Abstract
Feline immunodeficiency virus (FIV), the causative agent of an acquired immunodeficiency syndrome in cats (feline AIDS), is a ubiquitous health threat to the domestic and feral cat population, also triggering disease in wild animals. No registered antiviral compounds are currently available to treat FIV-infected cats. Several human antiviral drugs have been used experimentally in cats, but not without the development of serious adverse effects. Here we report on the treatment of six naturally FIV-infected cats, suffering from moderate to severe disease, with the antiretroviral compound (R)-9-(2-phosphonylmethoxypropyl)-2,6-diaminopurine ([R]-PMPDAP), a close analogue of tenofovir, a widely prescribed anti-HIV drug in human medicine. An improvement in the average Karnofsky score (pretreatment 33.2 ± 9.4%, post-treatment 65±12.3%), some laboratory parameters (ie, serum amyloid A and gammaglobulins) and a decrease of FIV viral load in plasma were noted in most cats. The role of concurrent medication in ameliorating the Karnofsky score, as well as the possible development of haematological side effects, are discussed. Side effects, when noted, appeared mild and reversible upon cessation of treatment. Although strong conclusions cannot be drawn owing to the small number of patients and lack of a placebo-treated control group, the activity of (R)-PMPDAP, as observed here, warrants further investigation.
Introduction
Feline immunodeficiency virus (FIV) was first isolated in 1986 as ‘a T-lymphotropic virus, causing an immunodeficiency-like syndrome’. 1 This retroviral lentivirus is an important pathogen of both wild and domestic cats worldwide, triggering a broad range of progressive diseases.2–4 Currently, no species-specific antiviral drugs are available for the treatment of FIV-infected cats; hence, treatment is, at best, supportive and symptomatic, sometimes combined with immunomodulatory therapy. 5
As FIV and human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT) enzymes are closely related, a number of nucleoside (NRTI) and/or nucleotide RT inhibitors (NtRTIs) of HIV also exert in vitro anti-FIV activity.6–8 In vivo treatment of FIV-infected cats with these drugs has raised major concerns regarding toxicity and clinical efficacy.9–13 The most extensively studied NRTI in cats is 3’-azido-3’deoxythymidine (AZT; zidovudine, Retrovir), as a single agent or combined with 2’,3’-dideoxy-3’-thiacytidine (3TC; lamivudine, Epivir). Despite an improvement in general health scores and recovery from opportunistic diseases, long-term use of AZT and the AZT/3TC combination treatment is jeopardised in cats owing to the development of non-regenerative anaemia.9,10,14 Although not reaching statistical significance, a thioether lipid–AZT conjugate, fozivudine, appears to exhibit antiviral activity by decreasing viraemia during acute experimental FIV infection, without the development of adverse effects. 15
A specific structural class of NtRTI—acyclic nucleoside phosphonates (ANPs)—exert broad-spectrum antiviral activity. Treatment of FIV-infected cats with the ANP 9-2-(phosphonylmethoxyethyl)adenine (PMEA; adefovir) showed greater potency and efficacy than AZT in improving clinical health parameters, but at the cost of a decline in packed cell volume (PCV) and haemoglobin levels.10,12 However, (S)-9-(3-fluoro-2-phosphonylmethoxypropyl)-adenine ([S]-FPMPA), an analogue of the HIV-drug tenofovir (ie, PMPA or [R]-9-[2-phosphonylmethoxypropyl]adenine) was better tolerated than PMEA in cats, but proved somewhat less antivirally and clinically effective for the treatment of FIV in cats. 11
Another more closely-related PMPA analogue, (R)-9-(2-phosphonylmethoxypropyl)-2,6-diaminopurine ([R]-PMPDAP), was reported to be not only a more potent inhibitor of FIV replication than PMEA, but also better tolerated in cats.12,16 Moreover, clinically healthy, experimentally infected cats showed a significant decrease in FIV viral load when treated with (R)-PMPDAP. 12 Although only a tendency towards improvement in clinical parameters was reported in naturally FIV-infected cats treated with (R)-PMPDAP, most cats included had mild general health problems, with a mean Karnofsky score (KS) >70% at the beginning of the treatment.16,17 Hence, the margin for amelioration was narrow. A quantitative monitoring of the viral load during (R)-PMPDAP treatment of naturally FIV-infected cats has not been reported.
Here, we report on the initial presentation of naturally FIV-infected cats with moderate-to-severe clinical disease and their clinical, as well as viral, response to treatment with the antiretroviral compound (R)-PMPDAP.
Materials and methods
Patient medical records
Medical records of client-owned FIV-positive cats treated with (R)-PMPDAP between September 2010 and August 2011 were reviewed. For all cats, owners had given their written informed consent for the treatment of their pets with (R)-PMPDAP. The compound had been manufactured in accordance with good manufacturing practices and administered as a formula magistralis.
