Abstract
Objectives
The objective of this study was to evaluate the clinical safety of the non-steroidal anti-inflammatory drug (NSAID) robenacoxib in cats with osteoarthritis. Degenerative joint disease, including osteoarthritis, is highly prevalent in cats and many cases have associated pain and impaired mobility. Although NSAIDs are used routinely to control pain and inflammation in cats with osteoarthritis, there are safety concerns because of the high concurrent prevalence of chronic kidney disease (CKD) and the paucity of data on the safety of these drugs in target clinical populations.
Methods
A total of 194 cats with osteoarthritis were recruited and randomly allocated to receive either robenacoxib at a dosage of 1.0–2.4 mg/kg (n = 95) or placebo (n = 99) tablets PO q24h for 28 days. Safety was assessed in 193 cats, including a subgroup of 40 animals with concurrent CKD, defined as serum creatinine concentration ⩾1.6 mg/dl and urine specific gravity <1.030. Safety endpoints included reports of adverse events, results of clinical examinations, including body weight, and clinical chemistry and hematology variables.
Results
In all 193 cats and the subgroup of 40 animals with concurrent CKD, there were no differences between groups in frequencies of reported adverse events, body weight change or results of serum or urine chemistry or hematology variables.
Conclusions and relevance
Robenacoxib was well tolerated when administered daily for 1 month in cats with osteoarthritis, including cats with evidence of concurrent CKD. There was no clinical indication of damage to the gastrointestinal tract, kidney or liver.
Introduction
Osteoarthritis (OA), which is an important subset of degenerative joint disease (DJD), has been identified to be an important clinical disease in cats.1,2 DJD, including OA, is relatively common in cats; in one study 92% of animals aged between 6 months and 20 years had radiographic signs of joint disease. 3 Given the importance and effects of this disease on mobility and behaviour, especially in older cats, there has been increased effort to find effective treatment options.1,2,4–8
Non-steroidal anti-inflammatory drugs (NSAIDs) are indicated in the treatment of pain and inflammation associated with OA, as they produce analgesic and anti-inflammatory effects. 4 Currently, in the USA there are no NSAIDs registered for long-term use in cats. Meloxicam is registered in Europe for the alleviation of pain and inflammation in acute and chronic musculoskeletal disorders, 9 but in the USA it is only registered as a single injectable dose for postoperative pain and inflammation in cats. Positive effects of meloxicam on signs of OA in cats have been reported in open-label studies,1,10 and two masked, placebo-controlled studies.8,11
Robenacoxib is an NSAID that has been recently introduced into canine and feline medicine. Presently, robenacoxib tablets are registered in the USA for cats for the control of postoperative pain and inflammation associated with orthopedic surgery, ovariohysterectomy and castration at an oral dosage of 1.0–2.4 mg/kg q24h, for up to 3 days. In Europe, robenacoxib tablets are registered for cats at the same dosage for the treatment of pain and inflammation associated with musculoskeletal disorders for up to 6 days use, and associated with surgery for up to 3 days use.12–14 Robenacoxib has been reported to have a good safety index in healthy cats, with dosages up to 20 mg/kg q24h for 42 days being well tolerated. 15 However, there are currently no published data on the tolerability of robenacoxib in cats with OA. The clinical safety results from a clinical trial conducted in cats with OA and treated with robenacoxib are presented here. The efficacy results will be reported in a separate paper.
Materials and methods
This study was a multicenter, prospective, randomized, blinded, clinical trial comparing robenacoxib with a placebo control in client-owned cats with OA.
The study was conducted in accordance with US Food and Drug Administration (FDA) Good Clinical Practices Regulations and USA Code of Federal Regulations.16,17 In addition, the study results are reported according to the Consolidated Standards of Reporting Trials (CONSORT) guidelines on randomised studies. 18 All procedures were reviewed and approved by the local institutional Animal Care and Use Committee, and were in compliance with Novartis Animal Health Animal Welfare Guidelines and the US Department of Agriculture’s Animal Welfare Act. 19
Owners were informed verbally about the methods and objectives of the study, and had to provide written consent before their cat was evaluated or enrolled.
Study outline
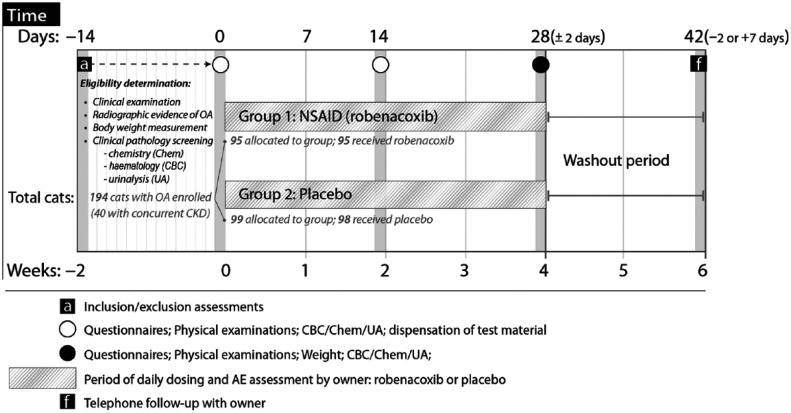
The study schedule is shown in Figure 1. In brief, cats were examined by the veterinary investigator at preselection (day −14 to −1) and enrolment (day 0) to determine study eligibility. If enrolled into the study, cats were administered placebo or robenacoxib once a day on days 1–28, and were re-examined by the investigator on days 14 and 28, and with a telephone interview of the owner on day 42.
Figure 1.
