Abstract
Enhanced knowledge on longevity and mortality in cats should support improved breeding, husbandry, clinical care and disease prevention strategies. The VetCompass research database of primary care veterinary practice data offers an extensive resource of clinical health information on companion animals in the UK. This study aimed to characterise longevity and mortality in cats, and to identify important demographic risk factors for compromised longevity. Crossbred cats were hypothesised to live longer than purebred cats. Descriptive statistics were used to characterise the deceased cats. Multivariable linear regression methods investigated risk factor association with longevity in cats that died at or after 5 years of age. From 118,016 cats attending 90 practices in England, 4009 cats with confirmed deaths were randomly selected for detailed study. Demographic characterisation showed that 3660 (91.7%) were crossbred, 2009 (50.7%) were female and 2599 (64.8%) were neutered. The most frequently attributed causes of mortality in cats of all ages were trauma (12.2%), renal disorder (12.1%), non-specific illness (11.2%), neoplasia (10.8%) and mass lesion disorders (10.2%). Overall, the median longevity was 14.0 years (interquartile range [IQR] 9.0–17.0; range 0.0–26.7). Crossbred cats had a higher median longevity than purebred cats (median [IQR] 14.0 years [9.1–17.0] vs 12.5 years [6.1–16.4]; P <0.001), but individual purebred cat breeds varied substantially in longevity. In cats dying at or after 5 years (n = 3360), being crossbred, having a lower bodyweight, and being neutered and non-insured were associated with increased longevity. This study described longevity in cats and identified important causes of mortality and breed-related associations with compromised longevity.
Introduction
Improved understanding of longevity and mortality in pet cats will support enhanced breeding, husbandry and clinical strategies that advance the health and welfare of owned cats.1–3 There are an estimated 8.5–10.3 million owned cats in the UK, with 19.0–25.5% of households owning a cat,4,5 suggesting substantial population effect from even moderate welfare gains. Recently, health issues associated with purebred dog breeding have been highlighted.6–8 However, breed-related disorder predispositions also affect cats and warrant exploration of effects on longevity and mortality.9–13
Hybrid vigour describes superior viability, production and fecundity of crossbred progeny compared with their purebred parents, and is an accepted phenomenon in production species.14,15 A recent report that hybrid vigour may influence longevity in dogs indicates that cats may similarly be affected. 16
Despite being stated to have a long life span, 17 few peer-reviewed reports have described population longevity values and mortality in domestic cats. 2 Analysis of veterinary clinical records from 460,000 cats in the USA identified an average longevity of 12.1 years and that neutering was associated with extended longevity. 18 Analyses of Swedish pet insurance records relating to 49,450 cats identified mortality variation between breeds but not between the sexes. 19 However, differing population and study design characteristics thwart subsequent attempts at generalisation to support disorder prioritisation and strategies to minimise disorder impacts. 2
Epidemiological analysis of electronic patient records (EPRs) collected from a large sample of primary care veterinary practices into a single national surveillance system has been recommended to investigate companion animal health.6,20 Veterinary EPRs provide longitudinal collection of clinical data that are contemporaneously recorded by veterinary health professionals and cover all presented patients and disorders. 20 The VetCompass (http://www.rvc.ac.uk/VetCompass/) database of merged primary care practice EPRs holds an extensive resource of clinical health information on companion animals in the UK and has been interrogated to report on longevity and mortality in dogs.16,21
Using the VetCompass database, this study aimed to estimate longevity, report common causes of mortality and identify demographic risk factors for compromised longevity in cats in England. Longevity in crossbred cats was hypothesised to exceed purebred cats.
Materials and methods
The VetCompass project collects de-identified EPR data from primary care veterinary practices in the UK for companion animal health surveillance. 22 This study included all cats with data uploaded to VetCompass from 1 September 2009 to 20 December 2012. Practice selection required willingness to participate and the use of an appropriately configured practice management system (PMS). Clinicians selected summary diagnosis terms at episodes of clinical care from an embedded VeNom Code list. 23 Clinical data extraction from PMSs used integrated clinical queries before being uploaded to a secure structured query language database. 24 Data fields collected included demographic (species, breed, date of birth, sex, neuter status, insurance status, microchip status, weight and deceased status) and clinical information (free-form text clinical notes, summary diagnosis terms, treatment and deceased status with relevant dates). Sample size calculation estimated that a cross-sectional study design with 314 purebred cats and 2826 crossbred cats would have an 80% power to detect a 1-year longevity difference between pure- and crossbred cats (α = 0.05), assuming that overall longevity was normally distributed with standard deviation of 6 years and that 10% of cats were purebreds. 25 Ethical approval of the project was granted by the Royal Veterinary College ethics and welfare committee (URN 2010 1076).