FIV infection status was determined using a commercial kit (Witness FeLV–FIV; Synbiotics Europe) and confirmed by RT quantitative polymerase chain reaction (qPCR) at a commercial laboratory (Okapi Sciences NV, Heverlee, Belgium).
Records were included if physical examination, complete blood count (CBC) and serum biochemistry findings before and during treatment were available, and if cats had an initial KS ⩽50%. Records were excluded if cats had received other antiviral therapy prior to treatment with (R)-PMPDAP.
The KS represents a scoring system to assess a cat’s wellbeing and quality of life. The index is based on a questionnaire for the owner or caretaker, considering various aspects of the cat’s behaviour, together with a clinician score assessing the general condition of the cat. 17
Treatment
(R)-PMPDAP was administered by subcutaneous injection twice a week at a dose rate of 25 mg/kg. The KSs were used to determine the duration of treatment. If the cat had reached a KS ≧60% after 3 weeks, treatment was stopped; otherwise, treatment was continued for another 3 weeks. (R)-PMPDAP was provided by Okapi Sciences NV, Heverlee, Belgium. Concurrent, supportive medication was administered when necessary. During treatment, all cats were hospitalised at the Small Animal Clinic of Ghent University.
Physical examination and KS
Weekly detailed physical examinations and KSs were available. A follow-up visit was advised 1 month after cessation of treatment. This information was also included if available.
Blood and urine analysis
Blood samples were taken from the jugular vein before the first administration of (R)-PMPDAP, every 3 weeks during treatment and at follow-up 1 month after last treatment. Urine samples were taken before and/or during treatment if clinically indicated. CBC, serum biochemistry profile and urinalysis were performed at a private commercial laboratory (Medic Lab Laboratories, Aalst, Belgium).
Viral load quantification
FIV viral load quantification in plasma was determined by Okapi Sciences NV, Heverlee, Belgium. Viral RNA was extracted out of 140 µl cat plasma using the QIAgen Viral RNA mini kit according to the manufacturer’s instructions. The extracted viral RNA was reverse transcribed to complementary DNA (cDNA) using 2 µl of the extract in an RT reaction containing 1 × RT reaction mix, 7 mM MgCl2, 2 µl deoxynucleotide triphosphates (2.5 mM each), 2.5 µM random hexamers, 0.2 µl RNase Inhibitor (20 U/µl; Life Technologies) and 0.25 µl Multiscribe RT (50 U/µl; Life Technologies) in a total volume of 10 µl. The RT programme consisted of following steps: 10 mins at 25°C, 30 mins at 45°C and 5 mins at 95°C. An FIV 1416p real-time PCR targeting the FIV gag gene was performed 14 using 2 µl of cDNA added to 20 nM probe; and 100 nM forward and reverse primers, and Fast Real Time Mastermix (Life Technologies) in a total volume of 20 µl. Amplification was done with a StepOnePlus thermocycler (Life Technologies) using the standard Fast programme.
Incorporation of a standard synthetic FIV RNA dilution series (using the RiboMax Large Scale RNA kit [Promega] following the manufacturer’s instructions) allowed for absolute FIV viral load quantification. The synthetic FIV RNA was synthesised from a 300-base pair PCR-amplified fragment of the gag gene of the FIV Glasgow-8 isolate, targeted by the FIV 1416p real-time PCR. The synthetic FIV RNA obtained was treated with DNase I (Life Technologies) and purified on a MicroSpin G-25 column (GE Healthcare). The synthetic RNA was separated and extracted from a formaldehyde agarose gel to remove remaining FIV DNA contaminants from the FIV RNA template. To generate a standard curve for viral load quantification, 2 µl of a 10-fold dilution series of the synthetic FIV RNA was used in each RT reaction ranging from 106 to 100 FIV RNA copies per reaction. The limit for quantification for this RT-qPCR is 103 virions per ml plasma.
Results
The records of 10 client-owned FIV-positive cats treated with (R)-PMPDAP were available. Three cats were excluded as they had previously been treated with antiviral drugs. One additional patient was not considered, as the initial KS was >50%.
Signalment and clinical signs
The age of the included cats ranged from 3 to 11 years; the majority (4/6) over 8 years of age. Male cats were overrepresented (4/6); all cats were neutered. The cats suffered from a variety of clinical diseases: oral disease (4/6), dermatological conditions (2/6) and conjunctivitis (2/6). All cats but one (cat K) were underweight, with a body condition score of 3 on a 9-point scale. 18 Cat M presented with a mandibular phlegmon, cat P concurrently suffered from hypertrophic cardiomyopathy, and cat F had a history of polydipsia and polyuria. Cat K was known to be feline leukaemia virus (FeLV)-positive and presented with vague symptoms—inappetence, occasional vomiting and lethargy—without abnormal findings on physical examination.