Schedule of events. OA = osteoarthritis; CBC = complete blood count; CKD = chronic kidney disease; NSAID = non-steroidal anti-inflammatory drug; AE = adverse event
Animals
Cats diagnosed with OA and having a history of impaired activity for at least 12 weeks were eligible for inclusion, as listed in Table 1. The diagnosis of OA was based on history and the presence of clinical and radiographic signs of OA. All radiographs were evaluated by a single board-certified radiologist. Exclusion criteria and prohibited concomitant treatments are described in Table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
| Age ⩾6 months |
| Body weight ⩾2.5 to ⩽12 kg |
| History of impaired activity for a minimum of the past 12 weeks |
| Moderate or severe impairment in at least two of the variables ‘activity level’, ‘ability and willingness to jump’ and ‘stiffness or lameness’ (assessed by four-point numerical rating scales) |
| Radiographic evidence of osteoarthritis in the appendicular or axial skeleton documented by radiography within 2 weeks prior to enrolment |
| Cat and owner available for duration of study |
| Owner able and willing to maintain a constant environment and housing |
| Owner informed consent provided in writing |
| Exclusion criteria |
| Clinical signs caused by an acute musculoskeletal disorder present for <12 weeks or which is likely to spontaneously resolve in the next 4 weeks |
| Clinical signs associated with neoplasia, a primary neurological disorder or known immunological disorder (ie, polyarthritis) |
| Uncontrolled endocrine or systemic disorders (eg, diabetes mellitus, hyperthyroidism or other). Cats requiring treatment for diabetes mellitus or hyperthyroidism must be stabilized for at least 28 days prior to inclusion in the study |
| Severe gastrointestinal disorders such as irritation and hemorrhage, impaired hepatic, cardiac or renal function, or hemorrhagic disorders |
| Cats intended for breeding, or known to be pregnant or lactating |
| Acupuncture therapy, homeopathy, herbal medicines or chiropractic care |
| Routine vaccinations within the previous 14 days |
| Surgery of any joint in the previous 90 days |
| Treatment within the defined time prior to study inclusion with short-acting or local corticosteroids (30 days), long-acting corticosteroids (90 days), local or systemic analgesics or non-steroidal anti-inflammatory drugs (14 days), pentosan polysulfate sodium or polysulfated glycosaminoglycans (30 days) |
| Administration of chondroitin sulfate, fatty acid supplements, glucosamine, or other nutraceuticals or diets specifically formulated for joint disease (except if administered at a constant dosage for at least 30 days before the study inclusion and continued at the same dosage throughout the study) |
| Concomitant treatments |
| Concomitant medications or treatments were allowed as long as they did not interfere with the objectives of the study Routine preventive antiparasitic treatments, angiotensin-converting enzyme inhibitors, calcium channel blockers, diuretics, hyperthyroid medications and insulin preparations were permitted during the trial. However, any cardiovascular or endocrine diseases had to be stabilized before starting the study |
Cats could be prematurely withdrawn from the study at any time for reasons that included inadequate efficacy of the test items, occurrence of an adverse event (AE) or other safety concerns, occurrence of concomitant disorders or administration of forbidden concomitant treatments.
Test item
Robenacoxib was administered as 6 mg flavored tablets (Onsior 6 mg; Novartis Animal Health). The placebo tablets were manufactured at the same site and consisted of tablets of identical appearance and composition with the exception that the active ingredient, robenacoxib, was replaced by an equal weight of lactose. Robenacoxib was administered at a target minimum dosage of 1 mg/kg, with a range of 1.0–2.4 mg/kg daily. Therefore, cats weighing 2.5 to ⩽6 kg received one tablet (placebo or robenacoxib) and cats weighing >6 to ⩽12 kg received two tablets.
The test items were administered q24h for 28 (± 2) days. Owners documented the administration in a daily diary. In addition, the number of tablets dispensed and returned by the owner for each cat was reconciled at each site. The tablets could be administered with or without food. 20 If a cat vomited or regurgitated within 5 mins of treatment administration, and the originally administered tablet was visible, the animal could be re-dosed. Otherwise, the owner was instructed to not dose the cat until the next scheduled treatment. The number of tablets administered was based on the weight at day 0 and was not adjusted during the 28 day treatment period.
Power analysis, randomisation and blinding
Once selected for the study at day 0, cats were allocated randomly to treatment groups in blocks of two (1:1 ratio placebo to robenacoxib) using a SAS/STAT software-generated randomization schedule (SAS System for Windows, version 9.1.2; SAS Institute). Randomization schedules were prepared by the statistician for each investigator. No separate randomization was made for age, body weight or sex of the cat, as there is no evidence of effect for these variables on the pharmacology of robenacoxib. Power analysis was based on the primary efficacy outcome variable (data to be presented separately), not on safety variables, and additionally on the FDA requirement to have at least 100 subjects treated with the active drug in field studies. A total of 90 cats per group was calculated to have >90% power, with an assumed 30% difference between groups in the primary efficacy variable.
Blinding of both investigators and owners was maintained as the placebo and robenacoxib tablets and packaging (blisters) had identical appearance. Furthermore, one or more dispensers were identified at each site to dispense the test items and reconcile the test items to and from the owners. The study was unblinded only after study conclusion and the database had been locked. It was not necessary to unmask any cases during the study, which could have been necessary in the event of human exposure to the test items or the occurrence of a serious AE.
Outcome measures: safety evaluation
Safety was assessed using the following data.
AE reporting
An AE was considered to be any observation, in a treated animal, that was unfavorable and unintended, and occurred after the use of the test items, whether or not considered to be related to the product. The following information was requested for each AE: a description of the suspected AE, duration (onset and end date, if known), magnitude of the event, presumed relationship of the event to the test items and outcome. The investigator made the determination whether the AE was clinically ‘serious’ or ‘not serious’. A ‘serious’ AE required active medical intervention and was considered by the investigator to be clinically significant.
Owner-assessed AEs
For owner-assessed AEs, owners were instructed to complete a daily diary. In the event the owner noted anything different from normal in their cat, they were instructed to contact their veterinarian.
Veterinarian examinations
Clinical examinations and body weight measurements were performed by the investigator at preselection and on days 0, 14 and 28, or at the time of exit from the study in cases of early withdrawal. An additional clinical examination, if possible, was performed for any animal that experienced a serious AE.
