Abstract
The objective of this study was to determine the influence of the observer’s level of experience on within- and between-day variability, and the percentage of successful systolic (SAP) and diastolic arterial blood pressure (DAP) measurements obtained by Doppler ultrasonography (DU) in awake cats. For this purpose, six healthy conscious cats were used and four observers with different levels of training performed 144 SAP and DAP measurements on 4 days using DU. Measurements were recorded five consecutive times, and mean values were used for statistical analysis. Only the two most skilled observers – a PhD student in cardiology and a Dipl ECVIM-CA (cardiology) – had within- and between-day coefficients of variation (CVs) for SAP ⩽16% (13–16%). Conversely, the two less experienced observers – a fifth-year student and an assistant – had high between-day CVs (61% and 73%). For DAP, only the most experienced observer (Dipl ECVIM-CA) succeeded in 100% of the attempts, with within- and between-day CVs of 11% and 4%, respectively. Conversely, DAP could not be measured by the other three observers in 8%, 19% and 56% of attempts (from the highest to the lowest level of experience); therefore, the corresponding CV values could not be calculated. In conclusion, SAP may be assessed using DU in healthy awake cats with good repeatability and reproducibility by a well-trained observer. Measurement of DAP is more difficult than of SAP, and needs a longer training period, which represents one of the limitations of DU in cats.
Introduction
Systemic arterial hypertension, which is defined as a sustained increase in systemic arterial blood pressure (BP), has been increasingly recognised in canine and, most of all, feline veterinary medicine during the last 20 years, either as a complication of various common systemic diseases, such as chronic kidney diseases1–7 and endocrinopathies (eg, hyperthyroidism, primary hyperaldosteronism, diabetes mellitus, hyperadrenocorticism), or as idiopathic hypertension, also called primary or essential hypertension.7–15
Systemic arterial hypertension, in both dogs and cats, and similarly to humans, has been shown to cause irreversible damage to target organs, including lesions of the kidneys, the eyes, the cardiovascular and central nervous systems.16–22 Therefore, repeatable and reproducible BP measurement is of great importance to identify hypertensive animals and for long-term monitoring of antihypertensive therapy in both clinical practice and clinical trials.23–29 Careful, routine BP measurement is also recommended by some authors in order to screen middle-aged to older cats, even if overtly normal, for early detection of those at risk for target organ damage. 3 However, obtaining repeatable and reproducible BP measurements may sometimes represent a technical challenge, particularly in cats and most of all when the animals are anxious or uncooperative. 3
Arterial BP can be directly measured by intra-arterial means or indirectly by devices that incorporate a compressive cuff. At the present time, indirect devices are the most commonly used in clinical practice because of their noninvasive aspect. 1 In the consensus statement of the American College of Veterinary Internal Medicine (ACVIM) on the identification, evaluation and management of systemic hypertension in dogs and cats, the authors propose both Doppler ultrasonography (DU) and oscillometry or high-definition oscillometry as reliable indirect techniques for BP measurement in small animals, 1 even if they emphasise the lack of standardised procedures for the validation of BP measuring devices. According to these ACVIM guidelines and whatever the method used, a well-defined standard protocol involving suitably trained observers should be followed because technical errors associated with personnel inexperience are major causes of unreliable BP measurements. 1 However, to the best of our knowledge, a precise definition of appropriate experience has never been established, and the within- and between-day variability of BP measurements according to the level of experience has been evaluated only in the awake dog, 30 but not in the cat, although several studies have focused on indirect BP measurements in both anaesthetised and conscious animals in this species.31–36
The aims of this prospective study were therefore to (1) determine the influence of the observer’s experience on the within- (repeatability) and between-day (reproducibility) variability, and on the percentage of successful systolic (SAP) and diastolic (DAP) BP measurements obtained using DU in healthy, awake cats; and to (2) assess the consequence of the observer-dependent variability on the minimum number of animals required to detect a treatment-associated difference in a clinical trial.
Materials and methods
Animals
Six intact healthy cats (median age 0.9 years [range 0.6–3.9 years]; median weight 3.8 kg [range 2.8–5.0 kg])—four Siamese (three females and one male) and two domestic shorthairs (two males) from a feline cohort at the National Veterinary School of Alfort—were used, and procedures were conducted in accordance with guidelines established by the National Institute of Health Guide for the Care and Use of Laboratory Animals. The cats were considered healthy on the basis of the results of a complete clinical examination, electrocardiography and conventional echocardiography performed before inclusion in the study. The cats were not familiar with the investigators or the BP measurement procedure.
