Abstract
Feline lungworms have long been known as pathogens of cats. However, an increased incidence of clinical cases in some areas has been the focus of a number of recent epidemiological and clinical studies. While Aelurostrongylus abstrusus causes respiratory signs in cats all over the world, Troglostrongylus brevior has recently been found in domestic cats from Spain and Italy (where it often causes severe clinical signs). Capillaria aerophila, a parasite that infects many wild carnivores, may cause respiratory distress in cats. A variety of treatment options are known for A abstrusus, while almost no information is available on the treatment of troglostrongylosis and capillariosis. This series describes two mixed infections in clinically affected kittens with T brevior, one with concurrent A abstrusus and the other with C aerophila. In both cases, the nematodes were identified and confirmed by copromicroscopic examination and specific DNA-based assays. Kittens showed respiratory signs that resolved after one or two administrations of a spot-on solution containing emodepside. Larval (T brevior and A abstrusus) and egg (C aerophila) shedding was also eliminated 2–4 weeks after treatment. New clinical insights into these parasitoses are discussed.
The cat lungworm Aelurostrongylus abstrusus (Metastrongyloidea, Angiostrongylidae) is a globally distributed parasitic nematode that inhabits the bronchi, bronchioles and alveoli of domestic cats. Adult stages, eggs and larvae may cause coughing, dyspnoea, sneezing and wheezing, although some cats may also be infected subclinically.1,2 This mollusc-transmitted nematode has long been regarded as the major lungworm affecting cats, while other species (ie, Troglostrongylus species, Oslerus rostratus) have been sporadically described and considered to be only occasional. 2 However, Troglostrongylus subcrenatus and, more often, Troglostrongylus brevior (Metastrongyloidea, Crenosomatidae), usually considered to be affiliated only with wild felids,2,3 have recently been identified as agents of bronchopneumonia in cats. These parasites live in bronchioles and bronchi (T brevior) or in the trachea and bronchi (T subcrenatus), and can result in severe damage to the affected airways.
Troglostrongylus brevior was described for the first time in the 20th century from the respiratory systems of wild felids from Palestine. 3 Another article from the 1950s described a Troglostrongylus species related to T brevior in a European wild cat (Felis silvestris silvestris) and in a feral cat caught in central Italy but of unknown origin. 4 No other reports have been published until recently. These articles describe the occurrence of T brevior in cats from Ibiza, Spain 5 and from different areas of Italy.6–8 Therefore, until 2010, the presence of T brevior in domestic cats received no attention. Similarities between the first larval stage (L1) of T brevior and that of A abstrusus may have resulted in misdiagnoses of aelurostrongylosis. 9 However, peer-reviewed literature and epidemiological and clinical features of these infections would not support the hypothesis that confusion between the two parasites had resulted in underdiagnosis of T brevior.2,10
General knowledge of cat troglostrongylosis is fragmentary, and information on clinical features of this disease and treatment options are poor. The data available indicate that signs in cats infected with T brevior are frequently severe and that the parasite is more frequent in kittens and young cats. The parasitosis is often fatal in infected kittens, even when a parasiticide is promptly administered.6,8 Interestingly, cats reported to have had severe clinical signs have been younger than 6 months of age, with only few kittens subclinically infected.7,8 Cats older than this have been subclinically infected or shown only mild signs.2,5,7
Although the numbers of reported infections are too small to draw any conclusion, it could be argued that Troglostrongylus species establish mostly in young cats that have severe illness due to lack of age-related immunity. The few cases in adult cats could be owing to a lower susceptibility of animals with a developed immune system to this (likely) non-specific parasite.
It is unknown whether the recent apparent increase in the incidence of troglostrongylosis in cats is because of increased awareness and thus diagnosis, or because of a genuine increase due to epidemiological and biological drivers promoting the likelihood of T brevior infection.1,9,10
Capillaria aerophila, a trichurid nematode living in the trachea, bronchi and bronchioles of wildlife, may also cause respiratory distress in cats.1,11,12 Interestingly, this nematode may also infect humans.2,13,14 There is an apparent increase in clinical cases of lung capillariosis in companion animals from some countries (eg, Italy), which, again, could be due to an increased awareness or to different epidemiological and biological drivers that are currently promoting the spread of extraintestinal nematodes.1,11
Overall, there is significant merit in promoting knowledge about clinical features of lungworm infections in cats and treatment options of practical importance in feline medicine, especially for T brevior. This case series describes two cases of mixed lungworm infections in kittens, with a focus on clinical features, diagnosis and therapy.
