminopurine. J Feline Med Surg 2015; 17: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Finco DR. Evaluation of renal functions. In: Osborne CA, Finco DR. (eds). Canine and feline nephrology and urology. Baltimore, MD: Williams and Wilkins, 1995, pp 216–229. [Google Scholar]
- 59. Stockham SL, Scott MA. Urinary system. In: Stockham SL, Scott MA. (eds). Fundamentals of veterinary clinical pathology. Oxford: Blackwell Publishing, 2008, 416–493. [Google Scholar]


