Abstract
Infection of inbred mouse strains with Borrelia burgdorferi results in the development of experimental Lyme arthritis. The degree of arthritic pathology has been suggested to correlate with the level of spirochete burden within tissues. To investigate this further, we infected resistant DBA/2 (DBA) and susceptible C3H/HeJ (C3H) mice in the hind footpads and monitored arthritis development for 21 days. To quantitate levels of spirochetes within tissues, we created a competitive PCR molecule containing modified ospA and fla gene segments. C3H mice developed severe arthritis of the tibiotarsal joints, while DBA mice developed only mild inflammation throughout the experimental period. At day 21, when the gross size and histologic composition of ankles revealed significant differences in arthritis between the strains, there was little difference in levels of spirochete DNA as determined by competitive PCR. Cultures of ankle tissue at day 21 were also uniformly positive in both C3H and DBA animals and contained relatively similar levels of spirochetes. These results indicate that the presence of spirochetes in the ankles of experimental animals is not sufficient for arthritis development. Since arthritic and nonarthritic animals can harbor relatively equal spirochete burdens yet retain their distinct phenotypic outcomes, an aberrant or overly exuberant immune response may be an additional requirement for pathology in arthritis-prone mice.
Lyme disease is a multisystemic illness caused by infection with the spirochete Borrelia burgdorferi (27). Left untreated, infected individuals usually develop a recurring arthritis in one or multiple joints and occasionally carditis or neurologic disease. Antibiotic treatment results in resolution of disease in most individuals, indicating that spirochetal presence is needed for the persistence of pathology. In some individuals, however, arthritis persists despite adequate antibiotic therapy and an inability to detect spirochetes in inflamed tissues (26). Individuals having the major histocompatibility complex class II allele HLA-DR2 or -DR4 appear to be more likely to develop severe arthritis of longer duration following Borrelia infection (28). This suggests that the host immune response is also an important determinant for development of pathology.
In experimental models, cellular and humoral immune responses to B. burgdorferi (12, 15, 24), T-helper cell phenotype (16, 18), and levels of spirochete virulence (1, 4) influence arthritis development. C3H/HeJ (C3H) mice develop severe arthritis and BALB/c mice develop mild arthritis upon intradermal infection with B. burgdorferi (6). Experimental Lyme arthritis in mice is transient, is usually most pronounced at 14 to 30 days, and then resolves over the next 60 days. Several studies have suggested that the development of arthritis in susceptible mouse strains correlates with higher numbers of spirochetes in these hosts (7, 21, 29). The presence of viable spirochetes in the joints is required for arthritis development, as injection of heat-killed spirochetes or Borrelia antigen does not result in pathology (5, 17). Mice can remain chronically infected, however, without developing arthritis (10). In addition, experimental intervention may result in a reduction of peak arthritis severity yet cause an increase in spirochete burden (2). The precise role that spirochete burden plays in determining disease phenotype, therefore, remains unclear.
We created a competitive PCR construct containing modified Borrelia ospA and fla genes. This technique allowed us to compare the relative presence of Borrelia DNA, both plasmid and genomic, in murine tissues after standardization to a single-copy mammalian gene (IL4pr). We found that following footpad injection of B. burgdorferi, arthritis-susceptible C3H and arthritis-resistant DBA/2 (DBA) mice contain similar levels of spirochetes within their ankles during the time of peak arthritis development. These results indicate that the presence of significant numbers of spirochetes in ankles of inbred mouse strains is not sufficient for the development of Lyme arthritis. This suggests that unique host factors, and not merely microbial presence, are needed for arthritis development in susceptible strains of mice.
MATERIALS AND METHODS
Mice and infections.
Female C3H and DBA mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). All mice were between 4 and 6 weeks of age at the time of infection. B. burgdorferi N40 was kindly provided by Steven Barthold (Yale University, New Haven, Conn.). Spirochetes were reisolated from severe combined immunodeficiency mice, passaged twice in Barbour-Stoenner-Kelly II medium (Sigma Chemical Co., St. Louis, Mo.), and frozen in aliquots at −80°C. For infections, an aliquot was thawed, placed in 7 ml of medium, and grown for 5 days at 32°C. Mice were inoculated in both hind footpads with 106 B. burgdorferi organisms in 50 μl of medium. Tibiotarsal joints were measured weekly with a metric caliper (Ralmike’s Tool-A-Rama, South Plainfield, N.J.) through the thickest anteroposterior diameter of the ankle. Blood, heart, spleen, urinary bladder, skin, and ankles were aseptically collected and cultured at 32°C for 14 days in Barbour-Stoenner-Kelly II medium. Cultures were scored by placing 10 μl of supernatant on a microscope slide under a cover- slip (22 by 22 mm) and examining 20 high-power fields by dark-field microscopy.
Histology.