BP measurements
An indirect measurement of BP was carefully obtained in conscious cats by use of a standardised method of the general technical protocol recommended for a BP measurement session by the ACVIM consensus statement, 1 and as previously described in the dog by our group. 30 The same DU device (811-BL; Parks Medical Electronics) was used throughout the study. Stress and anxiety were reduced as much as possible by performing all BP measurements in the same isolated quiet room and allowing the animals a 10-min period of acclimatisation each day before starting the measurements. 1 The cats were gently restrained in the most comfortable position (right, left lateral or sternal recumbency), and an appropriately sized inflatable cuff (Soft-cuf, Ref 2525, 4–8 cm; Parks Medical Electronics) was placed on the tail. 1 Coupling gel was applied between the 8-MHz probe and the skin to improve contact. The hair was not clipped before placing the probe. As described, 33 the sound volume of the Doppler device was adjusted to obtain a clearly audible signal. The cuff was manually inflated until the pulse signal was no longer audible and was then gradually deflated. The BP (read on the manometer) at which the audible pulse signal was again detected was considered to be the SAP. The cuff was further deflated until a change in tone of the flow sound was detected, which was recorded as the DAP.
In accordance with the ACVIM consensus statement, 1 several consecutive BP measurements were done during each session to obtain a stable set of five values; the mean was used for the statistical analyses. Observers could choose to discard the first values if they were considered abnormally high and anxiety-induced. 1 The time required to obtain the BP measurements, from cuff placement to end of recording of the five consecutive conserved BP values, was recorded by another person.
Observers
Four observers from the National Veterinary School of Alfort with different levels of experience were involved in the study. Observer 1 (HB) was a fifth-year student with only 1 h of training in BP measurements before starting the study. Observer 2 (DB) was a cardiology assistant who had undergone the same training as observer 1, but had also done some occasional BP measurements during his year as an assistant. Observer 3 (CM) was a PhD student in cardiology who had been performing daily BP measurements using DU for 3 years. Observer 4 (VG) was a Diplomate ECVIM-CA (Cardiology) with 8 years of experience in BP measurements, and was therefore considered as the reference observer.
Assessment of within- and between-day intra- and interobserver variability
All BP measurements were randomised (for the order of examinations, cats and investigators were randomly drawn). The same cat could not be used for two consecutive BP measurements.
The study was performed on four different days over a 2-week period. Each day, each observer (n = 4) took three BP measurements for three cats. Therefore, 36 BP measurements per day were obtained, representing a total of 144 BP measurements for the whole 4-day study period (ie, 720 BP recordings because each BP value used for the statistical analysis was the mean of five consecutive values). Each observer was blinded to the BP values recorded by the other three observers.
Statistical analysis
Data are expressed as median and minimum–maximum values. Systat (Systat, version 10.0; SPSS) was used to perform the statistical analysis as previously described. 37 The following linear model was used for each observer and each BP measurement:
where Yijkl is the first value measured for cat k on day j by observer i; μ is the general mean; catk is the differential effect (considered as fixed) of cat k; (day * cat)jk is the interaction term between day and cat; and ϵijkl is the model error. The SD of repeatability was determined from the residual SD of the model and the SD of reproducibility from the square root of the mean square of day.
Any interaction between cat and operator was determined using the following general linear model:
where Yijkl is the first value measured for cat k on day j by observer i; μ is the general mean; Obi is the differential effect (considered as fixed) of observer i; catk is the differential effect of cat k; (Ob * cat)ik is the interaction term between observer and cat; (day * cat)jk is the interaction term between day and cat; and ϵijkl is the model error. A similar general linear model was used to determine the observer effect on the time taken to measure BP for each technique. The level of significance was set at P <0.05.
The minimum number (n) of cats per group required to detect a difference of absolute value (Δ) between two groups with different treatments was determined according to the intra-(SDintra) and inter-day (SDinter) variability using the following equation, as previously described: 37
where α, the type I error of the test is set at 5%; ϵα/2 is the quantile α/2 of the standard Gaussian distribution; and β, the type II error of the test, is set at 20%.