Serum chemistry, hematology and urinalysis
At preselection and on day 28, blood and urine samples were collected for serum chemistry, including serum activities of alkaline phosphatase, alanine aminotransferase (ALT), aspartate aminotransferase and γ-glutamyl transferase, and concentrations of albumin, amylase, bilirubin, calcium, chloride, cholesterol, creatine phosphokinase (CPK), creatinine, globulin, glucose, lipase, magnesium, phosphate, potassium, sodium, triiodothyronine, thyroxine, total protein, triglycerides and blood urea nitrogen; hematology, including hematocrit, hemoglobin, concentrations and counts of platelets, red cells and white cells; and urinalysis, including urine specific gravity (USG).
Follow-up
A follow-up telephone call from the investigator or designate to the owner was performed on day 42, or 14 days after the final visit, to obtain an update on the cat’s condition since the last visit.
Defining chronic kidney disease
Cats were defined as having concomitant chronic kidney disease (CKD) based on serum creatinine concentration ⩾1.6 mg/dl plus USG <1.030; that is, representing IRIS stages 2, 3 or 4. 21 Cases were allocated to IRIS stages 2, 3 and 4 based on serum creatinine concentration ranges of 1.6–2.8 mg/dl, 2.9–5.0 mg/dl and >5.0 mg/dl, respectively.
Statistics
All statistical analyses were conducted using SAS/STAT version 9.1.2. Statistical significance was concluded with two-tailed P values <0.05. The frequency of AEs in the two groups was compared with Fisher’s exact test.
Change in body weight was analyzed by analysis of variance (ANOVA) using SAS PROC MIXED. The model included the fixed effect of ‘treatment’ with the main effect ‘site’ and the interaction term ‘treatment by site’ treated as random variables. Body weight change was also analyzed with respect to the animal’s age.
Clinical chemistry, hematology and urinalysis variables were evaluated statistically using analysis of covariance (ANCOVA) with the pretreatment value used as a covariate. The model included the fixed effect of ‘treatment’ with the main effect ‘site’ and the interaction term ‘treatment by site’ treated as random variables. In addition, for each variable, the frequency of cases with values higher, within or lower than the reference interval (RI) (denoted ‘high’, ‘normal’ or ‘low’, respectively) was calculated. A Cochran–Mantel–Haenszel (CMH) test was performed on the frequency of cats moving from ‘low’ or ‘normal’ at pretreatment to ‘high’ at study conclusion, and moving from ‘high’ or ‘normal’ at pretreatment to ‘low’ at the study conclusion.
Results
A total of 194 cats (108 females, 86 males) were recruited between July 2007 and October 2008 at 26 veterinary centres in various geographic locations within the USA. The cats were aged 8 months to 19 years 10 months (67% of the cats were ⩾12 years old) and weighed 2.8–9.7 kg at enrolment. A total of 14 breeds were represented, primarily domestic shorthair (126 cats). All cats were either castrated or spayed. Enrolment included cats with pre-existing endocrine (hyperthyroidism, diabetes mellitus) and cardiac (murmur) disorders, and CKD, in addition to a confirmed diagnosis of OA. Evidence of OA was observed most frequently in the elbow (55 in placebo group, 59 in robenacoxib group), hip (33 in placebo group, 31 in robenacoxib group), stifle (59 in placebo group, 49 in robenacoxib group) and lumbar-sacral area of the spine (57 in placebo group, 57 in robenacoxib group). For the spinal cases, it was not recorded if the synovial facets were affected, as is required for a diagnosis of OA. Spinal cases with no synovial involvement should be classified as DJD.
There were no clinically relevant differences between the cats randomized to the placebo or robenacoxib groups. Of the 194 cats, one cat did not receive any test item owing to treatment for a urinary tract infection; therefore, safety data were analyzed for 193 cats (98 received placebo and 95 robenacoxib).
Owner-reported AEs
One hundred and two AE reports from 70 cats were documented during the study (Table 2). Thirty-three cats with reported AEs (48 reports) were in the placebo group and 37 cats with reported AEs (54 reports) were in the robenacoxib group. Differences were not statistically significant (P = 0.46). Clinical signs of AEs reported at least twice are shown in Table 3. Gastrointestinal tract disorders, primarily vomiting, were the most frequently reported AEs. The following AEs were reported only once for cats in the placebo group: abdominal distension, adipsia, change in fecal color, ear irritation, elevated ALT, elevated thyroxine, headshake, hematochezia, conjunctival irritation, lung sound, nasal discharge, ocular discharge, polyuria, respiratory congestion and vocalization. The following AEs were reported only once for cats treated with robenacoxib: abnormal behavior, conjunctivitis, elevated renal enzymes (azotemia), loose stool, malodorous stool, oliguria, pain, scaling skin eruption, sleepiness, sneezing and trauma (bloody discharge on head).
Table 2.
Adverse event (AE) reports
| Treatment group | Seriousness of AE |
Total number of AE reports | Number of animals with reported AEs (% of treatment group)* | Total number of clinical signs reported as AEs | |
|---|---|---|---|---|---|
| Non-serious | Serious | ||||
| Placebo (n = 98) | 35 | 13 | 48 | 33 (33.7) | 64 |
| Robenacoxib (n = 95) | 46 | 8 | 54 | 37 (38.9) | 76 |
| Total (n = 193) | 81 | 21 | 102 | 70 (36.3) | 140 |
No significant difference between groups (P = 0.46) with Fisher’s exact test in percentage of AE occurrence (yes/no to at least one AE experienced)
Table 3.
Frequency of preferred terminology classification of adverse events (AEs)
| Preferred term | Placebo (n = 98) | Robenacoxib (n = 95) | Total (n = 193) |
|---|---|---|---|
| Vomiting | 21 | 19 | 40 |
| Periodic* | 3 | 10 | 13 |
| Multiple* | 7 | 0 | 7 |
| Single occurrence | 11 | 9 | 20 |
| Anorexia | 4 | 8 | 12 |
| Lethargy | 7 | 3 | 10 |
| Diarrhea | 5 | 2 | 7 |
| Hiding | 1 | 3 | 4 |
| Weight loss | 2 | 1 | 3 |
| Hyposthenuria | 0 | 2 | 2 |
| Malaise | 0 | 2 | 2 |
| Stiffness | 0 | 2 | 2 |
Only AEs reported twice or more are shown
Periodic corresponds to vomiting occurring occasionally throughout the study; multiple corresponds to vomiting occurring more than once in a sequence over a period of time
Reports that were deemed clinically serious included cases that were moderate to severe in severity and required medical intervention. Twenty-one AEs were considered clinically serious (13 reports from 10 cats treated with placebo, eight reports from eight cats treated with robenacoxib). There were no deaths or euthanasia cases reported during the study.