Mice were sacrificed 21 days following infection; the ankles were washed with 70% ethanol, and the skin was removed. The sample was excised by cutting just above and below the ankle joint and placing it in 10% buffered formalin. After several days, the sample was embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Arthritis severity scores were determined in a blinded manner and rated on a scale of 0 to 3 (9). Grade 0 represents no inflammation, grades 1 and 2 represent mild-to-moderate inflammation, and grade 3 represents severe inflammation.
Construction of BC3 PCR competitor.
The BC3 Borrelia competitive PCR molecule was constructed by utilizing a strategy similar to that employed by Reiner et al. (23), except that bacterial genomic or plasmid DNA, rather than mammalian cDNA, was modified. Briefly, genomic and plasmid DNAs were isolated from a B. burgdorferi culture or a mouse spleen with sodium dodecyl sulfate-Tris lysis buffer (0.1-mg/ml proteinase K in 200 mM NaCl–20 mM Tris-HCl [pH 8.0]–50 mM EDTA–0.2% sodium dodecyl sulfate). Segments of the genes of interest were amplified by PCR (see PCR conditions) using the primer sets listed below. The amplified products were isolated by gel purification from low-melting-point agarose and ligated into plasmid pGEM11Z(f), which had been linearized by blunt-end digestion and incubated with dTTP and Taq polymerase. The individual plasmids containing the three gene segments of interest were linearized by using unique internal restriction enzyme sites, and the ends were dephosphorylated. Digestions of genomic DNA were completed by using the same set of restriction enzymes, and approximately 75 bp of DNA was excised from a low-melting-point agarose gel. This small random DNA pool was then ligated into the wild-type fla, ospA, or IL4pr gene product to create addition mutations. Cloned colonies were picked after transformation. Once these addition mutations were made, the fla-containing plasmid was linearized with EcoRI and SacI. The ospA and IL4pr modified gene segment inserts were excised from their plasmids (ospA was excised with SacI and Xho; IL4pr was excised with Xho and EcoRI) and ligated as a trimolecular reaction. The completed competitor plasmid was called pBC3. The polycompetitor insert was excised from pBC3 with SacI and NotI for ease of use in PCR. This competitor insert was called BC3.
DNA extractions.
Tissue samples were collected for PCR analysis at each time point from both strains of mice. Extraction of ankles was performed by removing the skin and cutting just above and below the tibiotarsal joint. All excised ankle samples were approximately equal in size. Exact measurements were not needed, as the amount of tissue used in each PCR was equalized later by using the IL4pr competitor. To extract DNA from ankle tissue, samples were first digested overnight in 0.5 ml of 1% collagenase at 37°C. Supernatants were then transferred to new tubes, 0.25 ml of 3× lysis buffer (see above) was added, and the mixture was incubated at 55°C for 4 h. Following incubation, debris was pelleted and the supernatants were transferred to tubes containing 0.8 ml of isopropanol. Sample DNA was precipitated on ice for 60 min. DNA was pelleted at 4°C, air dried, and resuspended in 100 μl of Tris-EDTA buffer.
PCR conditions.
PCR amplification of both wild-type and BC3 gene segments was performed with the following sets of primers: fla 5′ primer GATGATGCTGCTGGTATGGGGGTTTCT plus fla 3′ primer CCTCTGTCTGCGTCTGAATATGTACCG, ospA 5′ primer TCTTGAAGGAAGTTTAACTGCTG plus ospA 3′ primer CAAGTTTTGTAATTTCAACTGCTGA, and IL4pr 5′ primer GATCAGCTGGGCTAGGATGCGAGA plus IL4pr 3′ primer GGGCCAATCAGCACCTCTCTTCCA. Sample reaction mixtures contained MgCl2 at 2.5 mM for IL4pr and ospA reactions and 1.5 mM for fla reactions. For IL4pr reactions, samples were initially denatured for 60 s, and then the cycling parameters were denaturation at 94°C for 50 s, annealing at 60°C for 30 s, and extension at 72°C for 50 s for 35 cycles. For ospA and fla amplification, an initial 60-s denaturation step was followed by 45 or 47 cycles of denaturation at 94°C for 60 s, annealing at 60°C for 60 s, and extension at 72°C for 90 s. All samples for a given primer set were spiked with equal amounts of BC3 to allow comparisons between samples.
Statistics.
Means of ankle diameters were compared by using the Student t test.
RESULTS
Development of arthritis in experimental animals.