Results
Median and ranges of repeated SAP and DAP values measured by the reference observer (observer 4) are reported in Table 1. Table 2 shows the number of successful SAP and DAP measurements, as well as the time taken for each observer to obtain the BP measurements. The within- and between-day coefficients of variation (CVs) and the corresponding SDs for all observers are given in Table 3.
Table 1.
Median, interquartile ranges and minimum–maximum ranges of repeated systemic arterial blood pressure values measured by the reference observer (observer 4) 36 times over 4 days in six healthy cats using Doppler ultrasonography
| Variable | Median | Minimum–maximum |
|---|---|---|
| Systolic arterial blood pressure (mmHg) | 134 | 98–169 |
| Diastolic arterial blood pressure (mmHg) | 63 | 50–80 |
Table 2.
Number of successful systolic (SAP) and diastolic (DAP) arterial blood pressure measurements and duration of the measurements in six healthy cats using Doppler ultrasonography performed by four observers with different levels of experience. Thirty-six attempts (six per cat) per observer were performed
| Variable | Observer 1 | Observer 2 | Observer 3 | Observer 4 |
|---|---|---|---|---|
| Number (%) of successful SAP measurements | 36 (100) | 36 (100) | 36 (100) | 36 (100) |
| Number (%) of successful DAP measurements | 16 (44) | 29 (81) | 33 (92) | 36 (100) |
| Duration of the measurements (mins) median (interquartiles range, minimum–maximum) |
6 (4–14, 2–22) | 6 (4–7, 3–20) | 5 (3–7, 2–15) | 7 (4–9, 2–26) |
Table 3.
Within- and between-day intra-observer standard deviations (SDs) and coefficients of variation (CVs) for indirect measurement of arterial blood pressure, using Doppler ultrasonography, performed in six healthy cats
| Within-day |
Between-day |
|||
|---|---|---|---|---|
| SD (mmHg) |
CV (%) |
SD (mmHg) |
CV (%) |
|
| Systolic arterial blood pressure | ||||
| Observer 1 | 23 | 17 | 84 | 61 |
| Observer 2 | 25 | 19 | 98 | 73 |
| Observer 3 | 17 | 13 | 20 | 15 |
| Observer 4 | 22 | 16 | 21 | 15 |
| Diastolic arterial blood pressure* | ||||
| Observer 4 | 7 | 11 | 3 | 4 |
Diastolic arterial blood pressure could not be measured by observers 1, 2 and 3 in 100% of cases (see Table 2); therefore, the corresponding variability could not be calculated
All four observers obtained successful SAP measurements in a total of 36 attempts per observer. Within- and between-day CVs for SAP were ⩽16% (13–16%) for only the two most experienced observers. Conversely, the two less experienced observers had high between-day CVs (61% and 73%, respectively).
For DAP, only the most experienced observer succeeded in 100% of the attempts, with within- and between-day CVs of 11% and 4% respectively. In contrast, DAP could not be measured in 56%, 19% and 8% of cases examined by observers 1, 2 and 3, respectively, because these observers were unable to detect a clear change from systolic to diastolic Doppler signal tone. Therefore, the corresponding repeatability and reproducibility could not be calculated.
No significant difference regarding duration of the measurements was observed between observers. No observer effect and no interaction between cat and observer were observed regarding SAP, and between cat and observer 4 for DAP.
However, a significant interaction was noted between cat and day with regard to SAP (P = 0.021). This was not the case for DAP and observer 4.
Results for the estimation of the minimal number of cats required per group in a clinical trial for observers 1 and 4 to detect a treatment-associated difference in SAP are shown in Figure 1. For example, the minimum number of cats in each group (eg, treated or placebo group) to detect an absolute difference of 10 mmHg should be 85 for the least trained observer (observer 1) and 54 for the most trained observer (observer 4) ie, a total of 170 and 108 animals respectively.
Figure 1.