A total of 60 cats (30 from each group) were reported with abnormal findings on post-study follow-up telephone contact with the owners. The majority of the findings consisted of OA-associated pain and behavior-related changes (eg, stiff, slow, reluctance to jump, lethargy).
Body weight
There was no statistically significant difference in body weight change (P = 0.83) between the placebo and robenacoxib groups. Mean (SD) values at baseline, study exit and the change from baseline were, respectively, 5.6 (1.6), 5.6 (1.5) and −0.03 (0.3) kg in the placebo group and 6.1 (1.6), 6.1 (1.7) and −0.02 (0.6) kg in the robenacoxib group. Body weight changes were further evaluated in ‘old’ cats (defined as 12 years or older in age) and ‘young’ cats (less than 12 years of age). No statistically significant differences existed between the groups in body weight change for old or young cats (P = 0.66 and P = 0.71, respectively). For the cats aged ⩾12 years, mean (SD) values at baseline, study exit and the change from baseline were, respectively, 5.2 (1.4), 5.1 (1.3) and −0.05 (0.2) kg in the placebo group and 5.8 (1.4), 5.7 (1.4) and −0.06 (0.2) kg in the robenacoxib group. For the cats aged <12 years, mean (SD) values at baseline, study exit and the change from baseline were, respectively, 6.5 (1.6), 6.5 (1.5) and +0.03 (0.4) kg in the placebo group and 6.9 (1.9), 7.0 (1.9) and +0.08 (1.0) kg in the robenacoxib group.
Clinical pathology
For serum chemistry, hematology and urinalysis variables, there were no significant differences, using ANCOVA, in change from baseline between groups. Group means were within normal ranges for all clinical pathology variables at baseline and study exit (Tables 4 and 5). There were no differences between groups in the number of cases with values higher or lower than the RI. In addition, there were no significant changes, using the CMH test, for any variable in either group in the number of cases moving from normal or above the RI ranges to below, or from normal or below the RI to above (data not shown).
Table 4.
Selected hepatic and hematological variables at baseline and at study exit in all cats
| Variable | Placebo (n = 98, baseline; n = 94, study exit) |
Robenacoxib (n = 95, baseline; n = 93, study exit) |
P value* | Reference interval | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | No. of cases
†
|
Mean | SD | No. of cases
†
|
|||||
| High Low | High Low | |||||||||
| ALP – baseline | 28.05 | 11.42 | 0 | 1 | 29.67 | 13.52 | 0 | 0 | 0.54 | 6.00–102.00 U/l |
| ALP – study exit | 29.91 | 14.01 | 0 | 1 | 32.04 | 17.44 | 0 | 0 | ||
| ALT – baseline | 63.36 | 47.24 | 10 | 0 | 62.59 | 31.71 | 11 | 0 | 0.55 | 10.00–100.00 U/l |
| ALT – study exit | 63.46 | 41.56 | 13 | 0 | 65.63 | 61.85 | 11 | 0 | ||
| AST – baseline | 31.10 | 18.78 | 2 | 1 | 30.14 | 9.96 | 0 | 2 | 0.44 | 10.00–100.00 U/l |
| AST – study exit | 29.51 | 14.39 | 0 | 0 | 30.43 | 20.04 | 2 | 0 | ||
| Bilirubin – baseline | 0.19 | 0.06 | 0 | 0 | 0.18 | 0.06 | 0 | 0 | 0.15 | 0.10–0.40 mg/dl |
| Bilirubin – study exit | 0.19 | 0.07 | 0 | 0 | 0.17 | 0.06 | 0 | 0 | ||
| Hematocrit – baseline | 36.48 | 5.47 | 3 | 8 | 36.81 | 5.36 | 1 | 9 | 0.90 | 29.00–48.00% |
| Hematocrit – study exit | 37.41 | 5.09 | 1 | 7 | 37.50 | 4.28 | 0 | 1 | ||
| Hemoglobin – baseline | 12.22 | 1.53 | 0 | 3 | 12.26 | 1.61 | 0 | 1 | 0.79 | 9.30–15.90 mg/dl |
| Hemoglobin – study exit | 12.44 | 1.52 | 0 | 1 | 12.51 | 1.38 | 0 | 3 | ||
| Platelet count – baseline ‡ | 260.52 | 100.19 | 1 | 29 | 252.93 | 101.36 | 0 | 32 | 0.91 | 200.00–500.00 103/µl |
| Platelet count – study exit ‡ | 252.27 | 103.02 | 1 | 28 | 247.72 | 106.34 | 0 | 37 | ||
| Red cell count – baseline | 8.03 | 1.10 | 1 | 3 | 8.11 | 1.23 | 1 | 0 | 0.83 | 5.92–9.93 106/µl |
| Red cell count – study exit | 8.24 | 1.03 | 1 | 4 | 8.30 | 1.10 | 2 | 1 | ||
P values are for the comparison of groups for change from baseline using analysis of covariance (ANCOVA)
The number of cases with values higher (high) or lower (low) than the reference interval
Platelet clumps were noted in several cases preventing precise determination of count and falsely decreasing the platelet number
ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase
Table 5.