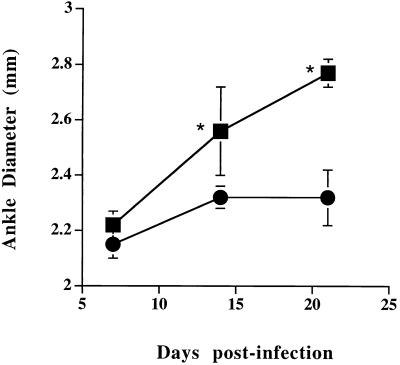
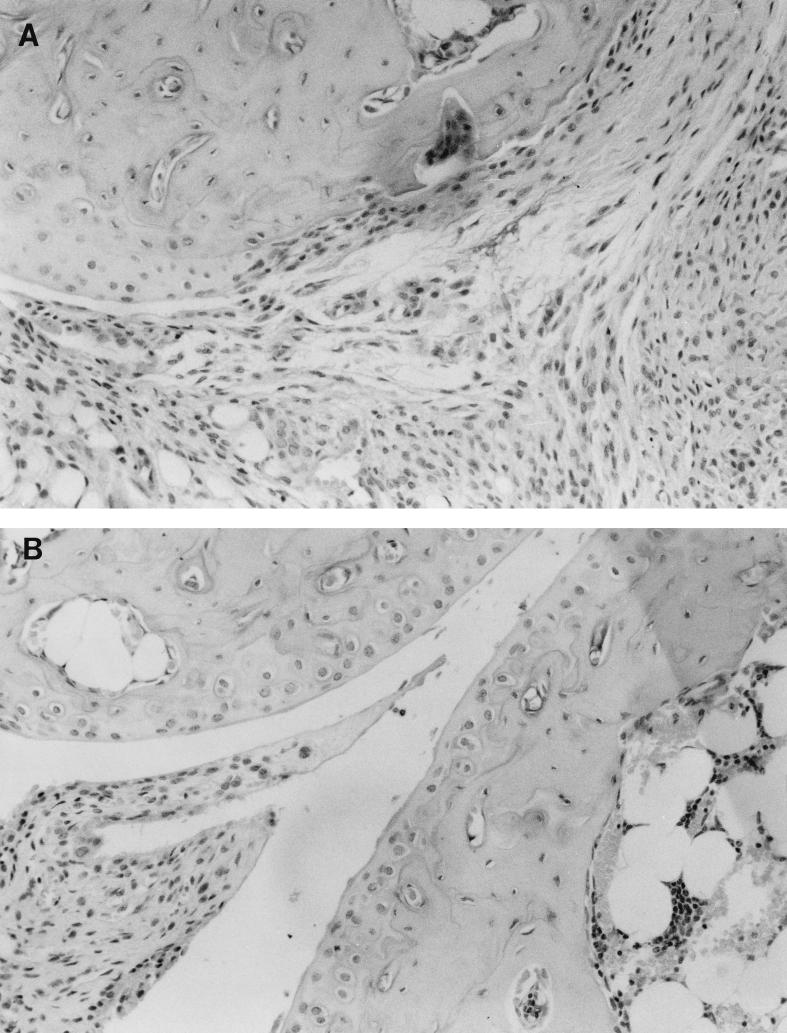
Mice were inoculated in the hind footpads, and arthritis development was monitored for 21 days. Ankle thickness versus time of infection of resistant DBA and susceptible C3H mice is shown in Fig. 1. At 7 days postinfection, there was very little difference in gross ankle size between the two experimental groups. At day 14, however, the ankles of C3H mice were significantly larger than those of DBA mice (P < 0.01). This increase in ankle swelling was still continuing in the C3H mice at day 21. Because ankle diameter does not always correlate with arthritis development (2), ankles of resistant and susceptible animals were examined histologically for the development of inflammatory cell infiltrates and changes typical of arthritis. In C3H mice, increasing ankle size correlated with the development of severe arthritis (Fig. 2). Ankle sections from C3H mice (Fig. 2A) had abundant inflammatory cell infiltrates in the synovia and bursa (not shown), while the ankles of DBA mice (Fig. 2B) had only mild inflammatory infiltrates. Other changes typical of arthritis development, such as pannus formation and thickening of the synovial lining, were easily seen in the ankle sections of C3H, but not DBA, mice. Ankle arthritis severity was scored in a blinded manner with weekly samples (Table 1). There was little difference in arthritis development between C3H and DBA animals at day 7 of infection. By day 14, however, ankles of C3H mice were developing a severe arthritis which was still evident at day 21. During this time, the ankles of resistant DBA mice were only mildly inflamed.
FIG. 1.
Measurements of hind tibiotarsal joints of mice infected with B. burgdorferi. Mice were 4 weeks old at the time of infection in the hind footpad. Squares represent C3H mice, and circles represent DBA mice. Error bars represent standard deviations. Asterisks indicate statistically significant difference (P < 0.01) by the Student t test.
FIG. 2.
Histopathology of tibiotarsal joints from C3H (A) or DBA (B) mice infected with B. burgdorferi. Joints were obtained on day 21 of infection, and paraffin sections were stained with hematoxylin and eosin. Magnification, ×20.
TABLE 1.