A random sample of potential deaths was selected from the ‘deceased status’ field for detailed study. 26 True cases of death were identified from the clinical notes, and the cause of mortality was extracted from the clinical note and VeNom diagnosis data relating to the death event. If a cause of mortality was not explicitly stated at the time of death, then no defined cause of mortality was included. The causes of mortality were grouped into appropriate pathophysiological or organ system categories. The mechanism of death (assisted [euthanasia] or non-assisted) and method of body disposal was noted. 27 The age at death relied on the date of birth values recorded in the PMS; no age at death was included for cats without date of birth information. Cats with recognised breed names were grouped as ‘purebred’, while cats described as mixed-breed, breed-specified crosses or domestic cats were grouped as ‘crossbred’. 11 The neuter and insurance statuses at death were used. Neuter and sex status were combined to create a sex/neuter variable with four categories: female entire, female neutered, male entire and male neutered. The maximum bodyweights recorded after 6 months of age were categorised into six groups (0.0–2.9 kg, 3.0–3.9 kg, 4.0–4.9 kg, 5.0–5.9 kg, ⩾6.0 kg, no weight recorded).
Following data checking and cleaning in Microsoft Excel 2007, statistical analyses were performed with Stata version 11.2 (StataCorp). 28 Overall and breed-specific (for breeds with ⩾10 cats in the study) longevities were reported using median, interquartile range (IQR) and range. Median overall longevity values for pure- and crossbred cats were compared using the Mann–Whitney U-test. The proportion of cats that were euthanased was compared between purebred and crossbred cats using the χ2 test. Causes of mortality were separately tabulated for cats of all ages, cats dying before 5 years of age and cats dying at or after 5 years. General linear regression modelling was used to evaluate associations between risk factors (purebred/crossbred, sex/neuter, weight category, microchip and insured) and longevity in cats dying at or after 5 years of age. The 5 year cut-off was chosen because the longevity data after this age approximated the normal distribution that is a required assumption for linear regression modelling. 29 Univariable risk factors liberally associated with longevity (P <0.20) were evaluated using multivariable models developed using backwards stepwise elimination. The final model was evaluated using the clinic attended as a random effect and for pairwise interaction effects. 30 The predictivity of the final non-random effect model was evaluated using the adjusted r2 value. Model diagnostics included visual inspection of residual and residual VS fitted plots to assess normality and homoscedasticity, respectively. 30 Statistical significance was set at P <0.05.
Results
From 118,016 cats attending 90 practices in central and south-east England with 12,012 potential death cases, a study sample of 4009 cats with confirmed deaths attending 87 practices was randomly selected. Data completeness varied between the variables: sex (98.9%), neutered (100.0%), breed (99.9%), date of birth (99.3%), insured (100.0%), microchipped (100.0%), weight (45.4%), cause of mortality (82.3%), mechanism of death (95.1%) and method of body disposal (84.2%). Demographic characterisation of deceased cats with information available indicated that 3660 (91.7%) were crossbred, 2009 (50.7%) were female, 2599 (64.8%) were neutered, 543 (13.5%) were insured and 238 (5.9%) were microchipped. Adult bodyweights were distributed as follows: <3.0 kg (440 cats, 24.2%), 3.0–3.9 kg (612 cats, 33.6%), 4.0–4.9 kg (461 cats, 25.3%), 5.0–5.9 kg (208 cats, 11.4%), ⩾6.0 kg (98, 5.4%). The median (IQR) bodyweight was 3.7 (3.0–4.6) kg. Euthanasia accounted for 3265 (85.7%) deaths overall, with a greater proportion of crossbred cats (3006, 86.0%) being euthanased compared with purebred cats (254, 81.9%) (P = 0.050). Overall, 2522 (74.7%) cats were cremated.
Median overall longevity was 14.0 years (IQR 9.0–17.0; range 0.0–26.7) and was bimodally distributed, peaking at years 1 and 16 (Figure 1). The median longevity of crossbred cats (14.0 years; IQR 9.1–17.0, range 0.0–26.7) was greater than purebred cats (12.5 years; IQR 6.1–16.4, range 0.0–22.0) (P <0.001). Female cats (15.0 years; IQR 11.0–17.4) had a higher median longevity than male cats (13.0 years; IQR 7.6–16.0) (P <0.001). Neutered cats (15.0 years; IQR 11.8–17.0) had a higher median longevity than non-neutered cats (11.0 years; IQR 2.13–16.0) (P <0.001). The longest-lived breeds were the Birman (n = 12; median 16.1 years, IQR 8.1–16.9) and Burmese (n = 31; 14.3 years, IQR 10.0–17.0). The shortest-lived breeds were the Bengal (n = 15; 7.3 years, IQR 2.2–11.5) and Abyssinian (n = 11; 10.0 years, IQR 1.1–18.1) (Table 1).
Figure 1.
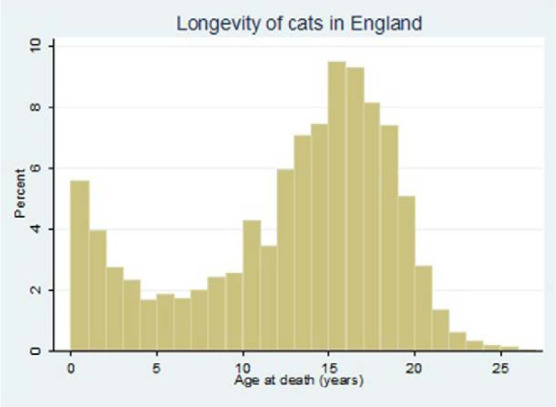
Distribution of ages at death in cats (n = 3979) attending primary care veterinary practices in England, showing the percentage of cats that died within 1 year age bands
Table 1.