Selected renal variables at baseline and study exit in all cats
| Variable | Placebo (n = 98, baseline; n = 94, study exit) |
Robenacoxib (n = 95, baseline; n = 93, study exit) |
P value* | Reference interval | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | No. of cases
†
|
Mean | SD | No. of cases
†
|
|||||
| High Low | High Low | |||||||||
| Urea nitrogen – baseline | 31.90 | 8.15 | 24 | 0 | 31.57 | 10.13 | 20 | 0 | 0.16 | 14.00–36.00 mg/dl |
| Urea nitrogen – study exit | 32.01 | 9.02 | 23 | 0 | 32.88 | 11.77 | 25 | 0 | ||
| Creatinine – baseline | 1.65 | 0.43 | 6 | 0 | 1.62 | 0.37 | 2 | 0 | 0.55 | 0.60–2.40 mg/dl |
| Creatinine – study exit | 1.67 | 0.45 | 8 | 0 | 1.67 | 0.43 | 4 | 0 | ||
| BUN:creatinine ratio – baseline | 19.89 | 5.20 | 2 | 0 | 19.81 | 4.93 | 2 | 0 | 0.72 | 4.00–33.00 |
| BUN:creatinine ratio – study exit | 19.96 | 6.00 | 3 | 0 | 20.16 | 5.64 | 4 | 0 | ||
| Total protein – baseline | 7.42 | 0.55 | 0 | 0 | 7.53 | 0.59 | 2 | 0 | 0.51 | 5.20–8.80 mg/dl |
| Total protein – study exit | 7.63 | 0.58 | 1 | 0 | 7.64 | 0.52 | 1 | 0 | ||
| Albumin – baseline | 3.29 | 0.27 | 1 | 0 | 3.28 | 0.30 | 0 | 0 | 0.54 | 2.50–3.90 mg/dl |
| Albumin – study exit | 3.32 | 0.25 | 1 | 0 | 3.34 | 0.30 | 1 | 0 | ||
| Placebo (n = 97, baseline; n = 90, study exit) | Robenacoxib (n = 95, baseline; n = 87, study exit) | P value* | Reference interval | |||||||
| Mean | SD | No. of cases
†
|
Mean | SD | No. of cases
†
|
|||||
| High Low | High Low | |||||||||
| USG – baseline | 1.04 | 0.02 | 6 | 0 | 1.04 | 0.01 | 8 | 2 | 0.12 | 1.015–1.060 |
| USG – study exit | 1.04 | 0.02 | 4 | 2 | 1.04 | 0.02 | 9 | 1 | ||
P values are for the comparison of groups for change from baseline using analysis of covariance (ANCOVA)
The number of cases with values higher (high) or lower (low) than the reference interval
BUN = blood urea nitrogen; USG = urine specific gravity
Safety assessment in cats with pre-existing CKD
A total of 40 cats of various breeds and a median of 15 years old (range 6–20 years) were identified as having pre-existing CKD. At baseline (day 0), the number of cases in the placebo and robenacoxib groups were IRIS stage 2 (21 in the placebo group and 18 in the robenacoxib group), stage 3 (1 in the placebo group and 0 in the robenacoxib group); there were no cases with stage 4 disease. None of the identified cases received specific treatment for CKD during the study or throughout the follow-up period.
In the cats with CKD, there was no significant change in body weight from baseline for either the placebo (P = 0.13) or robenacoxib (P = 0.55) groups, and no difference between groups (P = 0.47). Mean (SD) values at baseline, study exit and the change from baseline were, respectively, 5.9 (1.3), 5.8 (1.2) and −0.03 (0.2) kg in the placebo group and 4.9 (1.2), 4.8 (1.2) and −0.05 (0.1) kg in the robenacoxib group.
For clinical pathology (serum chemistry, hematology and urinalysis), the only variable with a significant difference between the groups was CPK, which was significantly lower after treatment with placebo (P = 0.04; Tables 6 and 7). This result was due to a marked decrease from baseline (216.38 to 137.33 U/l) in the placebo group, with no relevant change in the robenacoxib group (227.11 to 233.61 U/l).
Table 6.
Selected hepatic and hematological variables at baseline and at study exit in the subgroup of cats with chronic kidney disease
| Variable | Placebo (n = 21, baseline and study exit) |
Robenacoxib (n = 18, baseline and study exit) |
P value* | Reference interval | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | No. of cases
†
|
Mean | SD | No. of cases
†
|
|||||
| High Low | High Low | |||||||||
| ALP – baseline | 23.76 | 8.53 | 0 | 0 | 28.28 | 11.75 | 0 | 0 | 0.88 | 6.00–102.00 U/l |
| ALP – study exit | 26.62 | 9.64 | 0 | 0 | 31.33 | 20.56 | 0 | 0 | ||
| ALT – baseline | 50.29 | 15.06 | 0 | 0 | 53.72 | 19.98 | 1 | 0 | 0.33 | 10.00–100.00 U/l |
| ALT – study exit | 50.62 | 17.35 | 0 | 0 | 58.28 | 24.43 | 2 | 0 | ||
| AST – baseline | 25.14 | 7.70 | 0 | 0 | 30.70 | 8.78 | 0 | 0 | 0.31 | 10.00–100.00 U/l |
| AST – study exit | 23.48 | 5.61 | 0 | 0 | 34.83 | 26.57 | 0 | 0 | ||
| Bilirubin – baseline | 0.18 | 0.05 | 0 | 0 | 0.17 | 0.05 | 0 | 0 | 0.58 | 0.10–0.40 mg/dl |
| Bilirubin – study exit | 0.19 | 0.08 | 0 | 0 | 0.20 | 0.07 | 0 | 0 | ||
| Hematocrit – baseline | 33.05 | 5.59 | 0 | 5 | 35.70 | 5.22 | 0 | 1 | 0.93 | 29.00–48.00% |
| Hematocrit – study exit | 34.63 | 4.90 | 0 | 4 | 35.61 | 4.00 | 0 | 0 | ||
| Hemoglobin – baseline | 11.34 | 1.64 | 0 | 3 | 11.83 | 1.52 | 0 | 1 | 0.76 | 9.30–15.90 mg/dl |
| Hemoglobin – study exit | 11.63 | 1.24 | 0 | 2 | 11.94 | 1.30 | 0 | 0 | ||
| Platelet count – baseline ‡ | 247.71 | 118.33 | 0 | 8 | 251.89 | 112.26 | 0 | 6 | 0.80 | 200.00–500.00 103/µl |
| Platelet count – study exit ‡ | 248.81 | 122.51 | 1 | 8 | 255.39 | 113.10 | 0 | 7 | ||
| Red cell count – baseline | 7.54 | 1.23 | 0 | 4 | 7.75 | 1.40 | 1 | 1 | 0.51 | 5.92–9.93 106/µl |
| Red cell count – study exit | 7.90 | 1.14 | 1 | 1 | 7.83 | 1.11 | 0 | 0 | ||
P values are for the comparison of groups for change from baseline using analysis of covariance (ANCOVA)
The number of cases with values higher (high) or lower (low) than the reference interval
Platelet clumps were noted in several cases preventing precise determination of count and falsely decreasing the platelet number
ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase
Table 7.