Isolation of B. burgdorferi from selected tissues and arthritis development in ankles of DBA and C3H micea
| Strain | Postinfection day | No. of cultures positive
|
Mean arthritis severity ± SD | |||||
|---|---|---|---|---|---|---|---|---|
| Blood | Heart | Spleen | Bladder | Ear | Ankle | |||
| C3H | 7 | 0 | 0 | 0 | 1 | 0 | 3 | 1.0 ± 0.0 |
| DBA | 7 | 0 | 0 | 0 | 1 | 0 | 3 | 1.3 ± 0.6 |
| C3H | 14 | 0 | 2 | 0 | 3 | 0 | 3 | 2.3 ± 0.6 |
| DBA | 14 | 3 | 2 | 0 | 3 | 0 | 3 | 1.0 ± 0.0 |
| C3H | 21 | 0 | 1 | 0 | 3 | 3 | 3 | 2.3 ± 0.6 |
| DBA | 21 | 1 | 1 | 0 | 3 | 3 | 3 | 1.0 ± 0.0 |
Mice were infected with 106 B. burgdorferi spirochetes and sacrificed at 7, 14, and 21 days. Tibiotarsal arthritis development was scored on a scale of 0 to 3. Three cultures of each sample type were prepared.
Assessment of spirochete loads in ankles.
To assess the relative levels of spirochetes in tissues of infected arthritis-resistant and -susceptible mice, three C3H and three DBA mice were randomly sacrificed on days 7, 14, and 21 of infection and their ankles were removed for PCR analysis. We constructed a PCR competitor molecule, BC3, containing addition mutations of the Borrelia fla and ospA and mammalian IL4pr genes (Fig. 3). Both fla and ospA were used because of the possibility of unequal target amplification in tissues (22). The ospA gene is located on a plasmid (11), while fla is a single-copy gene located on the Borrelia linear chromosome (14). In another set of experiments, the levels of spirochetes in blood, hearts, spleens, urinary bladders, skin (ear punches), and ankles were assessed by culture to ensure the presence of live spirochetes within the tissues studied.
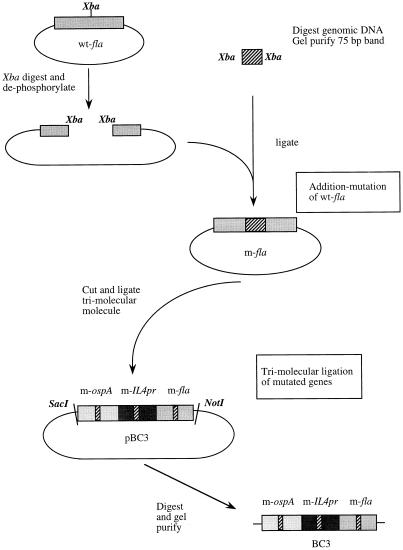
FIG. 3.
Schematic representation of cloning strategy for BC3. Addition mutation is shown for fla only but is the same for ospA and IL4pr. Wild-type gene segments (e.g., wt-fla) were amplified by PCR and cloned into pGEM plasmids. These were internally modified by the addition of a random 75-bp DNA segment (e.g., m-fla). BC3p was created by trimolecular ligation of the modified gene segments into a single molecule. BC3 is the linearized trimolecular gene segment cut from the pGEM plasmid.
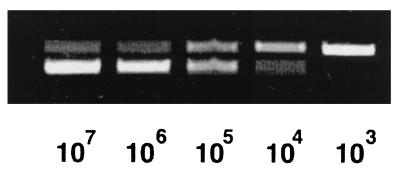
The BC3 polycompetitor insert was used to spike samples to enable the relative assessment of Borrelia DNA in experimental samples. Figure 4 shows a standard curve of ospA amplification. Tenfold dilutions of a DNA sample containing 3 × 107 B. burgdorferi organisms were made, and 5 μl was placed in a PCR. Each reaction was spiked with a constant amount (2.5 pg) of the BC3 competitor. The upper bands in each lane are the BC3 amplification products, and the lower bands are the wild-type DNA PCR products. With decreasing levels of Borrelia DNA, there is a decrease in the intensity of the lower band and a corresponding increase in the upper BC3 band. At the 105 sample dilution, the upper and lower bands are relatively equal, enabling an estimation that 2.5 pg of BC3 contains approximately 1,500 mutated B. burgdorferi genomic equivalents. At a higher cycle number (routinely 45), we were able to amplify sample concentrations 10- to 100-fold more dilute (or lower) than this. Levels of tissue DNA for each sample were equalized by using the single-copy mammalian gene for interleukin 4 (20). For example, DNA extracted from ankles harvested 7 days after infection were placed in PCR mixtures containing a constant amount of the BC3 competitor. Amounts of DNA in each sample were then adjusted during subsequent rounds of PCR amplification until they all contained similar ratios of wild-type to competitor bands. At that point, all samples contained the same amount of mammalian DNA, which controlled for differences in tissue sample sizes and also for variation in the efficiency of DNA extraction. Thus, the ankle samples from C3H and DBA mice can be compared to one another with respect to the levels of ospA or fla that they harbor.
FIG. 4.
Competitive PCR amplification of ospA from known numbers of Borrelia spirochetes. A 5-μl volume of BC3 (2.5 pg) was spiked into 5-μl samples of log dilutions of 3 × 107 B. burgdorferi genomic equivalents. Results are ospA PCR products from 35 cycles of amplification.