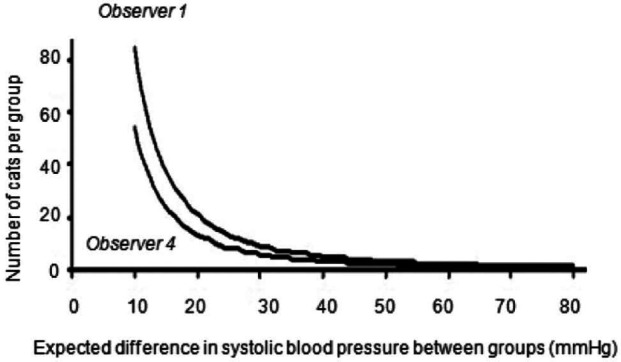
Minimum number of cats required per group to detect a given difference in systolic arterial blood pressure between each group when observer 1 and observer 4 are performing the blood pressure measurements by Doppler ultrasonography
Discussion
Accurate BP measurement, in conjunction with the identification of target organ damage (eg, hypertensive choroidopathy, left ventricular hypertrophy, neurological signs, renal lesions), is essential to the diagnosis of systemic arterial hypertension in veterinary medicine.1,3 Irrespective of whether DU or oscillometric methods are used, specific attention should be paid to limit a white coat effect (comfortable position, minimal restraint, quiet room) and to obtain repeatable and reproducible measurements (eg, use of a correctly sized cuff),1,3,38 as was done in this study. Inappropriate BP measurements can lead to over- or underdiagnosis of systemic arterial hypertension—a falsely elevated BP in normotensive patients (false-positive) being the most common issue. 3 Once hypertension has been confirmed, a monitoring plan based on repeated BP measurements should be scheduled to assess medical treatment efficacy, and the observer’s variability should always be taken into account when examining BP variations over time in order to avoid any misinterpretation of BP changes. Several previous studies have focused on BP variability in the cat33,39 and in the dog.30,40,41 Nevertheless, to the best of our knowledge, this report is the first to assess the influence of the observer’s experience on SAP and DAP measurements (ie, both variability and percentage of successful measurements) using DU in healthy, conscious cats.
Both animals and observers may influence BP variability for a given device. The main goal of this study was not to document the former (ie, the biological or intercat variability), but to obtain numerous data (144 sessions of BP measurements, representing 720 recordings) from different investigators on the same small number of animals (n = 6), and to determine the corresponding inter- and intra-observer variability. Healthy cats were selected to limit daily pathological variations in BP values during the protocol, which might have interfered with assessment of the intra- and inter-observer intrinsic variability. Nevertheless, in this study, a significant interaction between day and cat was observed for SAP, implying that BP values may vary according to the day of measurement (eg, they may decrease over time, as animals get used to the procedure and as stress is concomitantly minimised).
In this study, all four observers obtained SAP values in 100% of their attempts. These results are in accordance with those of Jepson et al, 33 which involved two observers, each with five BP attempts on 28 cats, and demonstrated the success of obtaining a systolic BP reading, using the DU technique, in all 280 attempts. Similarly, in one report comparing DU and high-definition oscillometry in healthy, awake dogs, all attempts to measure SAP using both methods were successful for the four investigators involved in the study (and who all had different levels of training). 30
With regard to SAP, our results also indicate that observers may have different variabilities, with the highest within- and between-day CVs being obtained by the least experienced observers. Similar results have been obtained in the dog. 30 However, in the latter report the within- and between-day CVs for SAP measured with DU by the two most experienced observers (4.1–9.4%) were lower than those obtained here in the cat by observers 3 and 4 (13–16%). The difference in SAP CVs between the two species were much greater for the two least experienced observers, particularly the between-day CVs (7.9% and 12.4% in the dog VS 61% and 73% in the cat for observers 1 and 2 respectively), although in both studies the level of training of the two least skilled observers was similar (1 h of training in BP measurements before starting the study for each and some occasional training during 1 year for one of them). These results suggest that DU is technically more difficult in the cat and necessitates more training than in the dog.
In this study, the between-day CVs for SAP were 15% for both experienced observers. Sparkes et al 39 previously reported lower between-day CVs (⩽7.9%) for SAP measured by DU. However, the objective of their study was to assess SAP variations over time in seven cats with a different protocol to that used in our study (only one measurement per day by one observer, seven times at intervals of at least 24 h over a 10-day period). 39 Although there is currently an emphasis on the diagnosis of systolic arterial hypertension in veterinary medicine, and although SAP seems to be the most important determinant of hypertensive tissue damage, true isolated diastolic hypertension can occur in dogs and cats, and DAP measurement is therefore relevant in these species. 1 However, the subjective nature of determining DAP using Doppler has already been highlighted in the ACVIM consensus statement 1 and nothing in our study suggests that this inherent problem could be overcome. Regardless of accuracy, which remains an important issue for DAP, another point of the present study is that except for the most trained observer, DAP could not be measured using DU on all occasions. Observer 4’s repeatability and reproducibility was good (CVs of 11% and 4% respectively), but the reliability of these results could not be assessed as no direct BP readings were undertaken, which represents a limitation of this study.