Treatment schedule and concurrent medication
Two cats (cats F and J) received the antiviral treatment for 3 weeks, three cats (cats P, N and K) for 6 weeks and cat M received the treatment for only 1 week (as described below). Cat K was treated four times a week instead of twice a week from the second week of treatment onwards. All cats but one (cat F) received concurrent medication, including antibiotics and non-steroidal anti-inflammatory drugs (NSAIDs).
General health
During treatment
The main clinical signs (ie, stomatogingivitis, dermatitis, polyuria and polydipsia, conjunctivitis) remained stable throughout the treatment period in four cats (cats F, J, P and N), but the condition of two cats (cats M and K) deteriorated markedly after 1 week of treatment to a point requiring intensive care therapy.
Cat M had to be humanely euthanased owing to recurrent hypotension not responding to treatment. Autopsy revealed acute diffuse interstitial pneumonia, liver necrosis, immune complex-mediated glomerulonephritis and non-suppurative encephalitis.
Cat K showed abnormalities on abdominal ultrasonography suggestive of cholangitis/cholangiohepatitis, together with hyperbilirubinemia and a moderate elevation of serum bile acids. The cat recovered following intensive treatment. Further supportive care for cat K consisted of corticosteroid administration for recurrent episodes of fever despite antibiotic therapy and the daily addition of insulin owing to the development of diabetes mellitus. Consequently, the frequency of antiviral treatment was doubled from a twice a week to a four-times weekly dosing schedule from the second week of (R)-PMPDAP treatment onward.
One month after cessation of antiviral therapy
Of the five cats completing (R)-PMPDAP treatment, four cats (cats F, P, N and K) underwent a follow-up visit 1 month after the last administration of the antiviral compound. The remaining cat (cat J) was euthanased 2 weeks after discharge because of persisting anorexia.
In three cats (cats F, P and K), clinical presentation seemed similar to their condition at the end of (R)-PMPDAP treatment. Cat N had received supportive treatment with an antibiotic and an NSAID for an episode of pyrexia that occurred after discharge and showed, still on this treatment, an ameliorated clinical condition (ie, ameliorated dermatitis and gingivitis) at follow-up.
KS
The evolution of KS is shown in Table 1. The average KS was 33.2 ± 9.4% at initial presentation (D0). Compared with D0, all cats (n = 5) showed an improvement in the KS at discharge (Tend), with an average increase of 28.6%.
Table 1.
Evolution of Karnofsky scores expressed in percentages before and during treatment of six FIV-positive cats with (R)-PMPDAP
| Cat | D0 | D0→ Tend | D0→ Tend + 1 m |
|---|---|---|---|
| F | 31 | +30 | +16 |
| J | 36 | +24 | – |
| P | 31 | +27 | +5 |
| N | 40 | +19 | +50 |
| K | 44 | +43 | +38 |
| M | 17 | – | – |
D = day of treatment; D0 = first day of (R)-PMPDAP treatment; Tend = end of treatment; D0→Tend = evolution from onset until end of treatment (ie, 3 or 6 weeks); D0→ Tend + 1 m = evolution from onset until one month following last treatment
At follow-up (Tend + 1 m), all four cats showed a higher KS when compared with their initial score (D0). However, compared with the end of the treatment period (Tend), 3/4 cats (cats F, P and K) presented with a lower score at follow-up (Tend + 1 m).
Laboratory results
Haematology
The main haematological parameters are summarised in Table 2.
Table 2.
Haematological parameters before and during treatment with (R)-PMPDAP of six FIV-positive cats
| Haematological variable | D0 (n = 6) |
D21 (n = 5) |
D42 (n = 3) |
D0→Tend |
Tend + 1 m (n = 4) |
D0→ Tend + 1 m |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | (Range) | Mean ± SD | (Range) | Mean ± SD | (Range) | ↓ | ↑ | Mean ± SD | (Range) | ↓ | ↑ | |
| PCV (%) | 23.7 ± 4.1 | (20–31) | 23.0 ± 6.4 | (18–33) | 29.0 ± 5.6 | (23–34) | 3/5 | 2/5 | 28.3 ± 4.6 | (22–33) | - | 4/4 |
| Leukocyte count (103/µl) | 10.9 ± 11.2 | (1.2–28.7) | 14.7 ± 7.8 | (4.8–23.7) | 17.5 ± 5.1 | (11.9–21.9) | 2/5 | 3/5 | 18.2 ± 8.3 | (6.3–25.2) | 2/4 | 2/4 |
| Lymphocyte count (103/µl) | 3.7 ± 3.8 | (0.2–10.6) | 3.4 ± 2.4 | (0.9–6.7) | 5.5 ± 3.9 | (1.0–8.3) | 2/5 | 3/5 | 4.3 ± 2.9 | (0.8–6.8) | 2/4 | 2/4 |
D = day of treatment; D0 = first day of (R)-PMPDAP treatment; Tend = end of treatment; D0→Tend = evolution from onset until end of treatment (ie, 3 or 6 weeks); D0→ Tend + 1 m = evolution from onset until 1 month following last treatment; PCV = packed cell volume
Four cats (cats F, J, P and M) had anaemia at presentation (PCV <24%), while at follow-up only one (cat F) was anaemic.