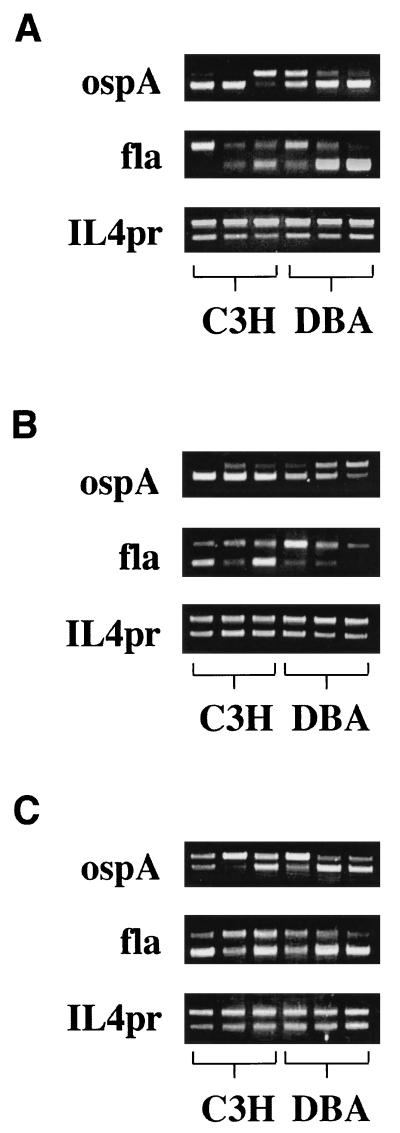
Figure 5 shows the PCR results for three C3H and three DBA ankles at each time point. On day 7, two of the C3H (lanes 1 and 2) and two of the DBA (lanes 5 and 6) mice had high levels of amplification of ospA from their ankles while the other C3H and DBA samples contained lower levels of ospA (Fig. 5A). The chromosomal target fla is less abundant than the plasmid target ospA. To amplify fla, more concentrated samples and higher dilutions of the BC3 competitor were needed to reach a point where two bands could be seen. Thus, some lanes (e.g., Fig. 5A, fla lane 2) contain one or two very light bands. On day 7, there was a high level of amplification of fla gene products from the ankles of two DBA mice (Fig. 5A, lanes 5 and 6), while the other DBA lane had higher amplification of the BC3 competitor than did wild-type fla. The ankles of two C3H mice had amplification of wild-type fla equal to or slightly greater than that of the BC3 competitor (lanes 2 and 3). No wild-type fla could be amplified from the remaining C3H ankle sample. These results indicate that at day 7, there are relatively equal levels of spirochetes in the ankles of resistant and susceptible mice following footpad inoculation. The level of variability seen in these samples was highly reproducible (11a).
FIG. 5.
Competitive PCR amplification of ospA, fla, and IL4pr from ankles of C3H and DBA mice infected 7 (A), 14 (B), or 21 (C) days earlier. Each lane represents data from an individual mouse. All samples at days 7 and 14 were diluted 1:10, and samples at day 21 were diluted 1:50. The amount of BC3 spiked into the samples was 0.25 pg for all samples except that for day 7 fla, which was 0.025 pg.
On day 14, the ankle samples from all three C3H mice had high levels of ospA amplification (Fig. 5B). The ankle samples from DBA mice were more heterogeneous, displaying high, intermediate, and low levels of ospA amplification (lanes 4, 5, and 6, respectively). Amplification of fla was also more heterogeneous, with two samples from C3H mice (lanes 1 and 3) having high levels and one having low levels of wild-type DNA. Very little wild-type fla DNA could be amplified from the 14-day DBA ankle samples, even though faint wild-type bands could be seen in two of the three samples (lanes 4 and 5). These results indicate that while all of the C3H mice appeared to have significant spirochete burdens in their ankles at day 14, some (but not all) of the DBA mice appeared to contain fewer spirochetes at that time.
By day 21 of infection, high levels of wild-type ospA amplification were present in two samples from the DBA mice (Fig. 5C, lanes 5 and 6) and high (but slightly less so) levels were present in two samples from the C3H samples (lanes 1 and 3). The other samples from C3H and DBA mice (lanes 2 and 4) had considerably less wild-type ospA gene amplification. All ankle samples from day 21 had high levels of wild-type fla amplification. These results demonstrate that high levels of spirochetes in the ankles of resistant mouse strains, as assessed by PCR amplification of either plasmid or genomic Borrelia DNA, do not necessitate the development of Lyme arthritis. Over the course of the experiment, there was a fivefold increase in the numbers of B. burgdorferi organisms in the ankles of both strains of mice. As shown in the legend to Fig. 5, with a constant level of BC3 competitor (0.25 pg), the samples from days 7 and 14 needed to be diluted only 1:10, while the day 21 samples required a 1:50 dilution for amplification of both products. This allowed us to estimate that the ankles on day 7 contained about 1.5 × 106 spirochetes, which increased to about 9 × 106 by day 21 in both strains.