Case 1
An approximately 7–8-week-old stray male kitten found in the outskirts of Teramo municipality (Abruzzo region, central Italy) was referred in November 2013 to a local private veterinary clinic. Clinical examination of the cat showed severe general respiratory signs, intense sneezing and coughing, hyperthermia (40.6°C) and depression. The kitten was immediately hospitalised and treated with 25 mg/kg amoxicillin clavulanate (Synulox; Pfizer), 0.1 mg/kg meloxicam (Metacam; Boehringer Ingelheim) and intravenous fluid therapy. Fever and depression resolved after 7 days of hospitalisation, while coughing and sneezing persisted. Therefore, a suspicion of lungworm infection was made, and radiographical imaging and copromicroscopic examination were performed. The faeces of the cat were subjected to a classical flotation procedure and to the Baermann method, 15 and showed the presence of coccidia (ie, Cystoisospora felis) and nematode L1s, respectively. A microscopical analysis of five Baermann sediments was carried out. All L1s found in each microscopic field were examined morphometrically and morphologically, and identified based on their size, and appearance of the head and tail.1,3,9 Two larval morphotypes were detected, and were compatible with A abstrusus and T brevior. In particular, L1s of A abstrusus were recognised based on their length (370.5 ± 21.78 μm), the terminal position of the oral opening and on the appearance of the tail, which was S-shaped, had ventral and dorsal incisures, and a subterminal spine (Figure 1). L1s of T brevior were 325.0 ± 10.25 μm long, with a subterminal position of the oral opening and a pointed tail, lacking the kink present in A abstrusus, and had a pronounced dorsal cuticular spine and a shallower ventral one (Figure 2). The identity of the larvae was confirmed upon genetic examinations specific for A abstrusus and T brevior carried out as previously described. 8 The radiographical examination of the cat showed the presence of nodular pulmonary lesions with a bronchiolar pattern (Figure 3).
Figure 1.
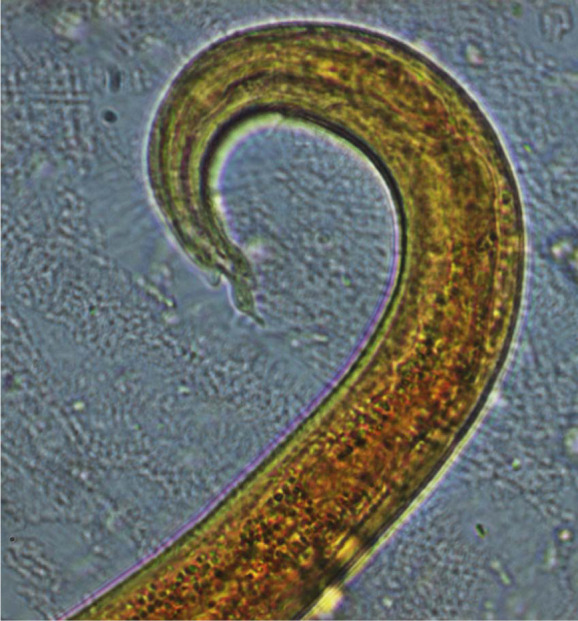
Case 1, caudal end of the first stage larva of Aelurostrongylus abstrusus
Figure 2.
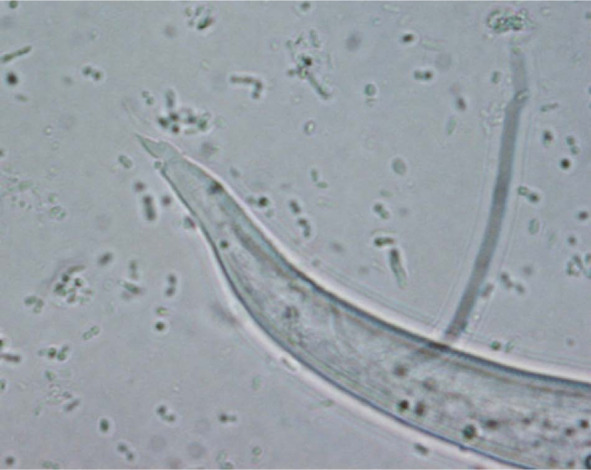
Case 1, caudal end of the first stage larva of Troglostrongylus brevior
Figure 3.