Before treatment, two cats (cats K and M) were leukopenic as a result of a lymphopenia. For only one of these cats (cat K) were follow-up data available. One month after the cessation of (R)-PMPDAP treatment, the leukocyte count had normalised, but the cat remained lymphopenic.
Three cats (cats F, P and N) initially presented with a leukocytosis, which was still present on follow-up. In one of these cats (cat P), an increased lymphocyte count was noted at initiation, which had returned to within normal limit values at follow-up examination.
Cat M showed a pancytopenia at the start of antiviral treatment, with an aggravated anaemia following 1 week of treatment.
Biochemical profile
Two cats (cats F and J) were mildly azotaemic; two others (cats N and M) showed elevated urea levels without an increased creatinine concentration. Mild-to-moderate hyperphosphataemia was seen in three cats (cats F, N and K). Four cats (cats F, J, P and N) presented with hyperproteinaemia and a persistent hypergammaglobulinaemia. At the end of treatment, total protein concentration had normalised in two cats (cats P and N). Electrophoresis showed a decreased gamma globulin fraction in one of them (cat N). Cat K showed persistent hypoproteinaemia with hypogammaglobulinaemia. The acute phase protein serum amyloid A (SAA) was elevated in all samples and decreased during treatment in all but one cat (cat F).
As previously mentioned, cat K developed diabetes mellitus. Blood glucose and serum fructosamine concentrations had normalised upon follow-up analysis while under daily insulin treatment. At the end of the (R)-PMPDAP treatment period, an elevated alanine aminotransferase (ALT) activity (172 U/l, reference interval [RI] <122 U/l) was noted for cat K. Subsequently, abdominal ultrasound showed a focal echoic structure within the gallbladder lumen without signs of cholangitis. During follow-up, several liver values increased, namely aspartate aminotransferase activity (AST) (80 U/l, RI <65 U/l) and ALT (164 U/l), total bilirubin (25.7 µmol/l, RI <17.1 µmol/l) and serum bile acids (96 µmol/l, RI <20 µmol/l). Abdominal ultrasound was not repeated.
Urinalysis
Urinalysis results of only four cats (cats F, J, N and K) were available. One cat (cat K) had a normal urinary protein:creatinine (UPC) ratio (<0.4) on repeated urinalysis from day 28 of (R)-PMPDAP treatment (D28) onwards.
The other three cats showed an increased UPC without active sediment. One of these (cat N) had a UPC ratio of 0.9, together with a urinary specific gravity >1.035 on D0. Unfortunately, no repeated measurements were available in this cat.
The remaining two cats (cats F and J), who were also azotaemic at the onset of antiviral treatment, showed isosthenuria and a substantial increase in the UPC ratio (average UPCs of 3.3 and 15.7, first measured on D0 and D7, respectively), which was persistent. An abdominal ultrasound was performed in the cat (cat F) with the highest UPC, which showed significant renomegaly. This cat had a systolic blood pressure (SBP) of 110 mmHg, measured at a single occasion during treatment.
Viral load
Figure 1 displays the FIV viral load in four cats before, during and after treatment. Cat M survived for only 1 week after the initiation of treatment with (R)-PMPDAP; therefore, only the initial viral load is shown. Viral load results below a reliable quantification limit (<103 virions/ml plasma) are not shown in Figure 1, namely at all time points for cat J and at D21 for cat F. Viral load decreased during antiretroviral treatment in 3/4 cats (cats F, P and N), while an increase was noted in the remaining cat (cat K). At follow-up, FIV viral load had decreased in two cats (cats N and K) compared with their levels at the end of treatment with (R)-PMPDAP. In the other two cats (cats F and P), FIV viral load had increased again.
Figure 1.