Selected renal variables at baseline and study exit in the subgroup of cats with chronic kidney disease
| Variable | Placebo (n = 21, baseline and study exit) |
Robenacoxib (n = 18, baseline and study exit) |
P value* | Reference interval | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | No. of cases
†
|
Mean | SD | No. of cases
†
|
|||||
| High Low | High Low | |||||||||
| Urea nitrogen – baseline | 38.48 | 8.23 | 9 | 0 | 40.89 | 15.27 | 11 | 0 | 0.66 | 14.00–36.00 mg/dl |
| Urea nitrogen – study exit | 37.95 | 10.18 | 13 | 0 | 41.61 | 19.45 | 12 | 0 | ||
| Creatinine – baseline | 2.21 | 0.37 | 3 | 0 | 2.00 | 0.32 | 2 | 0 | 0.76 | 0.60–2.40 mg/dl |
| Creatinine – study exit | 2.23 | 0.40 | 0 | 0 | 2.02 | 0.41 | 0 | 0 | ||
| BUN:creatinine ratio – baseline | 17.62 | 3.53 | 0 | 0 | 20.17 | 5.04 | 1 | 0 | 0.43 | 4.00–33.00 |
| BUN:creatinine ratio – study exit | 17.14 | 3.98 | 0 | 0 | 20.17 | 4.91 | 0 | 0 | ||
| Total protein – baseline | 7.53 | 0.54 | 0 | 0 | 7.56 | 0.58 | 0 | 0 | 0.19 | 5.20–8.80 mg/dl |
| Total protein – study exit | 7.78 | 0.59 | 1 | 0 | 7.64 | 0.34 | 0 | 0 | ||
| Albumin – baseline | 3.20 | 0.18 | 0 | 0 | 3.28 | 0.28 | 0 | 0 | 0.91 | 2.50–3.90 mg/dl |
| Albumin – study exit | 3.28 | 0.22 | 0 | 0 | 3.35 | 0.33 | 0 | 0 | ||
| Placebo (n = 20, baseline and study exit) |
Robenacoxib (n = 16, baseline and study exit) |
P value* | Reference interval | |||||||
| Mean | SD | No. of cases
†
|
Mean | SD | No. of cases
†
|
|||||
| High Low | High Low | |||||||||
| USG – baseline | 1.02 | 0.00 | 0 | 0 | 1.02 | 0.00 | 0 | 1 | 0.46 | 1.015–1.060 |
| USG – study exit | 1.02 | 0.01 | 0 | 1 | 1.02 | 0.00 | 0 | 0 | ||
P values are for the comparison of groups for change from baseline using analysis of covariance (ANCOVA)
The number of cases with values higher (high) or lower (low) than the reference interval
BUN = blood urea nitrogen; USG = urine specific gravity
There were no significant differences between groups for change from baseline for serum creatinine (P = 0.76), urea nitrogen (P = 0.66) or USG (P = 0.46). During the study, the study, the majority of cats across groups (87.5%) had no change in IRIS stage; three cases treated with the placebo and one case that received robenacoxib had an improvement in IRIS stage; and one case treated with robenacoxib had a change from IRIS stage 2 to stage 3 (creatinine increased from 2.3 mg/dl to 3.0 mg/dl and USG decreased from 1.044 to 1.039, with an absence of clinical signs and with no follow-up findings).
Thirteen of the 40 cats identified to have CKD reported AEs (seven cats treated with placebo and six with robenacoxib), including 15 clinical signs (Table 8). The only signs that occurred more than once were lethargy and vomiting (each sign was reported in both groups). A total of five cases were considered to have had a clinically serious AE (three cats treated with placebo, two cats treated with robenacoxib).
Table 8.
Summary of clinical abnormalities reported for cases with evidence of concurrent chronic kidney disease (CKD)
| Case | Treatment | Pre-study | During the study | Post-study follow-up |
|---|---|---|---|---|
| 1 | Placebo | Mild dental tartar, iris atrophy, overweight | Punctured ear pinnae and abscess | – |
| 2 | Robenacoxib | Hyperthyroidism, diabetes mellitus, bradycardia | Psoriasiform scale/crusting | – |
| 3 | Robenacoxib | Hyperthyroidism, CKD, dental disease | Conjunctivitis | – |
| 4* | Placebo | CKD | Vomited | – |
| 5 | Robenacoxib | Blood in urine, constipation | Loose stool | Owner reported cat became stiffer when walking after ending the trial |
| 6 | Robenacoxib | Blood in urine, increased amylase, low platelets | Vomited food | Owner reported cat much stiffer since ending trial |
| 7 | Placebo | Abscess right cheek | Head shake | Owner reported cat is worse since stopping study; falls, limps and is hesitant to jump |
| 8 | Placebo | Anemia, urinary tract infection, bilateral nuclear sclerosis, dental disease | Vomited once | – |
| 9* | Placebo | Congestion/wheezing | Congestion, coughing † | – |
| 10* | Robenacoxib | Bacteria in urine | Lethargy, anorexia | – |
| 11* | Placebo | Dental tartar | Lethargy | The cat’s behavior returned to normal with no lethargy or pain |
| 12* | Robenacoxib | Chin acne | Straining to urinate, bloody urine, frequent urination ‡ | – |
| 13 | Placebo | – | Vomited | – |
With reported serious adverse events
Associated with feline asthma
Diagnosed with masses in the bladder
Although there were no deaths or euthanasias reported for any animal in the study, one robenacoxib-treated cat with pre-existing CKD was euthanized after the follow-up period owing to a ruptured eye associated with a pre-existing eye injury. In addition, one 14-year-old cat treated with robenacoxib was euthanized more than 1 month after the study owing to CKD. The cat had pre-existing CKD prior to the study (serum creatinine concentration 2.0 mg/dl, USG 1.026) and had no reported AEs during or after robenacoxib treatment, although serum creatinine increased to 2.5 mg/dl after treatment (USG 1.021).