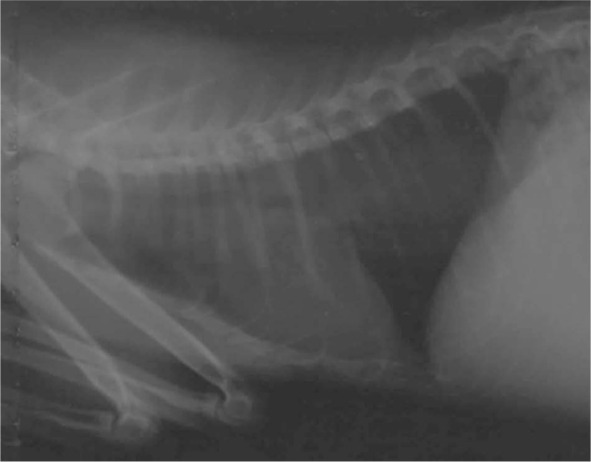
Lateral view of the thorax of case 1 showing nodular pulmonary lesions with a bronchiolar pattern
Given the age (approximately 2 months) and weight (ie, 1.2 kg) of the kitten, it was treated (day 0) with a spot-on solution containing emodepside 2.1%/praziquantel 8.6% (Profender; Bayer). All respiratory signs had resolved at day 7, and the faeces were examined again using the Baermann method at days 7 and 14. Dead L1s of A abstrusus and T brevior, and live and motile L1s of the latter species, were found during both examinations. Identification of the live L1s as T brevior was confirmed genetically. 8 Thus, at day 14 the cat received a second dose of Profender. After 1 and 2 weeks (days 21 and 28) faecal examination was performed again. The faeces were negative for A abstrusus and T brevior by conventional testing and also by PCR assay. The kitten was then adopted.
Case 2
An approximately 8–9-week-old stray female cat was referred to a private veterinary practice in Isola del Gran Sasso municipality (province of Teramo) in December 2013. Clinical examination of the cat showed coughing and severe respiratory distress. The kitten was hospitalised and treated with 10 mg/kg doxycycline (Ronaxan; Merial) for 6 days. Thoracic radiographs revealed an extensive and severe bronchial pattern.
A copromicroscopic examination performed with both flotation and Baermann techniques 15 showed the presence of C aerophila ova (Figure 4) and nematode L1, respectively. The L1s were identified microscopically and genetically as T brevior (323 ± 8.5 μm). 8 The kitten, weighing 1.1 kg, was treated (day 0) with Profender.
Figure 4.
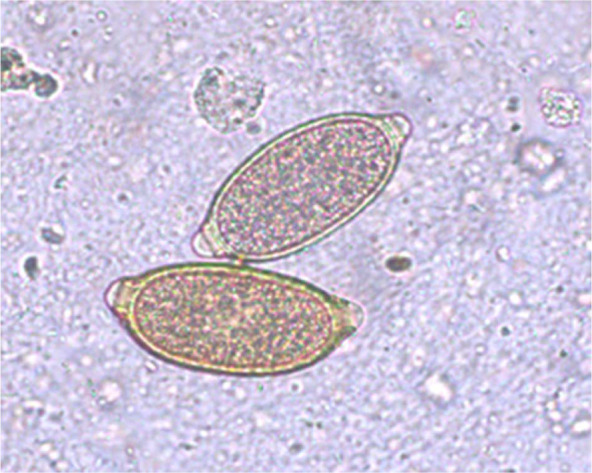
Case 2, Capillaria aerophila eggs
Clinical examination on day 7 revealed that while the cough had resolved, dyspnoea was still present, albeit less severe. At days 14, 21 and 28 the kitten was healthy. The copromicroscopic examinations at days 7, 14, 21 and 28 revealed the absence of T brevior L1s and C aerophila eggs. Faecal and pharyngeal swabs collected on the same days were negative for T brevior on PCR assays.