Breed longevity (breeds with ⩾10 study animals) for cats attending primary care veterinary practices in England ranked by median age at death. The interquartile range (IQR), range and number of study cats are also shown (n = 4009)
| Breed | Median (years) | IQR | Range | Number of cats |
|---|---|---|---|---|
| Birman | 16.1 | 8.1–16.9 | 1.0–20.7 | 12 |
| Burmese | 14.3 | 10.0–17.0 | 0.7–20.7 | 31 |
| Siamese | 14.2 | 10.8–19.0 | 0.9–21.1 | 31 |
| Persian | 14.1 | 12.0–17.0 | 0.0–21.2 | 70 |
| Crossbred | 14.0 | 9.2–17.0 | 0.0–26.7 | 3621 |
| British shorthair | 11.8 | 5.8–16.3 | 0.0–21.0 | 69 |
| Maine Coon | 11.0 | 4.0–15.5 | 0.2–19.0 | 14 |
| Ragdoll | 10.1 | 0.9–14.8 | 0.1–17.9 | 21 |
| Abyssinian | 10.0 | 1.1–18.1 | 1.0–20.8 | 11 |
| Bengal | 7.3 | 2.2–11.5 | 0.6–13.7 | 15 |
A cause of mortality was specified for 3309 (82.5%) cats. In cats of all ages with a cause of mortality specified, the most frequently attributed causes were trauma (n = 405; 12.2%), renal disorder (n = 399; 12.1%), non-specific illness (n = 370; 11.2%), neoplasia (n = 356; 10.8%) and mass lesion disorders (n = 336; 10.2%) (Table 2). Mass lesions described mass-associated disorders that did not have a more precise aetiological diagnosis. Of the 405 cats that died from trauma, 243 (60.0%) were ascribed to road traffic accidents (RTA). Younger and older cats differed markedly in cause of mortality. For cats dying before 5 years of age (n = 516), the most frequent causes were trauma (n = 244; 47.3%), viral infectious disorders (n = 34; 6.6%) and respiratory disorders (n = 23; 4.5%). For cats dying at or after 5 years of age (n = 2793), the most frequent causes were renal disorder (n = 379; 13.6%), non-specific illness (n = 352; 12.6%), neoplasia (n = 343; 12.3%) and mass lesion disorder (n = 324; 11.6%) (Table 3).
Table 2.
Attributed causes of mortality (top 20) specified in the clinical notes (n = 3309) in cats of all ages attending primary care veterinary practices in England. The median, interquartile range (IQR) and range for the age (years) at death are reported
| Attributed cause | Rank | Number of deaths (%) | Median | IQR | Range |
|---|---|---|---|---|---|
| Trauma | 1 | 405 (12.2) | 3.0 | 1.1–9.0 | 0.0–22.0 |
| Renal disorder | 2 | 399 (12.1) | 15.1 | 12.0–17.3 | 0.3–24.0 |
| Non-specific illness | 3 | 370 (11.2) | 16.0 | 13.3–18.0 | 0.0–25.0 |
| Neoplasia | 4 | 356 (10.8) | 13.6 | 11.3–16.0 | 1.2–22.1 |
| Mass lesion disorder | 5 | 336 (10.2) | 14.2 | 12.0–16.5 | 0.9–22.1 |
| Neurological disorder | 6 | 231 (7.0) | 15.1 | 13.0–18.0 | 0.1–25.0 |
| Respiratory disorder | 7 | 183 (5.5) | 13.7 | 10.0–16.8 | 0.1–22.0 |
| Cardiac disease | 8 | 139 (4.2) | 14.0 | 11.5–16.3 | 0.0–22.0 |
| Endocrine disorder | 9 | 124 (3.8) | 16.0 | 12.9–17.3 | 0.0–24.1 |
| Thromboembolism | 10 | 106 (3.2) | 12.0 | 8.1–15.0 | 2.0–21.3 |
| Enteropathy | 11 | 98 (3.0) | 14.7 | 10.4–17.0 | 0.1–23.0 |
| Hepatopathy | 12 | 61 (1.8) | 13.6 | 10.0–16.4 | 1.0–25.0 |
| Viral disorder | 13 | 60 (1.8) | 3.8 | 0.6–10.4 | 0.1–17.0 |
| Urinary disorder | 14 | 57 (1.7) | 7.0 | 3.5–13.0 | 0.1–20.9 |
| Abdominal disorder | 15 | 48 (1.5) | 15.7 | 10.9–18.1 | 0.2–22.0 |
| Oral cavity disorder | 16 | 47 (1.4) | 15.5 | 12.7–18.0 | 2.6–23.0 |
| Behavioural disorder | 17 | 43 (1.3) | 16.0 | 14.0–18.6 | 2.0–22.0 |
| Ocular disorder | 18 | 37 (1.1) | 16.0 | 9.0–18.0 | 0.1–23.0 |
| Anaemia | 19 | 36 (1.1) | 10.9 | 2.8–15.0 | 0.1–17.5 |
| Parasitic disorder | 20 | 31 (0.9) | 15.2 | 12.0–17.8 | 0.1–21.9 |
Table 3.