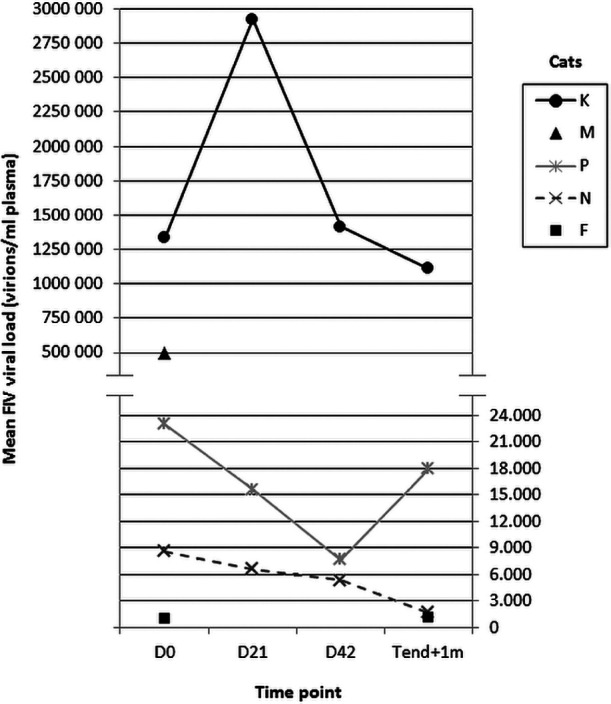
Viral load before and during treatment of five FIV-positive cats with (R)-PMPDAP. Cat M survived for only 1 week after the initiation of antiviral treatment. Viral load values <103 virions/ml plasma were considered unreliable for quantification and are not shown. Viral load results were below a quantification limit at all time points for cat J and at D21 for cat F. D = day of treatment, D0 = first day of (R)-PMPDAP treatment; Tend + 1 m = 1 month after last treatment
Discussion
Herein, we report on the efficacy and safety of (R)-PMPDAP treatment administered for 3–6 weeks to a small number of naturally FIV-infected cats. The following data were available: physical examinations, including a KS (modified for cats), 17 blood and urine examinations, and viral load quantification. Where available, follow-up data were reported to assess longer-term treatment effects and the possible reversibility of any adverse events.
Signalment and clinical presentation of the six included FIV-positive cats were consistent with the scientific literature.3,5,19,20
During (R)-PMPDAP treatment, the KS of all cats improved, suggesting an improved general health status and quality of life. An increase in the KS of FIV-positive cats following (R)-PMPDAP treatment has been described previously, although it was not found to be statistically significant compared with a placebo control arm. 16 However, the initial average KS in the aforementioned placebo-controlled study was about 40% higher than the scores observed in our study. Hence, cats included in our study presented with more severe clinical conditions at the start of treatment and thus had markedly lower KSs. The average KS increased from 33.2 ± 9.4% to 65.0 ± 12.3% at the end of antiviral treatment.
However, the potential beneficial effect of concurrent medication has to be considered as it is likely to have contributed to the improvement in the KSs. Indeed, FIV-infected cats may fully recover, even from a moribund condition, with adequate and proper care. 21
Historically, an objective assessment of the wellbeing and quality of life of cats has been challenging for veterinary clinicians. The modified KS for cats was proposed by Hartmann and Kuffer 17 as an attempt to facilitate this task. To the best of our knowledge, validation of this quantitative scoring system has not been performed. Moreover, the influence of confounding factors on the KS determination, for example, the acclimatisation of cats during hospitalisation and the unblinded aspect of the antiviral treatment, cannot be excluded.
The relapsing hypotension leading to euthanasia during treatment for one cat is, to the best of our knowledge, not a reported side effect of ANP antivirals. In contrast, antiviral treatment is rather associated with hypertension in HIV-infected humans. 22 The presence of a mandibular phlegmon, neutropenia, anaemia and hypoglycaemia in this cat could reflect sepsis. No blood culture was performed; hence, the diagnosis remains unclear.
Apart from the situation with the pancytopenic cat, haematological anomalies were common in the treated cats, both before and during treatment. Several haematological deficits in FIV-positive cats, such as the observation of anaemia, leukopenia, lymphopenia and neutropenia akin to the presence of these cytopenias in HIV-infected humans, have been reported in numerous papers.2,23–25 However, a clear association of these abnormalities with FIV infection appears inconsistent. When compared with FIV-negative control cats, matched by age and clinical condition, most haematological deficits were not associated with FIV infection, except for neutro- and monocytopenia.26–28 Consequently, and in contrast with the cytopenias traditionally assigned to FIV-infection, it is interesting to note that three cats showed a leucocytosis throughout the whole treatment period.
During treatment of FIV-infected cats, ANPs are known to cause a mild-to-severe anaemia.10–12,29 For (R)-PMPDAP specifically, a mild decrease in PCV is reported in naturally infected FIV cats, 16 whereas another study reported no toxic side effects in experimentally FIV- infected cats. 12 Therefore, two cats showing an improvement in PCV values at the end of treatment is noteworthy. Moreover, all four cats seen at follow-up examination had an increased PCV. Hence, as some cats developed a lower PCV during treatment, a negative haematological effect cannot be excluded, but reversibility of this potential adverse event seems likely.
Notable on serum biochemistry were elevated phosphorus, urea and creatinine levels in several cats. In humans, an HIV-associated nephropathy has been described, with proteinuria and progressive deterioration of renal function.30,31 Previous studies have also suggested an FIV-associated nephropathy in cats.32–36 However, recent studies could not assign an absolute association with renal azotaemia,37,38 but proteinuria did appear to be associated with FIV infection. 38 Two of the azotaemic cats in this study had persistent substantial proteinuria indicative of glomerular proteinuria. As hypertension can have an influence on proteinuria, and a previous study described a significantly higher SBP in FIV-positive cats, 39 further studies to evaluate proteinuria and SBP in naturally FIV-infected cats are recommended. In our cats, SBP was recorded in only one azotaemic cat, and appeared normal.