We also examined spirochete loads in ankles and other distant tissues by culture (Table 1). There were no differences in spirochete recovery from ankles of DBA or C3H mice at any of the time points tested. Cultures from ankle tissues were uniformly positive for both resistant and susceptible mice and contained similar numbers of spirochetes (data not shown). Urinary bladder was the only other tissue with a positive culture on day 7, with one of three mice of both strains positive. There were few differences in the culture results of distant sites at days 14 and 21 of infection, with the notable exception of blood at day 14. All of the cultures from DBA mice were positive, with high numbers of spirochetes, while the cultures from C3H mice were negative. Spirochetes were absent from the blood of two of three DBA mice by day 21, with the remaining positive culture containing fewer spirochetes. These results confirm the PCR analysis, demonstrating relatively small differences in spirochetal burden in the ankles of resistant and susceptible mouse strains following footpad infection during the peak of arthritis development.
DISCUSSION
The infection of inbred mouse strains with B. burgdorferi recapitulates a portion of the disease spectrum seen in human Lyme disease. C3H mice develop severe arthritis, while DBA mice develop only mild arthritis or joint inflammation (6). The presence of live B. burgdorferi spirochetes is required for the development of pathology, as repeated injections of heat-killed spirochetes or whole-B. burgdorferi antigen has no effect in rats (5), hamsters (17), or mice (11a). When injected intradermally, susceptible C3H mice are more permissive than resistant BALB/c mice to B. burgdorferi growth and dissemination and harbor higher numbers of spirochetes within their tissues (29). A higher spirochete tissue burden has been suggested to be the cause of the increased development of pathology in susceptible animals. However, whether resistant mice harboring relatively equal numbers of spirochetes in their ankles would develop pathology equivalent to that of susceptible mice has not been tested. To investigate this question more fully, we gave footpad injections of high numbers of B. burgdorferi spirochetes to resistant and susceptible mouse strains and monitored arthritis development. To quantify tissue spirochete burden, we created a competitive PCR construct containing modified Borrelia chromosomal (fla) and plasmid (ospA) gene segments. This molecule allows direct comparison of the levels of spirochetes in tissues of different mouse strains. Our data show that the presence of high numbers of spirochetes within the ankles of resistant mouse strains does not dictate the development of Lyme arthritis. With their ankles harboring relatively equal numbers of spirochetes, DBA mice remain arthritis resistant while C3H mice develop severe Lyme arthritis. Thus, an inappropriate or overly exuberant immune response, and not tissue spirochete burden, might contribute to genetic differences in the propensity to develop experimental Lyme arthritis.
Several studies have suggested a correlation between the development of Lyme arthritis and high numbers of spirochetes in tissue (7, 21, 29). Using in vitro cultivation of infected tissues, Keane-Myers and Nickell (16) compared spirochete levels in ankles and ear punches from BALB/c and C3H mice. The reported differences in spirochete burden were less than 10-fold between the resistant and susceptible animals. Using a similar subculturing technique, however, Anguita et al. (2) found that C3H mice treated with antibody to interleukin 12 had a decrease in arthritis severity but an increase in spirochete burden as assessed by ear punches. Those researchers suggested that both the tissue spirochete burden and the resulting immune response were likely to play important roles in the pathogenesis of Lyme arthritis.
Yang et al. (29) used PCR to determine differences in spirochete burden in tissues of arthritis-resistant BALB/c and arthritis-susceptible C3H mice. Following intradermal injection of 2 × 105 spirochetes into the shaved backs of experimental mice, they assayed various tissues at weekly intervals for the presence of Borrelia DNA. The susceptible C3H animals had tissues positive for ospA at weeks 1 through 4, whereas the BALB/c tissues were only positive for ospA at week 3. Hearts, ankles, and bladders were the most heavily infected tissues of both mouse strains. Quantitation of spirochete loads by two different methods indicated 10-fold-higher numbers of spirochetes in C3H hearts and 5-fold-higher numbers of spirochetes in C3H ankles by ospA analysis than in BALB/c mice. Similar differences were seen in the detection of fla, but 10 to 20% fewer organisms were detected than when ospA was analyzed. BALB/c mice also had better spirochetal clearance from tissues, while tissue loads of C3H mice remained high. The investigators concluded that pathology during infection with B. burgdorferi may be correlated with the presence of greater numbers of organisms in the tissues of susceptible animals. Our results do not support this conclusion and show that the presence of high numbers of spirochetes in ankles of resistant mice does not induce the development of severe Lyme arthritis. There are, however, several differences between the experimental protocols used in these two studies that most likely explain the differences between the conclusions reached. The major difference is likely to be the site of inoculation. Yang et al. (29) injected animals intradermally in the back, mimicking the natural route of infection. However, this requires that the spirochetes disseminate to reach the target organs, allowing potential differences in dissemination between resistant and susceptible animals to influence the level of organisms within each tissue type. It was for this reason that we chose footpad injection as our inoculation protocol. This delivers the organisms very near the target site (ankle) and minimizes the variable of differing rates of dissemination.