Attributed causes of mortality (top 15) specified in the clinical notes in cats aged <5 years (n = 516) and cats aged ⩾5 (n = 2793) that attended primary care veterinary practices in England
| Attributed cause of mortality | <5 years |
⩾5 years |
||
|---|---|---|---|---|
| Rank | Number (%) of deaths | Rank | Number (%) of deaths | |
| Trauma | 1 | 244 (47.3) | 6 | 161 (5.8) |
| Viral disorder | 2 | 34 (6.6) | ||
| Respiratory disorder | 3 | 23 (4.5) | 7 | 160 (5.7) |
| Renal disorder | 4 | 20 (3.9) | 1 | 379 (13.6) |
| Urinary disorder | 5 | 19 (3.7) | ||
| Non-specific illness | 6 | 18 (3.5) | 2 | 352 (12.6) |
| Congenital disorder | 7 | 17 (3.3) | ||
| Thromboembolism | 8 | 14 (2.7) | 10 | 92 (3.3) |
| Enteropathy | 9 | 13 (2.5) | 11 | 85 (3.0) |
| Intoxication (poisoning) | 10 | 13 (2.5) | ||
| Neoplasia | 11 | 13 (2.5) | 3 | 343 (12.3) |
| Mass lesion disorder | 12 | 12 (2.3) | 4 | 324 (11.6) |
| Neurological disorder | 13 | 12 (2.3) | 5 | 219 (7.8) |
| Anaemia | 14 | 10 (1.9) | ||
| Cardiac disease | 15 | 10 (1.9) | 8 | 129 (4.6) |
| Endocrine disorder | 9 | 118 (4.2) | ||
| Hepatopathy | 12 | 55 (2.0) | ||
| Oral cavity disorder | 13 | 46 (1.7) | ||
| Abdominal disorder | 14 | 45 (1.6) | ||
| Behavioural disorder | 15 | 42 (1.5) | ||
For cats dying at or after 5 years (n = 3360), all risk factors evaluated using univariable linear regression modelling were associated with longevity. Multivariable modelling identified being crossbred, having a lower bodyweight, and being neutered and non-insured as associated with increased longevity. After accounting for the effects of the other risk factors, the mean additional longevity for crossbred cats was 0.6 years (95% confidence interval [CI] 0.2–1.1; P = 0.008) compared with purebred cats. Compared with entire female cats, neutered female cats had a greater longevity of 0.6 years (95% CI 0.1–1.0; P = 0.007) and entire male cats had a shorter longevity of 1.8 years (95% CI −1.3 to −2.3; P <0.001). Increasing bodyweight was associated with decreasing longevity (P <0.001). The longevity of insured cats was 1.1 years (95% CI −0.7 to −1.5; P <0.001) shorter than non-insured cats (Table 4).
Table 4.
Final multivariable linear regression results for risk factors associated with longevity (years) in cats attending primary care veterinary practices in England that died at or after 5 years of age (n = 3310). The coefficient indicates the average longevity difference in years compared with the baseline group
| Variable | Coefficient | 95% confidence interval | P value |
|---|---|---|---|
| Purebred status | |||
| Crossbred | Baseline | – | – |
| Purebred | −0.6 | −0.2 to −1.1 | 0.008 |
| Sex/neuter status | |||
| Female entire | Baseline | – | – |
| Female neutered | 0.6 | 0.1–1.0 | 0.007 |
| Male entire | −1.8 | −1.3 to −2.3 | <0.001 |
| Male neutered | 0.1 | −0.4 to 0.5 | 0.756 |
| Bodyweight (kg) | |||
| <3.0 | Baseline | − | – |
| 3.0–3.9 | −0.8 | −0.3 to −1.3 | 0.001 |
| 4.0–4.9 | −1.7 | −1.2 to −2.2 | <0.001 |
| 5.0–5.9 | −2.0 | −1.4 to −2.7 | <0.001 |
| ⩾6.0 | −3.3 | −2.5 to −4.2 | <0.001 |
| No weight recorded | −0.4 | −0.8 to 0.0 | 0.047 |
| Insurance status | |||
| Not insured | Baseline | – | – |
| Insured | −1.1 | −0.7 to −1.5 | <0.001 |
Adjusting for clustering within veterinary clinics did not materially affect the results. No significant interactions or major departures from normality or homoscedasticity were identified. A relatively low adjusted r2 value (0.085) suggested that the final model accounted for 8.5% of data variation.