The hyperproteinaemia with high gamma globulin fractions in all but one cat is in agreement with previous observations in naturally FIV-infected cats.24,26,35,40 –42 The only cat with hypogammaglobulinaemia was co-infected with FeLV, which can be the cause of hypogammaglobulinaemia through a B-lymphocyte function deficit. 43
At the end of antiviral treatment, two cats showed total protein concentrations within the normal RI, with decreased gamma globulin levels in one of them. As FIV infection can cause a polyclonal B-cell activation, 44 this decrease may be considered favourable.
SAA is one of the major and most rapidly responsive acute phase proteins in cats. It appears to be a useful marker for the status of inflammatory diseases and for evaluating the response to treatment in cats.45–47 Therefore, it is interesting to note that several cats showed a decline in their overall increased SAA level at the end of treatment.
Viral load declined during treatment in all but one cat, confirming previous studies describing significant decreases in viral load in experimentally FIV-infected, (R)-PMPDAP-treated cats.12,48 Plasma viraemia appears to be, akin to HIV-1 infected humans, 49 a possible indicator of the disease stage in FIV-infected cats, and quantitative analysis might act as a predictive value of disease progression.50,51 In accordance, the two cats that developed a clinical deterioration also had the highest viral loads. Again, a potential beneficial influence of concurrent medication on the viral load levels cannot be excluded, 21 as viral load decreased when concurrent diseases were better regulated (eg, diabetes mellitus in one cat).
Conclusions
This study suggests that treatment with (R)-PMPDAP of six naturally FIV-infected cats, suffering from moderate to severe clinical disease might lead to an improvement of the cats’ quality of life and wellbeing, as measured by the modified KS, as well as a decrease in FIV viral load in plasma. In addition, some biochemical parameters appear to improve. Concurrent medication likely contributed to this amelioration.
A mild decrease in PCV was observed during treatment in three cats, but these changes seemed reversible within 1 month of stopping the antiviral therapy.
Owing to the small number of patients and lack of a control group, no definite conclusions can be drawn. Nevertheless, the data suggest that the potential use of (R)-PMPDAP should be further explored for the treatment of FIV infections in cats.
Acknowledgments
We thank Okapi Sciences NV, Heverlee, Belgium, for providing the drug, the financial support of the study, assistance with recruiting cats and FIV viral load quantification. We would also like to thank Dr Aino Billiet for the veterinary assistance.
Footnotes
Nesya Goris, Joeri Auwerx and Johan Neyts have a financial interest in Okapi Sciences NV.
Funding: This work was financially supported by Okapi Sciences NV, Heverlee, Belgium.
Accepted: 21 March 2014
References
- 1. Pedersen NC, Ho EW, Brown ML, et al. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science 1987; 235: 790–793. [DOI] [PubMed] [Google Scholar]
- 2. Yamamoto JK, Hansen H, Ho EW, et al. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental United States and Canada and possible mode of transmission. J Am Vet Med Assoc 1989; 194: 213–220. [PubMed] [Google Scholar]
- 3. Ishida T, Washizu T, Toriyabe K, et al. Feline immunodeficiency virus infection in cats of Japan. J Am Vet Med Assoc 1989; 194: 221–225. [PubMed] [Google Scholar]
- 4. Levy J, Crawford C, Hartmann K, et al. 2008 American Association of Feline Practitioners’ feline retrovirus management guidelines. J Feline Med Surg 2008; 10: 300–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dunham SP, Graham E. Retroviral infections of small animals. Vet Clin North Am Small Anim Pract 2008; 38: 879–901. [DOI] [PubMed] [Google Scholar]
- 6. North TW, Cronn RC, Remington KM, et al. Characterization of reverse transcriptase from feline immunodeficiency virus. J Biol Chem 1990; 265: 5121–5128. [PubMed] [Google Scholar]
- 7. North TW, North GL, Pedersen NC. Feline immunodeficiency virus, a model for reverse transcriptase-targeted chemotherapy for acquired immune deficiency syndrome. Antimicrob Agents Chemother 1989; 33: 915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stickney AL, Dunowska M, Cave NJ. Sequence variation of the feline immunodeficiency virus genome and its clinical relevance. Vet Rec 2013; 172: 607–614. [DOI] [PubMed] [Google Scholar]
- 9. Arai M, Earl DD, Yamamoto JK. Is AZT/3TC therapy effective against FIV infection or immunopathogenesis? Vet Immunol Immunopathol 2002; 85: 189–204. [DOI] [PubMed] [Google Scholar]