In this study, the time taken for observer 4 to complete the BP measurements was highly variable, the longest times (up to 26 mins) being related to the time sometimes required to record DAP, which may represent a limitation of the DU technique in the feline species. In the study by Lin et al, 42 which only focused on SAP in clinically normal, conscious cats, all BP measurements using DU were completed in only 6 mins. Similarly, Jepson et al 33 were able to perform five BP readings within 5 mins in approximately 40% of the animals, but DAP was not obtained systematically.
In our study, observers 1, 2 and 3 could not obtain DAP values in 56%, 19% and 8%, respectively, of the 36 sessions because they were unable to detect a clear change from systolic to diastolic Doppler signal tone. The difficulty of measuring DAP by DU has already been shown in both dogs and cats.30,33 In the report by Jepson et al, 33 the two observers obtained SAP values in 100% of their 280 attempts with the DU device, whereas DAP measurements were only obtained in 144 of 280 attempts (51%). Similarly, in one study focusing on the comparative variability of DU and high definition oscillometry for BP measurements in healthy awake dogs, DAP could not be measured by the least skilled observer in 17% of 36 attempts using DU. 30 This represents an additional limitation of the use of the DU technique in small animals.
In this study, the impact of the observer-dependent variability on the minimum number of animals required to detect a given difference in SAP between two groups of animals was also assessed. The most experienced observer needed a lower number of animals compared with the least experienced one. Similar results were found in a study by our group, which assessed the minimum number of dogs required to detect a given difference between two groups of animals in various two-dimensional and M-mode echocardiographic variables according to the observers’ level of experience (the more experienced the observer, the lower the number of animals needed, and therefore the lower the cost of the clinical trial). 37 Both results emphasise the need to document observer-dependent variability before scheduling the protocol design of any clinical trial using quantitative clinical variables selected as endpoints.
In this study, the procedure used for BP measurements was in accordance with the ACVIM consensus statement, 1 which therefore allowed each observer to discard the first BP values if these were considered abnormally high. This could have led to an underestimation of BP variability, which might represent a limitation of this study. Nevertheless, the objective of this study was to assess the variability of BP measurements as actually done by general practitioners following published guidelines. 1 Finally, and most importantly, direct BP readings were not undertaken, and therefore accuracy of the measurements could not be determined. Although accuracy was beyond the scope of the present report, validation of DAP by using a gold standard with regard to accuracy and a trained observer with regard to repeatability/reproducibility would have been relevant.
Conclusions
SAP may be assessed with good repeatability and reproducibility by trained observers using DU in healthy awake cats. DAP is more difficult to measure, depending on the experience of the observer, and further studies are needed to assess the accuracy of the DU technique for this measurement in cats.
Acknowledgments
We would like to thank sincerely Dr Alain Fontbonne and Emeline Leblond (CERCA, National Veterinary School of Alfort) for providing the animals involved in the study.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Accepted: 21 March 2014
References
- 1. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007; 21: 542–558. [DOI] [PubMed] [Google Scholar]
- 2. Jacob F, Polzin DJ, Osborne CA, et al. Association between initial systolic blood pressure and risk of developing a uremic crisis or of dying in dogs with chronic renal failure. J Am Vet Med Assoc 2003; 222: 322–329. [DOI] [PubMed] [Google Scholar]