Discussion
This study of over 4000 cats attending primary care veterinary practices aimed to report longevity and mortality values that would generalise well to the overall owned cat population in England, and further aimed to explore the variation in longevity between pure- and crossbred cats. The hope was that the novel information derived from the study would support evidence-based approaches to advance feline breeding, husbandry and medicine.1–3
The non-normal and bimodal distribution of longevity in this study (Figure 1) suggested the median as a more appropriate statistic for longevity than the mean because extreme values from non-normally distributed distributions exert disproportionate effects on the mean. 29 Few published studies have reported the longevity of domestic cats. A report based on clinical records from 800 Banfield Pet Hospitals in the USA suggested an ‘average’ longevity of 12.1 years for cats, but did not specify whether this was a median or a mean value. 18 Improved nutrition, healthcare and management are stated to promoted increased life expectancy in domestic cats, but specific supporting data are limited.2,17,19
This study identified a bimodal longevity distribution peaking in years 1 and 16, which suggested the existence of two distinct subpopulations of cats: those with a propensity for earlier death and cats that survive to an older age (Figure 1). Longevity is similarly bimodal in dogs, 16 indicating that mortality studies should be separated for each longevity group. Of deaths occurring before 5 years of age, almost half (47.3%) resulted from trauma, with the majority of these from RTA. This compares with just 5.8% of trauma-related deaths in cats dying at or after 5 years of age. The preponderance for RTA-related deaths among younger cats concurs with a UK practice-based study that reported a reduction in RTA death rates as cats got older. 31 A Swedish study of insured cats that died before 12 years of age also identified RTA as important to cat mortality, with an age-standardised mortality rate (ASMR) of 411 per 10,000 cat-years at risk. 19 Differing international attitudes to outdoor access for owned cats may affect trauma- and RTA-related mortality: over 90% of UK cats have daily outdoor access compared with 80% in Australia and 50–60% in the USA.3,32,33
The most common causes of mortality at or after 5 years identified in this study were renal disorder (13.6%), non-specific illness (12.6%), neoplastic disorder (12.3%) and mass lesion disorder (11.6%). The clinical importance of renal disease in older cats is supported by previous reports showing that 15–30% of cats over the age of 15 years were azotaemic. 34 Kidney and ureter disorders were the most common cause of mortality in insured cats dying before the age of 12 years in Sweden (ASMR: 713 per 10,000 cat-years at risk). 19 The frequent identification of physical and biochemical abnormalities in apparently healthy older cats35,36 emphasises the value of clinical vigilance and routine health checks to optimise the detection and management of renal disease, especially in older cats.37–39 Although 12% of deaths in this study were from neoplasia, an equivalent number of deaths were also ascribed to non-specific masses. Although mass lesions could include cysts, inflammation and infection, 40 it is possible that many may have been undiagnosed neoplastic disorders, suggesting that neoplasia could account for up to a quarter of deaths in older cats. Neoplastic disorders were reported to have an ASMR of 528 per 10,000 cat-years at risk in insured cats in Sweden, but the study was limited by including only cats that died before the age of 12 years. 19 Although malignant neoplasia is often life-limiting, routine veterinary evaluation of older cats for neoplastic disorders has been recommended because earlier diagnosis may enable interventions that increase longevity and improve palliative care, as well as the provision of more-informed choices for owners. 36
The results of this study support the hypothesis that longevity in crossbred cats exceeds that of purebred ones. Direct comparison of median overall longevity values showed that crossbred cats outlived purebred cats by 1.5 years (14.0 years for crossbred cats compared with 12.5 years for purebred cats). Within those cats dying at or after 5 years of age, after accounting for bodyweight, sex, and neutering and insurance status, crossbred cats outlived purebred cats by 0.6 years. In support of this, an Australian survey on currently living pet cats reported a significantly higher median age for crossbred cats (7.0 years) compared with purebred ones (5.5 years). 3
Cats have been kept as pets by humans for 10,000 years, 41 but it is during the past 150 years that cats have been selectively bred for show and novelty, in particular. 12 Purebred cats comprised 8.3% of the population in the current study. Although the proportion of UK-owned cats that is purebred is currently estimated around 10.0%, 42 this value is predicted to rise, bringing an expansion in both the recognition and impact of breed-related anomalies and genetic disorders in cats.43,44 The substantial longevity deficit identified for purebred cats in this study warrants further investigation to better understand and manage the mechanisms involved. It may be that purebred cats express more recessive disorders because of greater homozygosity for deleterious genes (inbreeding depression) 45 but other genetic and non-genetic differences may also contribute. It is also worth noting that, although purebred status was significantly associated with a reduced longevity, only 8.5% of longevity variation was explained by the final model used in this study. This implies substantial roles in feline longevity for factors not included in the current study such as diet, 46 vaccination, 47 outdoor access 33 and obesity. 48
Despite the superior longevity identified in crossbred cats, it is notable that longevity varied widely between pure cat breeds, suggesting the importance of improved understanding of associations between breed and longevity. The Birman, Burmese, Siamese and Persians lived as long as or longer than crossbreeds, whereas the Bengal, Abyssinian, Ragdoll, Maine Coon and British Shorthair breeds showed reduced longevity. Similarly, a Swedish insurance study identified equivalent or greater age-based survival for Birman, Norwegian, Persian and Siamese cats compared with domestic cats. 2 Longevity variation between breeds may be partially explained by differing breed bodyweights: lighter breeds have greater longevity than heavier breeds. 49 Differing breed predispositions to specific diseases may also contribute to variation in longevity. 9 Larger breed-specific studies would enable greater longevity precision and reporting of within-breed risk factors for compromised longevity. Such studies are especially important for less popular breeds where smaller gene pools for breeding may increase predispositions for inherited disease. 10
It is worth noting that both overall and breed-specific longevity is influenced by the right of owners to opt for termination of life for their cats. In this study, >85% of deaths involved euthanasia, with statistically different proportions of crossbred and purebred cats being euthanased (86.0% VS 81.9% respectively). Euthanasia decision-making is complex and emotionally intense both for owners and veterinarians.50,51 Greater understanding of the human factors involved in pet euthanasia decisions may ameliorate the psychological burden on owners and veterinarians, as well as improving the quality of the decisions made.