- 10. Hartmann K, Donath A, Beer B, et al. Use of two virustatica (AZT, PMEA) in the treatment of FIV and of FeLV seropositive cats with clinical symptoms. Vet Immunol Immunopathol 1992; 35: 167–175. [DOI] [PubMed] [Google Scholar]
- 11. Hartmann K, Kuffer M, Balzarini J, et al. Efficacy of the acyclic nucleoside phosphonates (S)-9-(3-fluoro-2-phosphonylmethoxypropyl)adenine (FPMPA) and 9-(2-phosphonylmethoxyethyl)adenine (PMEA) against feline immunodeficiency virus. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 17: 120–128. [DOI] [PubMed] [Google Scholar]
- 12. Vahlenkamp TW, Deronde A, Balzarini J, et al. (R)-9-(2-phosphonylmethoxypropyl)-2,6-diaminopurine is a potent inhibitor of feline immunodeficiency virus-infection. Antimicrob Agents Chemother 1995; 39: 746–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gómez NV, Fontanals A, Castillo V, et al. Evaluation of different antiretroviral drug protocols on naturally infected feline immunodeficiency virus (FIV) cats in the late phase of the asymptomatic stage of infection. Viruses 2012; 4: 924–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klein D, Leutenegger CM, Bahula C, et al. Influence of preassay and sequence variations on viral load determination by a multiplex real-time reverse transcriptase-polymerase chain reaction for feline immunodeficiency virus. J Acquir Immune Defic Syndr 2001; 26: 8–20. [DOI] [PubMed] [Google Scholar]
- 15. Fogle JE, Tompkins WA, Campbell B, et al. Fozivudine tidoxil as single-agent therapy decreases plasma and cell-associated viremia during acute feline immunodeficiency virus infection. J Vet Intern Med 2011; 25: 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hartmann AD, Wilhelm N, Balzarini J, et al. Clinical efficacy of the acyclic nucleoside phosphonate 9-(2-phosphonylmethoxypropyl)-2,6-diaminopurine (PMPDAP) in the treatment of feline immunodeficiency virus-infected cats. J Feline Med Surg 2012; 14: 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hartmann K, Kuffer M. Karnofsky’s score modified for cats. Eur J Med Res 1998; 3: 95–98. [PubMed] [Google Scholar]
- 18. Laflamme D. Development and validation of a body condition score system for cats: a clinical tool. Feline Pract 1997; 25: 13–18. [Google Scholar]
- 19. Courchamp F, Yoccoz NG, Artois M, et al. At-risk individuals in feline immunodeficiency virus epidemiology: evidence from a multivariate approach in a natural population of domestic cats (Felis catus). Epidemiol Infect 1998; 121: 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fleming EJ, McCaw DL, Smith JA, et al. Clinical, hematologic, and survival data from cats infected with feline immunodeficiency virus: 42 cases (1983–1988). J Am Vet Med Assoc 1991; 199: 913–916. [PubMed] [Google Scholar]
- 21. Hartmann K. Clinical aspects of feline retroviruses: a review. Viruses 2012; 4: 2684–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilson SL, Scullard G, Fidler SJ, et al. Effects of HIV status and antiretroviral therapy on blood pressure. HIV Med 2009; 10: 388–394. [DOI] [PubMed] [Google Scholar]
- 23. Shelton GH, Linenberger ML, Grant CK, et al. Hematologic manifestations of feline immunodeficiency virus infection. Blood 1990; 76: 1104–1109. [PubMed] [Google Scholar]
- 24. Hopper CD, Sparkes AH, Gruffydd-Jones TJ, et al. Clinical and laboratory findings in cats infected with feline immunodeficiency virus. Vet Rec 1989; 125: 341–346. [DOI] [PubMed] [Google Scholar]
- 25. Gleich S, Hartmann K. Hematology and serum biochemistry of feline immunodeficiency virus-infected and feline leukemia virus-infected cats. J Vet Intern Med 2009; 23: 552–558. [DOI] [PubMed] [Google Scholar]
- 26. Thomas JB, Robinson WF, Chadwick BJ, et al. Leukogram and biochemical abnormalities in naturally occurring feline immunodeficiency virus infection. J Am Anim Hosp Assoc 1993; 29: 272–278. [Google Scholar]
- 27. Liem BP, Dhand NK, Pepper AE, et al. Clinical findings and survival in cats naturally infected with feline immunodeficiency virus. J Vet Intern Med 2013; 27: 798–805. [DOI] [PubMed] [Google Scholar]
- 28. Walker C, Canfield PC. Haematological findings in cats naturally infected with feline immunodeficiency virus. Comp Haematol Int 1996; 6: 77–85. [Google Scholar]
- 29. Kuffer M, Balzarini J, Rolinski B, et al. Comparison of the efficacy of two nucleosid analogues against FIV in naturally infected cats. Tierarztl Prax Ausg K Klientiere Heimtiere 1997; 25: 671–677. [PubMed] [Google Scholar]