Discussion
While a number of treatment protocols are reported for treatment of A abstrusus, very little information is available on the treatment of T brevior and C aerophila in cats.
Two kittens with severe respiratory signs due to T brevior and T subcrenatus, respectively, were unsuccessfully treated with a single spot-on formulation containing imidacloprid 10%/moxidectin 1% or with fenbendazole 50 mg/kg PO q24h. 6 However, the same compounds were effective in stopping larval shedding and eliminating clinical signs in cats with natural aelurostrongylosis, with a percentage efficacy of 99.29% and 100.00%, respectively.16,17
In another study, two kittens belonging to the same litter and infected with both A abstrusus and T brevior received a single administration of milbemycin oxime 2 mg/kg. 8 While one kitten was negative for L1s after administration, the other remained infected by T brevior, despite treatment. Indeed, the latter kitten showed severe respiratory signs at referral and died after treatment, while the other had only mild signs before treatment and recovered fully. 8 These two kittens were likely exposed to the same source of A abstrusus and T brevior, but it is hard to understand why one animal developed severe signs and died despite treatment, while the second was subclinically infected and fully recovered after the administration of the same parasiticide. 8
The cats that died from T brevior infection despite the administration of a parasiticide were young subjects with severe coughing and dyspnoea, and other intense respiratory signs. It is possible that a single administration of a macrocyclic lactone (eg, moxidectin, milbemycin oxime) is not effective or has a partial efficacy in the case of a very high T brevior burden and/or extensive lesions within the respiratory tract.6,8 However, it is also plausible that there was insufficient time for the damaged lungs to recover after treatment, and no definitive conclusions can be drawn on so few cases.6,8
Injectable or oral levamisole at different dosages with repeated administrations and off-label abamectin showed a certain efficacy against C aerophila in cats.1,12 The same spot-on containing moxidectin 1% effective against cat aelurostrongylosis succeeded also in the treatment of lung capillariosis. 18 No other information is currently available for the treatment of C aerophila in cats.
Emodepside was chosen for use in the clinical cases described herein as it is known to have high efficacy against A abstrusus 17 and was convenient with respect to the age (approximately 2 months) and weight (approximately 1 kg) of the kittens. A single administration of Profender in case 1 resulted in clinical recovery after 1 week, and a full and partial efficacy, respectively, against larval shedding of A abstrusus and T brevior after 2 weeks. Complete efficacy in treating troglostrongylosis was achieved with a second administration 2 weeks apart.
A single dose of emodepside was effective against A abstrusus in case 1. This was consistent with the findings of a previous study, which showed 100.00% clinical recovery and a 99.38% efficacy in the cessation of L1 shedding 4 weeks post-administration in naturally infected cats. 17 Emodepside was also successful in resolving clinical signs within 14 days of treatment in case 2. Cessation in the shedding of C aerophila eggs and T brevior L1s was noted 7 days after this single administration.
The efficacy of Profender in treating C aerophila seems promising for further studies to evaluate on a large scale the potential use of this formulation for treatment of cat lung capillariosis.
The susceptibility of T brevior to a single administration of emodepside was different in the two kittens in both stopping L1s shedding and providing a clinical recovery. These results suggest that the efficacy of anthelmintics in treating T brevior in cats is dependent upon parasite burden, lung damage and/or host immunity.
Reliable identification of L1s shed by infected cats is essential, given the possible increase in incidence of clinical lungworm in cats, the high pathogenic potential of T brevior in kittens and the possible difference in terms of susceptibility of different feline lungworms to current anthelmintic compounds. This is of practical importance considering the pathogenic impact not only of A abstrusus and C aerophila shown by both clinical and pathological studies,1,11,19 but also of T brevior, in particular.2,6,8
Indeed, an accurate diagnosis is crucial also if one bears in mind the occurrence of mixed lungworm infections. This is especially true in the case of A abstrusus and T brevior, which have overlapping biological cycles20,21 and may share the same ecological niches, as demonstrated by past and herein presented cases of natural co-infections.5,6,8 More importantly, L1s (ie, the diagnostic stage) of these two nematodes have similar morphological and morphometric features, which may impair a correct species-specific identification. 2
These drawbacks and the lack of awareness of Troglostrongylus species may result in a missed or incorrect diagnosis of natural infections. Erroneous diagnoses may lead to a choice of anthelmintic with partial or null efficacy, and/or to number of administrations insufficient for the treatment of T brevior. Hence, a careful appraisal of morphometric (ie, length and width) and morphological characteristics (ie, position of the oral opening and tail appearance) of L1s shed by cats is necessary in clinical settings. In cases of doubtful diagnosis, the unequivocal molecular discrimination of A abstrusus and T brevior can be achieved via a duplex PCR recently validated on faeces from a cat with a mixed infection 22 and the species-specific nested PCR applicable to faeces and pharyngeal swabs of cats with mono-specific and mixed infections.8,23 Indeed, these tools are powerful in achieving a prompt diagnosis and an effective treatment regimen.