In this study, maximum bodyweight values recorded after 6 months of age were negatively associated with longevity. In cats that died at 5 years of age or older, cats weighing <3 kg lived 1.7 years longer, on average, than cats weighing between 4 and 5 kg. A negative association between bodyweight and longevity has previously been reported in dogs, 16 and was hypothesised to result from the genetic and pathological effects induced by artificial selection for extremes of size and growth.52–55 The bodyweight associations with longevity identified in this study may have been partially confounded by breed and obesity effects, which could be explored in future studies. 48
Although the study indicated an association between insurance and reduced longevity, the direction of any causality requires careful consideration. Insured animals may revert to being non-insured as they age because of increasing insurance costs or exclusions.56,57 This study used insurance values at death; thus, the negative association identified may have resulted from increasing insurance policy cancellation with advancing age.
Neutering was associated with 0.6 years greater longevity in females and 1.7 years greater longevity in males. Neutering offers health benefits, including reduced risk of asthma, gingivitis and hyperactivity in both sexes, and decreased abscesses, aggression toward veterinarians, undesirable sexual behaviours and urine spraying in males.58,59 However, the current neutering results should be interpreted cautiously because the neuter status at death was used and modelled as time-independent (ie, a single value applies throughout life) because of the nature of the available data. Cats aged <5 months tend to be entire, whereas a consistent proportion of older cats tend to be neutered, 60 suggesting that, ideally, neutering should be modelled as a time-dependent variable. 61 With the ongoing accrual of data by VetCompass, future studies will increasingly use time-dependent modelling.
This study had some limitations. Many of the study cats were privately owned and therefore the results may not reflect longevity in unowned or feral cats. Data on some cats that died and were buried at home or that went missing may not have been included. The exclusion of records with unconfirmed cause of mortality in this study could have resulted in under estimation of deaths caused by RTA or animal attacks.62,63 The veterinary practices included in the study had progressive attitudes to data sharing and were situated mainly in central and south-east England, and thus may not be representative of all veterinary practices in England. The validity of the data relied heavily on owner-reported information and on the clinical acumen and note-making of attending practitioners. 16
Conclusions
On average, crossbred cats showed greater longevity than purebred cats, but individual purebred cat breeds varied substantially in longevity. Increasing bodyweight in adult cats was negatively associated with longevity. The most common cause of mortality in younger cats was trauma; in older cats, the most common causes were renal disorders, non-specific illness, neoplasia and mass lesion disorders. This study identified important breed and phenotypic associations with variation in longevity that can be used to direct breeding and research strategies. Increased awareness of the common causes of mortality within subdemographics of cats should promote improved management and diagnostic methods that will improve feline welfare.
Acknowledgments
We thank Peter Dron (RVC) for VetCompass database development, and Noel Kennedy (RVC) for software and programming development. We are especially grateful to the Medivet Veterinary Partnership and other UK practices and clients for participating in VetCompass.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: This work was supported by an Royal Society for the Prevention of Cruelty to Animals (RSPCA) Animal Welfare Research Grant.
Accepted: 24 April 2014
References
- 1. Rochlitz I. Feline welfare issues. In: Turner DC, Bateson PPG. (eds) The domestic cat: the biology of its behaviour. 2nd ed. Cambridge: Cambridge University Press; 2000, pp 131–154. [Google Scholar]
- 2. Bonnett BN, Egenvall A. Age patterns of disease and death in insured Swedish dogs, cats and horses. J Comp Pathol 2010; 142 Suppl 1: S33–S38. [DOI] [PubMed] [Google Scholar]
- 3. Toribio JALM, Norris JM, White JD, et al. Demographics and husbandry of pet cats living in Sydney, Australia: results of cross-sectional survey of pet ownership. J Feline Med Surg 2009; 11: 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murray JK, Browne WJ, Roberts MA, et al. Number and ownership profiles of cats and dogs in the UK. Vet Rec 2010; 166: 163–168. [DOI] [PubMed] [Google Scholar]
- 5. Pet Food Manufacturers’ Association. Pet population 2013.. http://www.pfma.org.uk/pet-population/ (accessed December 9, 2013).
- 6. Bateson P. Independent inquiry into dog breeding. http://www.ourdogs.co.uk/special/final-dog-inquiry-120110.pdf. Cambridge: University of Cambridge, 2010. [Google Scholar]
- 7. Collins LM, Asher L, Summers J, et al. Getting priorities straight: risk assessment and decision-making in the improvement of inherited disorders in pedigree dogs. Vet J 2011; 189: 147–154. [DOI] [PubMed] [Google Scholar]
- 8. Adams VJ, Evans KM, Sampson J, et al. Methods and mortality results of a health survey of purebred dogs in the UK. J Small Anim Pract 2010; 51: 512–524. [DOI] [PubMed] [Google Scholar]
- 9. Gough A, Thomas A. Breed predispositions to disease in dogs and cats. 2nd ed. Chicester: Wiley-Blackwell, 2010. [Google Scholar]
- 10. Gunn-Moore D, Bessant C, Malik R. Breed-related disorders of cats. J Small Anim Pract 2008; 49: 167–168. [DOI] [PubMed] [Google Scholar]
- 11. International Cat Care. Inherited disorders in cats. http://www.icatcare.org/advice/cat-breeds/inherited-disorders-cats (accessed 10 December 2013).