Risk and number needed to harm
The number needed (to be treated) to harm (NNH) over the one month treatment period was calculated as follows. For the number of cats with any AE, the incidence of harm was 37/95 (0.39) with robenacoxib and 33/98 (0.34) with placebo. Therefore, the relative risk was 1.16 (incidence robenacoxib/incidence placebo), the attributable risk (AR) was 0.053 (incidence robenacoxib minus incidence placebo) and the NNH was 19.0 (1/AR). For serious AEs, the incidence of harm was 8/95 (0.084) with robenacoxib and 10/98 (0.10) with placebo, the relative risk was 0.83, and both the AR and NNH were negative as robenacoxib was associated with fewer serious AEs than placebo.
Similar results were obtained if the total number of AEs are considered; that is, with multiple AEs in individual cats treated as independent events. For that method, for any AE, the incidence of harm was 54/95 (0.57) with robenacoxib and 48/98 (0.49) with placebo, the relative risk was 1.16, the AR 0.079 and the NNH 12.7. For serious AEs, the incidence of harm was 8/95 (0.084) with robenacoxib and 13/98 (0.13) with placebo, the relative risk was 0.63 and the AR and NNH were both negative.
In the subgroup of 40 cats with CKD, for the number of cats with all AEs, the relative risk was 1.05, the AR 0.015 and the NNH 66. For the cats with CKD, the relative risk for serious AEs was <1 (0.81) and therefore both the AR and NNH were negative.
Discussion
Robenacoxib was well tolerated with no clinically detected evidence of damage to the gastrointestinal tract, kidney or liver when administered for one month to cats with OA, including in the subgroup of cats with concurrent CKD. The presence of CKD was based on the IRIS criteria of serum creatinine concentration ⩾1.6 mg/dl and USG <1.030, 21 but additional evidence of renal dysfunction was not obtained, so the full extent of renal impairment in the subgroup is not known.
Interestingly, the incidence of reported AEs (48 with placebo, 54 with robenacoxib) and serious AEs (13 with placebo, eight with robenacoxib) was similar in both groups. The most frequent AE was vomiting, but it occurred with similar frequency across treatment groups. There was no indication of clinically relevant changes from baseline or differences between the two treatment groups for any of the serum chemistry, hematology or urinalysis variables. Distinct age, breed or sex predilection for AE reporting was not observed. These data emphasize the need for prospective, placebo-controlled, blinded studies when assessing clinical safety in the target population. If there had been no placebo group in this study it would have appeared that there was a high number of AEs reported with the drug, when, in fact, the frequency of AEs was nearly identical to the placebo group.
Furthermore, it is important to note that the study showed no differences in any safety endpoints in cats with concurrent CKD. The present study found no indication of increased risk in the frequency of reported AEs, or deterioration in renal variables, in the subgroup of cats with concurrent CKD. It was reported that cats commonly have concurrent CKD, 22 and thus they are often considered to be at particular risk for NSAID-related renal toxicity. 4 However, whether cats with CKD are actually more at risk or not has not been established. The data presented here concur with research data that showed no changes in renal function assessed by glomerular filtration rate and urine protein:creatinine ratio in normal and reduced renal mass cats when given meloxicam and acetylsalicylic acid. 23 In addition, in a clinical study, prolonged treatment with very low dose meloxicam (0.02 mg/kg/day) did not result in significant worsening of renal values in cats with CKD. 24
The relative risk of a cat experiencing an AE with robenacoxib compared with placebo was marginally >1 for all AEs (1.16) but was <1 for the more clinically relevant serious AEs (0.83). Similar results were obtained in the subgroup of cats with CKD, with relative risks of 1.05 (all AEs) and 0.81 (serious AEs). Therefore, for serious AEs, the risk of harm was lower with robenacoxib compared with placebo for all cats and the cats with CKD, and consequently the AR and NNH were negative in both groups.
The strengths of this study include the prospective, randomized and blinded design with a relatively large population of cats (n = 98, placebo; n = 95, robenacoxib) and a duration of treatment of 1 month compared with the 3–6 day approved dosing duration for robenacoxib. The main limitation of the study is its relatively low power to detect uncommon, but potentially serious, AEs. With a total of 95 cats in the robenacoxib group, the study only had 95% power to detect AEs with a true incidence ⩾3%, and had only 62% power to detect AEs with a true incidence of 1%. As only 18 cats (10 receiving placebo and eight receiving robenacoxib) had reported serious AEs, the calculated standard harm parameters – relative risk, AR and NNH – are not highly reliable. In addition, the methods employed do not have high sensitivity for detection of some potential NSAID-related adverse effects, notably damage to the gastrointestinal tract or changes in renal function.
Conclusions
Robenacoxib was well tolerated with no clinically detected evidence of damage to the gastrointestinal tract, kidney or liver when administered for 1 month to cats with OA, including a subgroup of cats with evidence of concurrent CKD (IRIS stages 1, 2 and 3). Further studies are recommended in a larger population of cats with CKD.
Acknowledgments
We thank the following investigators for managing the clinical cases: Drs Christa Branch, Jane Brunt, Jay Butan, Kathyrn Christensen, Deborah Edwards-Pettey, Sam Geller, Cay Gilbertson, Mary Gray, Kelly Jones, Alice Johns, Jason Laramore, Marcia Levine, David Loehndorf, Catherine Lund, Elizabeth Martinez, Darryl Millis, Karen Mueller, Cynthia Rigoni, Kristi Rowland, Tammy Sadek, Linda Schoenberg, Leigh Sheridan, Wendy Simpson, Craig Smith, Susan Streeter, Emily Walker, Elaine Wexler-Mitchell and Judy Zinn. We also thank colleagues from Novartis Animal Health for monitoring the study, and Dr Michael Metcalf for evaluating the radiographs.