Analogously, a thorough microscopic analysis of key features (ie, size, wall surface and plug morphology and position) of the bipolar lemon-like eggs of C aerophila allows their unequivocal identification and a reliable diagnosis of lung capillariosis. Although these eggs could be mistaken for the eggs of Trichostrongylus vulpis, this nematode does not infect cats. 2 Feline Trichuris species are absent in the vast majority of the world, being confined to limited areas of the Americas and a few other regions, where they occur rarely, as shown by the scanty records in the scientific literature over the past 40 years.24,25 The scenario is more complicated bearing in mind that cats may shed eggs of rare intestinal or urinary capillariids, and trichurid eggs from prey.14,24
Conclusions
Lungworms should be considered in the differential diagnosis in cats with respiratory signs and a diagnosis at the species level should be achieved with a careful microscopic appraisal. The treatment of choice may vary depending upon several factors: the species involved, the presence of co-infections, the age and weight of the animal, and the severity of the clinical signs.
Although successful treatment was only described in two kittens, emodepside could be considered a drug of choice for the treatment of T brevior. Further studies on emodepside efficacy in the treatment of T brevior (and C aerophila) are required to confirm these preliminary finding.
Footnotes
AdC holds a postgraduate fellowship on research fields other than cat lungworms. The activities presented in the article have been performed according to her freedom of scientific research.
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case series.
Accepted: 7 April 2014
References
- 1. Traversa D, Di Cesare A, Conboy G. Canine and feline cardiopulmonary parasitic nematodes in Europe: emerging and underestimated. Parasit Vectors 2010; 3: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Traversa D, Di Cesare A. Feline lungworms: what a dilemma. Trends Parasitol 2013; 29: 423–430. [DOI] [PubMed] [Google Scholar]
- 3. Gerichter CB. Studies on the nematodes parasitic in the lungs of Felidae in Palestine. Parasitology 1949; 39: 251–262. [DOI] [PubMed] [Google Scholar]
- 4. Paggi L. Segnalazione, in Italia Centrale, di Troglostrongylus sp. parassita dei polmoni di felidi. Parassitologia 1959; 1: 80–81. [Google Scholar]
- 5. Jefferies R, Vrhovec MG, Wallner N, et al. Aelurostrongylus abstrusus and Troglostrongylus sp. (Nematoda: Metastrongyloidea) infections in cats inhabiting Ibiza, Spain. Vet Parasitol 2010; 173: 344–348. [DOI] [PubMed] [Google Scholar]
- 6. Brianti E, Gaglio G, Giannetto S, et al. Troglostrongylus brevior and Troglostrongylus subcrenatus (Strongylida: Crenosomatidae) as agents of broncho-pulmonary infestation in domestic cats. Parasit Vectors 2012; 5: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brianti E, Gaglio G, Napoli E, et al. Evidence for direct transmission of the cat lungworm Troglostrongylus brevior (Strongylida: Crenosomatidae). Parasitology 2013; 140: 821–824. [DOI] [PubMed] [Google Scholar]
- 8. Di Cesare A, Frangipane di Regalbono A, Tessarin C, et al. Mixed infection by Aelurostrongylus abstrusus and Troglostrongylus brevior in kittens from the same litter in Italy. Parasitol Res 2014; 113: 613–618. [DOI] [PubMed] [Google Scholar]
- 9. Otranto D, Brianti E, Dantas-Torres F. Troglostrongylus brevior and a nonexistent ‘dilemma’. Trends Parasitol 2013; 29: 517–518. [DOI] [PubMed] [Google Scholar]