The kinetics of dissemination of B. burgdorferi into various tissues of inbred mouse strains have been studied by several groups (7, 9, 10). B. burgdorferi spreads quickly from the site of inoculation to almost all tissues within the host. Following intradermal inoculation of C3H mice, blood, spleen, kidney, bladder, ankle, and heart are all spirochete positive by day 7 and the ears are positive by day 10 (7). Arthritis-susceptible strains have a more rapid dissemination and higher levels of spirochetes in most of the tissues studied than do arthritis-resistant mice (29). Thus, resistant mice may be better able to sequester Borrelia spirochetes at the site of infection than are susceptible mice (25). The infection site has been shown to be an important determinant in the development of pathology (8, 13). It is known that the presence of live spirochetes within joints is required for arthritis development. The failure of high-passage strains to disseminate and reach joints has been suggested to be one possible reason why they are nonpathogenic (19).
Since PCR does not distinguish between dead and live organisms, it is possible that DBA mice have a more effective mechanism for killing Borrelia spirochetes in ankle tissue than do C3H mice. Viable spirochetes are needed for arthritis development, and thus, resistant animals would be protected from pathology by this mechanism. This explanation does not seem plausible to us, however, for the following reasons. The relatively stable or increasing levels of spirochetal DNA in ankle samples indicate that the numbers of B. burgdorferi spirochetes within ankles of resistant mice are being maintained or even increased over the course of the experiment. In addition, spirochetal DNA has been reported to be eliminated along with viable organisms (3). Finally, the increasing numbers of culture-positive tissues at distant sites (e.g., the heart) over time indicate that viable organisms are migrating into these sites, which could not occur if the spirochetes were killed at the site of inoculation.
This report demonstrates that differences in pathology between resistant and susceptible mouse strains need not be due solely to differences in spirochete burden. More rapid dissemination and higher tissue spirochete burdens would be expected to play a role in arthritis development following intradermal infection. The current study demonstrates that when resistant and susceptible mice harbor relatively equivalent levels of spirochetes in their joints, they can still retain their distinct disease phenotype. Thus, other host factors besides degree of microbial clearance are likely to be important for disease outcome. Identification of these other factors may help elucidate some of the genetic contributions to inflammatory diseases.
ACKNOWLEDGMENTS
This work was supported by the Burroughs Wellcome Fund and by the NIH (AR 44042).
We thank Dan Brown, Kevin Swier, and Joseph Opferman for critical reading of the manuscript and Jennifer Bird for technical assistance.
REFERENCES
- 1.Anderson J, Barthold S, Magnarelli L. Infectious but nonpathogenic isolate of Borrelia burgdorferi. J Clin Microbiol. 1990;28:2693–2699. doi: 10.1128/jcm.28.12.2693-2699.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anguita J, Persing D H, Rincon M, Barthold S W, Fikrig E. Effect of anti-interleukin 12 treatment on murine Lyme borreliosis. J Clin Invest. 1996;97:1028–1034. doi: 10.1172/JCI118494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong A L, Barthold S W, Persing D H, Beck D S. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am J Trop Med Hyg. 1992;47:249–258. doi: 10.4269/ajtmh.1992.47.249. [DOI] [PubMed] [Google Scholar]
- 4.Barthold S, Moody K, Beck D. Susceptibility of laboratory rats to isolates of Borrelia burgdorferi from different geographic areas. Am J Trop Med Hyg. 1990;42:596–600. doi: 10.4269/ajtmh.1990.42.596. [DOI] [PubMed] [Google Scholar]
- 5.Barthold S W, Moody K D, Terwilliger G A, Duray P H, Jacoby R O, Steere A C. Experimental Lyme arthritis in rats infected with Borrelia burgdorferi. J Infect Dis. 1988;157:842–846. doi: 10.1093/infdis/157.4.842. [DOI] [PubMed] [Google Scholar]
- 6.Barthold S W, Beck D S, Hansen G M, Terwilliger G A, Moody K O. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 7.Barthold S W, Persing D H, Armstrong A L, Peeples R A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 8.Barthold S W. Infectivity of Borrelia burgdorferi relative to route of inoculation and genotype in laboratory mice. J Infect Dis. 1991;163:419–420. doi: 10.1093/infdis/163.2.419. [DOI] [PubMed] [Google Scholar]
- 9.Barthold S W, Sidman C L, Smith A L. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am J Trop Med Hyg. 1992;47:605–613. doi: 10.4269/ajtmh.1992.47.605. [DOI] [PubMed] [Google Scholar]
- 10.Barthold S W, de Souza M S, Janotka J L, Smith A L, Persing D H. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:959–972. [PMC free article] [PubMed] [Google Scholar]
- 11.Bergstrom S, Bundoc V G, Barbour A G. Molecular analysis of linear plasmid-encoded major surface proteins, ospA and ospB, of the Lyme disease spirochete, Borrelia burgdorferi. Mol Microbiol. 1989;3:479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 11a.Brown, C. Unpublished data.