- 3. Stepien RL. Feline systemic hypertension: diagnosis and management. J Feline Med Surg 2011; 13: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wehner A, Hartmann K, Hirschberger J. Associations between proteinuria, systemic hypertension and glomerular filtration rate in dogs with renal and non-renal diseases. Vet Rec 2008; 162: 141–147. [DOI] [PubMed] [Google Scholar]
- 5. Syme HM, Barber PJ, Markwell PJ, et al. Prevalence of systolic hypertension in cats with chronic renal failure at initial evaluation. J Am Vet Med Assoc 2002; 220: 1799–1804. [DOI] [PubMed] [Google Scholar]
- 6. Finco DR. Association of systemic hypertension with renal injury in dogs with induced renal failure. J Vet Intern Med 2004; 18: 289–294. [DOI] [PubMed] [Google Scholar]
- 7. Chetboul V, Lefebvre HP, Pinhas C, et al. Spontaneous feline hypertension: clinical and echocardiographic abnormalities, and survival rate. J Vet Intern Med 2003; 17: 89–95. [DOI] [PubMed] [Google Scholar]
- 8. Kobayashi DL, Peterson ME, Graves TK, et al. Hypertension in cats with chronic renal failure or hyperthyroidism. J Vet Intern Med 1990; 4: 58–61. [DOI] [PubMed] [Google Scholar]
- 9. Flood SM, Randolph JF, Gelzer AR, Refsal K. Primary hyperaldosteronism in two cats. J Am Anim Hosp Assoc 1999; 35: 411–416. [DOI] [PubMed] [Google Scholar]
- 10. Hoenig M. Feline hyperadrenocorticism: where are we now? J Feline Med Surg 2002; 4: 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Howard EB, Nielsen SW. Pheochromocytomas associated with hypertensive lesions in dogs. J Am Vet Med Assoc 1965; 147: 245–252. [PubMed] [Google Scholar]
- 12. Struble AL, Feldman EC, Nelson RW, et al. Systemic hypertension and proteinuria in dogs with diabetes mellitus. J Am Vet Med Assoc 1998; 213: 822–825. [PubMed] [Google Scholar]
- 13. Ortega TM, Feldman EC, Nelson RW, et al. Systemic arterial blood pressure and urine protein/creatinine ratio in dogs with hyperadrenocorticism. J Am Vet Med Assoc 1996; 209: 1724–1729. [PubMed] [Google Scholar]
- 14. Sennello KA, Schulman RL, Prosek R, et al. Systolic blood pressure in cats with diabetes mellitus. J Am Vet Med Assoc 2003; 223: 198–201. [DOI] [PubMed] [Google Scholar]
- 15. Reusch CE, Schellenberg S, Wenger M. Endocrine hypertension in small animals. Vet Clin North Am Small Anim Pract 2010; 4: 335–352. [DOI] [PubMed] [Google Scholar]
- 16. Carlos Sampedrano C, Chetboul V, Gouni V, et al. Systolic and diastolic myocardial dysfunction in cats with hypertrophic cardiomyopathy or systemic hypertension. J Vet Intern Med 2006; 20: 1106–1115. [DOI] [PubMed] [Google Scholar]
- 17. Henik RA, Stepien RL, Bortnowski HB. Spectrum of M-mode echocardiographic abnormalities in 75 cats with systemic hypertension. J Am Anim Hosp Assoc 2004; 40: 359–363. [DOI] [PubMed] [Google Scholar]
- 18. Nelson L, Reidesel E, Ware WA, et al. Echocardiographic and radiographic changes associated with systemic hypertension in cats. J Vet Intern Med 2002; 16: 418–425. [DOI] [PubMed] [Google Scholar]
- 19. Crispin SM, Mould JRB. Systemic hypertensive disease and the feline fundus. Vet Ophthalmol 2001; 4: 131–140. [DOI] [PubMed] [Google Scholar]
- 20. Stiles J, Polzin DJ, Bistner SI. The prevalence of retinopathy in cats with systemic hypertension and chronic renal failure or hyperthyroidism. J Am Anim Hosp Assoc 1994; 30: 564–572. [Google Scholar]
- 21. Sansom J, Rogers K, Wood JL. Blood pressure assessment in healthy cats and cats with hypertensive retinopathy. Am J Vet Res 2004; 65: 245–252. [DOI] [PubMed] [Google Scholar]
- 22. Maggio F, De Francesco TC, Atkins CE, et al. Ocular lesions associated with systemic hypertension in cats: 69 cases (1985–1998). J Am Vet Med Assoc 2000; 217: 695–702. [DOI] [PubMed] [Google Scholar]
- 23. Elliott J, Barber PJ, Syme HM, et al. Feline hypertension: clinical findings and response to antihypertensive treatment in 30 cases. J Small Anim Pract 2001; 42: 122–129. [DOI] [PubMed] [Google Scholar]