- 10. Traversa D. Response to Otranto et al.: lungworms in domestic and wild felids: dilemmas still persisting. Trends Parasitol 2014; 30: 53–54. [DOI] [PubMed] [Google Scholar]
- 11. Traversa D, Di Cesare A, Milillo P, et al. Infection by Eucoleus aerophilus in dogs and cats: is another extra-intestinal parasitic nematode of pets emerging in Italy? Res Vet Sci 2009; 87: 270–272. [DOI] [PubMed] [Google Scholar]
- 12. Barrs VR, Martin P, Nicoll RG, et al. Pulmonary cryptococcosis and Capillaria aerophila infection in an FIV-positive cat. Aust Vet J 2000; 78: 154–158. [DOI] [PubMed] [Google Scholar]
- 13. Lalosević D, Lalosević V, Klem I, et al. Pulmonary capillariasis miming bronchial carcinoma. Am J Trop Med Hyg 2008; 78: 14–16. [PubMed] [Google Scholar]
- 14. Di Cesare A, Castagna G, Otranto D, et al. Molecular detection of Capillaria aerophila, an agent of canine and feline pilmonary capillariosis. J Clin Microbiol 2012; 50: 1958–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sloss MW, Kemp RL, Zajac AM. Veterinary clinical parasitology. 6th ed. Ames, IA: Iowa State University Press, 1994. [Google Scholar]
- 16. Traversa D, Di Cesare A, Milillo P, et al. Efficacy and safety of imidacloprid 10%/moxidectin 1% spot-on formulation in the treatment of feline aelurostrongylosis. Parasitol Res 2009; 105 Suppl 1: S55–62. [DOI] [PubMed] [Google Scholar]
- 17. Traversa D, Milillo P, Di Cesare A, et al. Efficacy and safety of emodepside 2.1%/praziquantel 8.6% spot-on formulation in the treatment of feline aelurostrongylosis. Parasitol Res 2009; 105 Suppl 1: S83–89. [DOI] [PubMed] [Google Scholar]
- 18. Traversa D, Di Cesare A, Di Giulio E, et al. Efficacy and safety of imidacloprid 10%/moxidectin 1% spot-on formulation in the treatment of feline infection by Capillaria aerophila. Parasitol Res 2012; 111: 1793–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dennler M, Bass DA, Gutierrez-Crespo B, et al. Thoracic computed tomography, angiographic computed tomography, and pathology findings in six cats experimentally infected with Aelurostrongylus abstrusus. Vet Radiol Ultrasound 2013; 54: 459–469. [DOI] [PubMed] [Google Scholar]
- 20. Di Cesare A, Crisi PE, Di Giulio E, et al. Larval development of the feline lungworm Aelurostrongylus abstrusus in Helix aspersa. Parasitol Res 2013; 112: 3101–3108. [DOI] [PubMed] [Google Scholar]
- 21. Giannelli A, Ramos RA, Annoscia G, et al. Development of the feline lungworms Aelurostrongylus abstrusus and Troglostrongylus brevior in Helix aspersa snails. Parasitology 2014; 141: 563–569. [DOI] [PubMed] [Google Scholar]
- 22. Annoscia G, Latrofa MS, Campbell BE, et al. Simultaneous detection of the feline lungworms Troglostrongylous brevior and Aelurostrongylus abstrusus by a newly developed duplex-PCR. Vet Parasitol 2014; 199, 172–178. [DOI] [PubMed] [Google Scholar]
- 23. Traversa D, Iorio R, Otranto D. Diagnostic and clinical implications of a nested PCR specific for ribosomal DNA of the feline lungworm Aelurostrongylus abstrusus (Nematoda, Strongylida). J Clin Microbiol 2008; 46: 1811–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bowman DD, Hendrix CM, Lindsay DS, et al. Feline clinical parasitology. Ames, IA: Iowa State University Press, 2002. [Google Scholar]
- 25. Traversa D, Di Cesare A, Lia RP, et al. New insights into morphological and biological features of Capillaria aerophila (Trichocephalida, Trichuridae). Parasitol Res 2011; 109 Suppl 1: S97–104. [DOI] [PubMed] [Google Scholar]