- 12. Bessant C. Breed-related disorders of cats. SPVS Rev 2009: 56–58. [DOI] [PubMed] [Google Scholar]
- 13. Malik R, Sparkes A, Bessant C. Brachycephalia – a bastardisation of what makes cats special. J Feline Med Surg 2009; 11: 889–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dechow CD, Rogers GW, Cooper JB, et al. Milk, fat, protein, somatic cell score, and days open among Holstein, Brown Swiss, and their crosses. J Dairy Sci 2007; 90: 3542-3549. [DOI] [PubMed] [Google Scholar]
- 15. Nicholas FW. Introduction to Veterinary Genetics. 3rd ed. Oxford: Wiley-Blackwell, 2010. [Google Scholar]
- 16. O’Neill DG, Church DB, McGreevy PD, et al. Longevity and mortality of owned dogs in England. Vet J 2013; 198: 638–643. [DOI] [PubMed] [Google Scholar]
- 17. Day MJ. Ageing, immunosenescence and inflammageing in the dog and cat. J Comp Pathol 2010; 142 Suppl 1: S60–S69. [DOI] [PubMed] [Google Scholar]
- 18. Banfield Pet Hospital. State of pet health 2013 report. http://www.stateofpethealth.com/state-of-pet-health/Arthritis (accessed August 31, 2013).
- 19. Egenvall A, Nødtvedt A, Haggstrom J, et al. Mortality of life-insured Swedish cats during 1999–2006: age, breed, sex, and diagnosis. J Vet Intern Med 2009; 23: 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McGreevy PD. Breeding for quality of life. Anim Welfare 2007; 16: 125–128. [Google Scholar]
- 21. O’Neill D. Pointing the way to improved welfare for companion animals. Vet Rec 2013; 173: 240–242. [DOI] [PubMed] [Google Scholar]
- 22. VetCompass. VetCompass: Health surveillance for UK companion animals. London: Royal Veterinary College Electronic Media Unit, 2013. [Google Scholar]
- 23. The VeNom Coding Group. VeNom Veterinary Nomenclature, http://www.venomcoding.org (accessed May 13, 2013).
- 24. Kearsley-Fleet L, O’Neill DG, Volk HA, et al. Prevalence and risk factors for canine epilepsy of unknown origin in the UK. Vet Rec 2013; 172: 338. [DOI] [PubMed] [Google Scholar]
- 25. Dupont WD, Plummer WD., Jr. Power and sample size calculations for studies involving linear regression. Control Clin Trials 1998; 19: 589–601. [DOI] [PubMed] [Google Scholar]
- 26. Haahr M. RANDOM.ORG: true random number service, http://www.random.org/ (accessed March 17, 2012).
- 27. Rollin BE. Ethics and euthanasia. Can Vet J 2009; 50: 1081–1086. [PMC free article] [PubMed] [Google Scholar]
- 28. StataCorp. Stata statistical software: Release 112009. http://www.stata.com/ (accessed May 6, 2014).
- 29. Kirkwood BR, Sterne JAC. Essential medical statistics. 2nd ed. Oxford: Blackwell Science, 2003. [Google Scholar]
- 30. Dohoo I, Martin W, Stryhn H. Veterinary epidemiologic research. 2nd ed. Charlottetown: VER, 2009. [Google Scholar]
- 31. Rochlitz I. Study of factors that may predispose domestic cats to road traffic accidents: part 1. Vet Rec 2003; 153: 549–553. [DOI] [PubMed] [Google Scholar]
- 32. Murray JK, Gruffydd-Jones TJ. Proportion of pet cats registered with a veterinary practice and factors influencing registration in the UK. Vet J 2012; 192: 461–466. [DOI] [PubMed] [Google Scholar]
- 33. Rochlitz I. A review of the housing requirements of domestic cats (Felis silvestris catus) kept in the home. Appl Anim Behav Sci 2005; 93: 97–109. [Google Scholar]
- 34. Lulich J, Osborne C, O’Brien T, et al. Feline renal failure: questions, answers, questions. Comp Contin Educ Pract Vet 1992; 14. [Google Scholar]
- 35. Paepe D, Verjans G, Duchateau L, et al. Routine health screening: findings in apparently healthy middle-aged and old cats. J Feline Med Surg 2013; 15: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Epstein M, Kuehn NF, Landsberg G, et al. AAHA senior care guidelines for dogs and cats. J Am Anim Hosp Assoc 2005; 41: 81–91. [DOI] [PubMed] [Google Scholar]
- 37. Syme HM, Markwell PJ, Pfeiffer D, et al. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J Vet Intern Med 2006; 20: 528–535. [DOI] [PubMed] [Google Scholar]
- 38. Elliott J, Rawlings JM, Markwell PJ, et al. Survival of cats with naturally occurring chronic renal failure: effect of dietary management. J Small Anim Pract 2000; 41: 235–242. [DOI] [PubMed] [Google Scholar]
- 39. Elliott J, Watson ADJ. Chronic kidney disease: staging and management. In: Bonagura JD, Kirk RW. (eds). Kirk’s current veterinary therapy XIV. Philadelphia, PA: Elsevier Saunders, 2008, pp 883–892. [Google Scholar]
- 40. Beaman FD, Kransdorf MJ, Andrews TR, et al. Superficial soft-tissue masses: analysis, diagnosis, and differential considerations. Radiographics 2007; 27: 509–523. [DOI] [PubMed] [Google Scholar]
- 41. Driscoll CA, Menotti-Raymond M, Roca AL, et al. The Near Eastern origin of cat domestication. Science 2007; 317: 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pet Food Manufacturers’ Association. The Pet Food Manufacturers’ Association ‘statistics’. http://www.pfma.org.uk/statistics/ (accessed September 14, 2012).