Footnotes
Funding: The study was funded by Novartis Animal Health, now owned by Elanco Animal Health, which manufactures and markets robenacoxib (Onsior®).
At time of the study JNK, SK, SEB, LMR and ESR were employees of Novartis. BDXL has received honoraria from Novartis Animal Health for consulting services and for contributing to Novartis Animal Health-sponsored continuing education. BDXL has also received funding from Novartis Animal Health for research support.
Accepted: 18 May 2015
References
- 1. Clarke SP, Bennett D. Feline osteoarthritis: a prospective study of 28 cases. J Small Anim Pract 2006; 47: 439–445. [DOI] [PubMed] [Google Scholar]
- 2. Sul RM, Chase D, Parkin T. Comparison of meloxicam and a glucosamine-chondroitin supplement in management of feline osteoarthritis. A double-blind randomised, placebo-controlled, prospective trial. Vet Comp Orthop Traumatol 2014; 27: 20–26. [DOI] [PubMed] [Google Scholar]
- 3. Lascelles BDX, Henry JB, Brown J, et al. Cross-sectional study of the prevalence of radiographic degenerative joint disease in domesticated cats. Vet Surg 2010; 39: 535–544. [DOI] [PubMed] [Google Scholar]
- 4. Lascelles BDX, Court MH, Hardie EM. Nonsteroidal anti-inflammatory drugs in cats: a review. Vet Anaesth Analg 2007; 34: 228–250. [DOI] [PubMed] [Google Scholar]
- 5. Benito J, DePuy V, Hardie E, et al. Reliability and discriminatory testing of a client-based metrology instrument, feline musculoskeletal pain index (FMPI) for the evaluation of degenerative joint disease-associated pain in cats. Vet J 2013; 196: 368–373. [DOI] [PubMed] [Google Scholar]
- 6. Benito J, Hansen B, DePuy V, et al. Feline musculoskeletal pain index (FMPI): Responsiveness and testing of criterion validity. J Vet Intern Med 2013; 27: 474–482. [DOI] [PubMed] [Google Scholar]
- 7. Charlton AN, Benito J, Simpson W, et al. Evaluation of the clinical use of tepoxalin and meloxicam in cats. J Feline Med Surg 2013; 15: 678–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gruen ME, Jiamachello KN, Thomson A. Clinical trials involving cats: what factors affect owner participation? J Feline Med Surg 2014; 16: 727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. European Medicines Agency. Scientific discussion (Metacam). http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/veterinary/000033/WC500065773.pdf (2010, accessed January 24, 2014).
- 10. Gunew MN, Menrath VH, Marshall RD. Long-term safety, efficacy and palatability of oral meloxicam at 0.01–0.03 mg/kg for treatment of osteoarthritic pain in cats. J Feline Med Surg 2008; 10: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lascelles BDX, Hansen BD, Roe S, et al. Evaluation of client-specific outcome measures and activity monitoring to measure pain in cats with osteoarthritis. J Vet Intern Med 2007; 21: 410–416. [DOI] [PubMed] [Google Scholar]
- 12. Giraudel JM, Gruet P, Alexander DG, et al. Evaluation of orally administered robenacoxib versus ketoprofen for treatment of acute pain and inflammation associated with musculoskeletal disorders in cats. Am J Vet Res 2010; 71: 710–719. [DOI] [PubMed] [Google Scholar]
- 13. King S, Roberts ES, Roycroft LM. Evaluation of oral robenacoxib for the treatment of postoperative pain and inflammation in cats; results of a randomized clinical trial. ISRN Vet Sci 2012; 794148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sano T, King JN, Seewald W, et al. Comparison of oral robenacoxib and ketoprofen for the treatment of acute pain and inflammation associated with musculoskeletal disorders in cats: a randomized clinical trial. Vet J 2012; 193: 397–403. [DOI] [PubMed] [Google Scholar]
- 15. King JN, Hotz R, Reagan EL, et al. Safety of oral robenacoxib in the cat. J Vet Pharmacol Ther 2012; 35: 290–300. [DOI] [PubMed] [Google Scholar]
- 16. US Food and Drug Administration. Guidance for industry. Good clinical practice. VICH GL9. Final guidance. http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/ucm052417.pdf (2001, accessed July 11, 2014).
- 17. US Food and Drug Administration. Code of federal regulations (Title 21, Part 511.1 New animal drugs for investigational use). http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=511.1. (2004, accessed July 11, 2014).
- 18. CONSORT. CONSORT 2010 checklist. www.consort-statement.org (2010, accessed February 10, 2015).
- 19. United States Department of Agriculture National Agricultural Library. Legislative history of the Animal Welfare Act: introduction. www.nal.usda.gov/awic/pubs/AWA2007/intro.shtml (1996, accessed July 11, 2014).
- 20. King JN, Jung M, Maurer MP, et al. Effects of route of administration and feeding schedule on pharmacokinetics of robenacoxib in cats. Am J Vet Res 2013; 193: 397–403. [DOI] [PubMed] [Google Scholar]
- 21. International Renal Interest Society. IRIS staging of CKD (modified 2013). www.IRIS-kidney.com (accessed January 24, 2014).
- 22. Marino CL, Lascelles BDX, Vaden SL, et al. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg 2013; 16: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Surdyk KK, Brown CA, Brown SA. Evaluation of glomerular filtration rate in cats with reduced renal mass and administered meloxicam and acetylsalicylic acid. Am J Vet Res 2013; 74: 648–51. [DOI] [PubMed] [Google Scholar]
- 24. Gowan RA, Lingard AE, Johnston L, et al. Retrospective case-control study of the effects of long-term dosing with meloxicam on renal function in aged cats with degenerative joint disease. J Feline Med Surg 2011; 13: 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]