- 30. D’Agati V, Appel GB. HIV infection and the kidney. J Am Soc Nephrol 1997; 8: 138–152. [DOI] [PubMed] [Google Scholar]
- 31. Bourgoignie JJ, Pardo V. The nephropathology in human immunodeficiency virus (HIV-1) infection. Kidney Int 1991; 35: S19–23. [PubMed] [Google Scholar]
- 32. Hofmann-Lehmann R, Holznagel E, Ossent P, et al. Parameters of disease progression in long-term experimental feline retrovirus (feline immunodeficiency virus and feline leukemia virus) infections: Hematology, clinical chemistry, and lymphocyte subsets. Clin Diagn Lab Immunol 1997; 4: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reinacher M, Frese K. Untersuchungen zur Glomerulonephritis bei Hund und Katze. Tierarztl Prax 1991; 19: 175–180. [PubMed] [Google Scholar]
- 34. Poli A, Abramo F, Taccini E, et al. Renal involvement in feline immunodeficiency virus infection: a clinicopathological study. Nephron 1993; 64: 282–288. [DOI] [PubMed] [Google Scholar]
- 35. Poli A, Abramo F, Matteucci D, et al. Renal involvement in feline immunodeficiency virus infection: p24 antigen detection, virus isolation and PCR analysis. Vet Immunol Immunopathol 1995; 46: 13–20. [DOI] [PubMed] [Google Scholar]
- 36. Thomas JB, Robinson WF, Chadwick BJ, et al. Association of renal-disease indicators with feline immunodeficiency virus-infection. J Am Anim Hosp Assoc 1993; 29: 320–326. [Google Scholar]
- 37. White JD, Malik R, Norris JM, et al. Association between naturally occurring chronic kidney disease and feline immunodeficiency virus infection status in cats. J Am Vet Med Assoc 2010; 236: 424–429. [DOI] [PubMed] [Google Scholar]
- 38. Baxter KJ, Levy JK, Edinboro CH, et al. Renal disease in cats infected with feline immunodeficiency virus. J Vet Intern Med 2012; 26: 238–243. [DOI] [PubMed] [Google Scholar]
- 39. Paepe D, Verjans G, Duchateau L, et al. Routine health screening: findings in apparently healthy middle-aged and old cats. J Feline Med Surg 2013; 15: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shelton GH, Linenberger ML, Persik MT, et al. Prospective hematologic and clinicopathologic study of asymptomatic cats with naturally acquired feline immunodeficiency virus infection. J Vet Intern Med 1995; 9: 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sparkes AH, Hopper CD, Millard WG, et al. Feline immunodeficiency virus infection. Clinicopathologic findings in 90 naturally occurring cases. J Vet Intern Med 1993; 7: 85–90. [DOI] [PubMed] [Google Scholar]
- 42. Miro G, Domenech A, Escolar E, et al. Plasma electrophoretogram in feline immunodeficiency virus (FIV) and/or feline leukaemia virus (FeLV) infections. J Vet Med A Physiol Pathol Clin Med 2007; 54: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hoover EA, Mullins JI, Quackenbush SL, et al. Experimental transmission and pathogenesis of immunodeficiency syndrome in cats. Blood 1987; 70: 1880–1892. [PubMed] [Google Scholar]
- 44. Flynn JN, Cannon CA, Lawrence CE, et al. Polyclonal B-cell activation in cats infected with feline immunodeficiency virus. Immunology 1994; 81: 626–630. [PMC free article] [PubMed] [Google Scholar]
- 45. Tamamoto T, Ohno K, Ohmi A, et al. Time-course monitoring of serum amyloid A in a cat with pancreatitis. Vet Clin Path 2009; 38: 83–86. [DOI] [PubMed] [Google Scholar]
- 46. Kajikawa T, Furuta A, Onishi T, et al. Changes in concentrations of serum amyloid A protein, alpha 1-acid glycoprotein, haptoglobin, and C-reactive protein in feline sera due to induced inflammation and surgery. Vet Immunol Immunopathol 1999; 68: 91–98. [DOI] [PubMed] [Google Scholar]
- 47. Sasaki K, Ma Z, Khatlani TS, et al. Evaluation of feline serum amyloid A (SAA) as an inflammatory marker. J Vet Med Sci 2003; 65: 545–548. [DOI] [PubMed] [Google Scholar]
- 48. Vahlenkamp TW, DeRonde A, Horzinek MC, et al. Quantification of feline immunodeficiency virus (FIV) RNA in the plasma of infected cats. Berl Munch Tierarztl Wochenschr 1996; 109: 265–269. [PubMed] [Google Scholar]
- 49. Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4(+) lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med 1997; 126: 946–954. [DOI] [PubMed] [Google Scholar]
- 50. Diehl LJ, Mathiason-DuBard CK, O’Neil LL, et al. Longitudinal assessment of feline immunodeficiency virus kinetics in plasma by use of a quantitative competitive reverse transcriptase PCR. J Virol 1995; 69: 2328–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Diehl LJ, Mathiason-Dubard CK, O’Neil LL, et al. Plasma viral RNA load predicts disease progression in accelerated feline immunodeficiency virus infection. J Virol 1996; 70: 2503–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]