- 43. Malik R. Genetic diseases of cats. J Feline Med Surg 2001; 3: 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Walsh F. Human-animal bonds I: the relational significance of companion animals. Fam Proc 2009; 48: 462–480. [DOI] [PubMed] [Google Scholar]
- 45. McGreevy PD, Nicholas FW. Some practical solutions to welfare problems in dog breeding. Anim Welfare 1999; 8: 329–341. [Google Scholar]
- 46. Plantinga EA, Everts H, Kastelein AMC, et al. Retrospective study of the survival of cats with acquired chronic renal insufficiency offered different commercial diets. Vet Rec 2005; 157: 185–187. [DOI] [PubMed] [Google Scholar]
- 47. Horzinek MC. Vaccine use and disease prevalence in dogs and cats. Vet Microbiol 2006; 117: 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. German AJ. The growing problem of obesity in dogs and cats. J Nutr 2006; 136: 1940S–1946S. [DOI] [PubMed] [Google Scholar]
- 49. Kienzle E, Moik K. A pilot study of the body weight of pure-bred client-owned adult cats. Br J Nutr 2011; 106: S113–S115. [DOI] [PubMed] [Google Scholar]
- 50. Sanders CR. Killing with kindness: veterinary euthanasia and the social construction of personhood. Sociol Forum 1995; 10: 195–214. [Google Scholar]
- 51. Yeates JW, Main DCJ. Veterinary opinions on refusing euthanasia: justifications and philosophical frameworks. Vet Rec 2011; 168: 263. [DOI] [PubMed] [Google Scholar]
- 52. Galis F, Van Der Sluijs I, Van Dooren TJM, et al. Do large dogs die young? J Exp Zool B Mol Dev Evol 2007; 308B: 119–126. [DOI] [PubMed] [Google Scholar]
- 53. Fleming JM, Creevy KE, Promislow DEL. Mortality in North American dogs from 1984 to 2004: an investigation into age-, size-, and breed-related causes of death. J Vet Intern Med 2011; 25: 187–198. [DOI] [PubMed] [Google Scholar]
- 54. Urfer SR, Gaillard C, Steiger A. Lifespan and disease predispositions in the Irish Wolfhound: a review. Vet Q 2007; 29: 102–111. [DOI] [PubMed] [Google Scholar]
- 55. Salvin HE, McGreevy PD, Sachdev PS, et al. The effect of breed on age-related changes in behavior and disease prevalence in cognitively normal older community dogs, Canis lupus familiaris. J Vet Behav Clin Appl Res 2012; 7: 61–69. [Google Scholar]
- 56. Dobson JM, Samuel S, Milstein H, et al. Canine neoplasia in the UK: estimates of incidence rates from a population of insured dogs. J Small Anim Pract 2002; 43: 240–246. [DOI] [PubMed] [Google Scholar]
- 57. Egenvall A, Nødtvedt A, Penell J, et al. Insurance data for research in companion animals: benefits and limitations. Acta Vet Scand 2009; 51: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Spain CV, Scarlett JM, Houpt KA. Long-term risks and benefits of early-age gonadectomy in cats. J Am Vet Med Assoc 2004; 224: 372–379. [DOI] [PubMed] [Google Scholar]
- 59. Kustritz MV. Determining the optimal age for gonadectomy of dogs and cats. J Am Vet Med Assoc 2007; 231: 1665–1675. [DOI] [PubMed] [Google Scholar]
- 60. Murray JK, Roberts MA, Whitmars A, et al. Survey of the characteristics of cats owned by households in the UK and factors affecting their neutered status. Vet Rec 2009; 164: 137–141. [DOI] [PubMed] [Google Scholar]
- 61. van Hagen MAE, Ducro BJ, van den Broek J, et al. Life expectancy in a birth cohort of Boxers followed up from weaning to 10 years of age. Am J Vet Res 2005; 66: 1646–1650. [DOI] [PubMed] [Google Scholar]
- 62. Rochlitz I. Clinical study of cats injured and killed in road traffic accidents in Cambridgeshire. J Small Anim Pract 2004; 45: 390–394. [DOI] [PubMed] [Google Scholar]
- 63. Harris S. The food of suburban foxes (Vulpes vulpes), with special reference to London. Mammal Rev 1981; 11: 151–168. [Google Scholar]