- 12.Dattwyler R J, Volkman D J, Luft B J, Halperin J J, Thomas J, Golightly M G. Dissociation of specific T- and B-lymphocyte responses to Borrelia burgdorferi. N Engl J Med. 1988;319:1441–1446. doi: 10.1056/NEJM198812013192203. [DOI] [PubMed] [Google Scholar]
- 13.de Souza M, Smith A L, Beck D S, Kim L J, Hansen G M, Jr, Barthold S W. Variant responses of mice to Borrelia burgdorferi depending on the site of intradermal inoculation. Infect Immun. 1993;61:4493–4497. doi: 10.1128/iai.61.10.4493-4497.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gassmann G S, Jacobs E, Deutzmann R, Gobel U B. Analysis of the Borrelia burgdorferi GeHo fla gene and antigenic characterization of its gene product. J Bacteriol. 1991;173:1452–1459. doi: 10.1128/jb.173.4.1452-1459.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson R C, Kodner C, Russell M. Passive immunization of hamsters against experimental infection with Borrelia burgdorferi. Infect Immun. 1986;53:713–714. doi: 10.1128/iai.53.3.713-714.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keane-Myers A, Nickell S P. T cell subset-dependent modulation of immunity to Borrelia burgdorferi in mice. J Immunol. 1995;154:1770–1776. [PubMed] [Google Scholar]
- 17.Kenefick K B, Lim L C L, Alder J D, Schmitz J L, Czuprynski C J, Schell R F. Induction of interleukin-1 release by high- and low-passage isolates of Borrelia burgdorferi. J Infect Dis. 1993;167:1086–1092. doi: 10.1093/infdis/167.5.1086. [DOI] [PubMed] [Google Scholar]
- 18.Matyniak J, Reiner S L. T helper phenotype and genetic susceptibility in experimental Lyme disease. J Exp Med. 1995;181:1251–1254. doi: 10.1084/jem.181.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norris S J, Howell J K, Garza S A, Ferdows M S, Barbour A G. High- and low-infectivity phenotypes of clonal populations of in vitro-cultured Borrelia burgdorferi. Infect Immun. 1995;63:2206–2212. doi: 10.1128/iai.63.6.2206-2212.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otsuka T, Villaret D, Yokota T, Takebe Y, Lee F, Arai A, Arai K. Structural analysis of the mouse chromosomal gene encoding interleukin 4 which expresses B cell, T cell and mast cell stimulating activities. Nucleic Acids Res. 1987;15:333–344. doi: 10.1093/nar/15.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pennington P M, Allred C D, West C S, Alvarez R, Barbour A G. Arthritis severity and spirochete burden are determined by serotype in the Borrelia turicatae-mouse model of Lyme disease. Infect Immun. 1997;65:285–292. doi: 10.1128/iai.65.1.285-292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Persing D H, Rutledge B J, Rys P N, Podzorski D S, Mitchell P D, Reed K D, Liu B, Fikrig E, Malawista S E. Target imbalance: disparity of Borrelia burgdorferi genetic material in synovial fluid from Lyme arthritis patients. J Infect Dis. 1994;169:668–672. doi: 10.1093/infdis/169.3.668. [DOI] [PubMed] [Google Scholar]
- 23.Reiner S, Zheng S, Corry D, Locksley R. Constructing polycompetitor cDNAs for quantitative PCR. J Immunol Methods. 1993;165:37–46. doi: 10.1016/0022-1759(93)90104-f. [DOI] [PubMed] [Google Scholar]
- 24.Schaible U E, Wallich R, Kramer M D, Nerz G, Stehle T, Museteanu C, Simon M M. Protection against Borrelia burgdorferi infection in SCID mice is conferred by presensitized spleen cells and partially by B cells but not by T cells alone. Int Immunol. 1994;6:671–681. doi: 10.1093/intimm/6.5.671. [DOI] [PubMed] [Google Scholar]
- 25.Shih C-M, Telford III S R, Pollack R J, Spielman A. Rapid dissemination by the agent of Lyme disease in hosts that permit fulminating infection. Infect Immun. 1993;61:2396–2399. doi: 10.1128/iai.61.6.2396-2399.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steere A C, Schoen R T, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107:725–731. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 27.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 28.Steere A C, Dwyer E, Winchester R. Association of chronic Lyme arthritis with HLA-DR4 and HLA-DR2 alleles. N Engl J Med. 1990;323:219–225. doi: 10.1056/NEJM199007263230402. [DOI] [PubMed] [Google Scholar]
- 29.Yang L, Weis J H, Eichwald E, Kolbert C P, Persing D H, Weis J J. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect Immun. 1994;62:492–500. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]