- 24. Helms SR. Treatment of feline hypertension with transdermal amlodipine: a pilot study. J Am Anim Hosp Assoc 2007; 43: 149–156. [DOI] [PubMed] [Google Scholar]
- 25. Henik RA. Systemic hypertension and its management. Vet Clin North Am Small Anim Pract 1997; 27: 1355–1372. [DOI] [PubMed] [Google Scholar]
- 26. Mathur S, Syme H, Brown CA, et al. Effects of the calcium channel antagonist amlodipine in cats with surgically induced hypertensive renal insufficiency. Am J Vet Res 2002; 63: 833–839. [DOI] [PubMed] [Google Scholar]
- 27. Jepson RE, Elliott J, Brodbelt D, et al. Effect of control of systolic blood pressure on survival in cats with systemic hypertension. J Vet Intern Med 2007; 21: 402–409. [DOI] [PubMed] [Google Scholar]
- 28. Mishina M, Watanabe T. Development of hypertension and effects of benazepril hydrochloride in a canine remnant kidney model of chronic renal failure. J Vet Med Sci 2008; 70: 455–460. [DOI] [PubMed] [Google Scholar]
- 29. Watanabe T, Mishina M. Effects of benazepril hydrochloride in cats with experimentally induced or spontaneously occurring chronic renal failure. J Vet Med Sci 2007; 69: 1015–1023. [DOI] [PubMed] [Google Scholar]
- 30. Chetboul V, Tissier R, Gouni V, et al. Comparison of Doppler ultrasonography and high-definition oscillometry for blood pressure measurements in healthy awake dogs. Am J Vet Res 2010; 71: 766–772. [DOI] [PubMed] [Google Scholar]
- 31. Binns SH, Sisson DD, Buoscio DA, et al. Doppler ultrasonographic, oscillometric, sphygmomanometric, and photoplethysmographic techniques for noninvasive blood pressure measurement in anesthetized cats. J Vet Intern Med 1995; 9: 405–414. [DOI] [PubMed] [Google Scholar]
- 32. Petric AD, Petra Z, Jerneja S, Alenka S. Comparison of high definition oscillometric and Doppler ultrasonic devices for measuring blood pressure in anaesthetized cats. J Feline Med Surg 2010; 12: 731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jepson RE, Hartley V, Mendl M, et al. A comparison of CAT Doppler and oscillometric Memoprint machines for non-invasive blood pressure measurement in conscious cats. J Feline Med Surg 2005; 7: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klevans LR, Hirkaler G, Kovacs JL. Indirect blood pressure determined by Doppler technique in renal hypertensive cats. Am J Physiol 1979; 276: 720–723. [DOI] [PubMed] [Google Scholar]
- 35. Grandy JL, Dunlop CI, Hodgson DS, et al. Evaluation of the Doppler ultrasonic method of measuring systolic arterial blood pressure in cats. Am J Vet Res 1992; 53: 1166–1169. [PubMed] [Google Scholar]
- 36. Littman MP. Spontaneous systemic hypertension in 24 cats. J Vet Intern Med 1994; 8: 79–86. [DOI] [PubMed] [Google Scholar]
- 37. Chetboul V, Athanassiadis N, Concordet D, et al. Observer-dependent variability of quantitative clinical endpoints: the example of canine echocardiography. J Vet Pharmacol Ther 2004; 27: 49–56. [DOI] [PubMed] [Google Scholar]
- 38. Belew AM, Barlett T, Brown SA. Evaluation of the white coat effect in cats. J Vet Intern Med 1999; 13: 134–142. [DOI] [PubMed] [Google Scholar]
- 39. Sparkes AH, Caney SMA, King MKA, Gruffydd-Jones TJ. Inter- and intraindividual variability in indirect (Doppler) systolic blood pressure measurements in cats. J Vet Intern Med 1999; 13: 314–318. [DOI] [PubMed] [Google Scholar]
- 40. Bodey AR, Michell AR. Longitudinal studies of reproducibility and variability of indirect (oscillometric) blood pressure measurements in dogs: evidence for tracking. Res Vet Sci 1997; 63: 15–21. [DOI] [PubMed] [Google Scholar]
- 41. Rattez EP, Reynolds BS, Concordet D, et al. Within-day and between-day variability of blood pressure measurement in healthy conscious Beagle dogs using a new oscillometric device. J Vet Cardiol 2010; 12: 35–40. [DOI] [PubMed] [Google Scholar]
- 42. Lin CH, Yan CJ, Lien YH, Huang HP. Systolic blood pressure of clinically normal and conscious cats determined by an indirect Doppler method in a clinical setting. J Vet Med Sci 2006; 68: 827–832. [DOI] [PubMed] [Google Scholar]


