Abstract
The aim of this study was to evaluate the analgesic effects of maxillary and/or inferior alveolar nerve blocks with lidocaine and bupivacaine in cats undergoing dental extractions. Twenty-nine cats were enrolled. Using an adapted composite pain scale, cats were pain scored before the dental procedure and 30 mins, and 1, 2 and 4 h after isoflurane disconnection. Cats were sedated with buprenorphine (20 µg/kg), medetomidine (10 µg/kg) and acepromazine (20 µg/kg) intramuscularly. Anaesthesia was induced using alfaxalone (1–2 mg/kg) intravenously and maintained with isoflurane in oxygen. Each cat was randomly assigned to receive maxillary and/or inferior alveolar nerve blocks or no nerve blocks prior to dental extractions. Each nerve block was performed using lidocaine (0.25 mg/kg) and bupivacaine (0.25 mg/kg). Heart rate, systolic arterial blood pressure, respiratory rate, end tidal carbon dioxide and isoflurane vaporiser settings were recorded 5 mins before and after the dental extractions, and the difference calculated. Group mean differences (mean ± SD) for heart rate (−9.7 ± 10.6 vs 7.6 ± 9.5 beats/min [nerve block vs control group, respectively], P <0.0001), systolic arterial blood pressure (−10.33 ± 18.44 vs 5.21 ± 15.23 mmHg, P = 0.02) and vaporiser settings (−0.2 ± 0.2 vs 0.1 ± 0.4, P = 0.023) were significantly different between groups. The control group had higher postoperative pain scores (median [interquartile range]) at 2 h (3 [1.75–4.00] vs 1 [0–2], P = 0.008) and 4 h (4 [2–6] vs 2 [1–2], P = 0.006) after the dental extractions. Maxillary and inferior alveolar nerve blocks with lidocaine and bupivacaine administered prior to dental extractions resulted in a reduction in heart rate and blood pressure while allowing for a reduction in isoflurane. Cats receiving nerve blocks had lower postoperative pain scores than the group without nerve blocks.
Introduction
In human dentistry, dental nerve blocks have been successfully used for decades. The analgesia provided by a nerve block prevents hyperalgesia and peripheral sensitisation, decreases N-methyl-D-aspartate receptor activity and allows a reduction in inhalation agent requirements.1,2
In the feline oral cavity, as in other mammals (including humans), A-δ fibres transmit sharp, localised pain, A-β fibres are responsible for conducting touch and pressure, and C fibres provide burning, aching and throbbing sensations. These fibres are all present in the sensory branches of the trigeminal nerve, more specifically the maxillary and the inferior alveolar nerves and their branches. 2
Five dental nerve blocks have been described in cats: maxillary, inferior alveolar, major palatine, infraorbital and middle mental. The combination of maxillary and inferior alveolar nerve blocks is able to desensitise the oral cavity completely.1,2
The equipment necessary for these local anaesthesia techniques is minimal, inexpensive and readily available in the veterinary clinic.2,3 The most common local anaesthetic drugs used in veterinary patients are lidocaine, bupivacaine and mepivacaine. 3 The combination of lidocaine and bupivacaine has been described to achieve a fast onset and have a long duration of action. 4
The use of the brachial plexus nerve block prior to orthopaedic surgery for the forelimb has been investigated in cats. 5 Compared with a control group, a reduction in intraoperative isoflurane requirements, as well as pain during the early postoperative period, was demonstrated when bupivacaine was used to block the brachial plexus. Infiltration of bupivacaine around the surgical wound failed to reduce postoperative analgesic requirements following ovariohysterectomy in cats. 6
Gross et al 7 documented the efficacy of the infraorbital and inferior alveolar nerve blocks providing analgesia for dental procedures in dogs as they were shown to abolish reflex-evoked muscle action potentials in the digastricus muscle during non-invasive stimulation of tooth pulp in halothane-anaesthetised dogs. However, to our knowledge no studies have investigated the analgesic effects of local anaesthetic techniques prior to dental extractions in cats, despite the techniques being widely described. The aim of this study was to evaluate the intraoperative and postoperative analgesic effects of maxillary and inferior alveolar nerve blocks performed with lidocaine and bupivacaine in cats undergoing dental extractions.
Materials and methods
Study population
Cats presented to the hospital between July 2011 and May 2012 for dental procedures requiring extractions were included in the study. The project received approval from the hospital department’s ethics and welfare committee (reference: CR25), and permission was obtained from the cats’ caregivers before enrolment in the study. Only American Society of Anesthesiologists I and II health status cats were included. 8
Procedures
All cats were pain scored before the procedure, using an adapted composite scale (Table 1). 9 Before the dental procedure, cats were assigned a pain score by a single observer, where 0 represented minimum and 19 the worst pain score possible. A total of 24 could not be achieved at this point as appetite could not be assessed and only baseline systolic arterial blood pressure measurements were taken.
Table 1.
Pain score scale
| Factor | Item | Description | Score |
|---|---|---|---|
| Psychomotor changes | Posture | Cat is in any usual posture for the species; cat looks comfortable and relaxed | 0 |
| Cat in lateral recumbency; facial muscles tensed; eyelids semi-closed | 1 | ||
| Cat reluctant to move; head held low; facial muscles tensed; eyelids closed | 2 | ||
| Cat adopts different postures in an attempt to find a comfortable position | 3 | ||
| Comfort | Cat is awake or asleep; when stimulated, is interested in its surroundings | 0 | |
| Cat is quiet and dissociated from its environment; when stimulated is not interested | 1 | ||
| Cat is uncomfortable, agitated and restless | 2 | ||
| Activity | Cat moves with a normal gait | 0 | |
| Cat is quieter than usual | 1 | ||
| Cat is reluctant to move | 2 | ||
| Cat frequently changes its body position | 3 | ||
| Mental status | Cat is alert and interacts with the observer | 0 | |
| Cat does not interact with the observer | 1 | ||
| Cat is not interested in its surroundings | 2 | ||
| Cat is nervous and frightened, and attempts to hide or escape | 3 | ||
| Cat bites or scratches the observer after the slightest stimulation | 4 | ||
| Protection of the surgical area | Palpation | Cat does not react when the surgical area is touched or pressedCat does not react if touched but flinches or vocalises if the surgical area is pressed | 0 |
| 1 | |||
| Cat flinches, vocalises or moves the head when the surgical area is touched | 2 | ||
| Cat shows a fast movement of the head and tries to bite if touched in the surgical area | 3 | ||
| Cat vocalises and tries to bite or scratch when the observer approaches (no touch) | 4 | ||
| Physiological variables | Blood pressure | 0–15% >preoperative value16–29% >preoperative value | 0 |
| 1 | |||
| 30–45% >preoperative value | 2 | ||
| >45% above preoperative value | 3 | ||
| Appetite | Cat eats 1/4 of a tin of Hill’s a/d | 0 | |
| Eats less than a 1/4 of a tin of Hill’s a/d | 1 | ||
| Cat is not interested in food | 2 | ||
| Vocal expression of pain | Vocalisation | Cat purrs when touched or meows, but does not groan, hiss or growlCat vocalises when the observer approaches, but calms down when touched | 0 |
| 1 | |||
| Cat vocalises when the observer approaches, but does not calm down when touched | 2 | ||
| Cat vocalises spontaneously | 3 |
Systolic arterial blood pressure (SAP) was measured following the American College of Veterinary Internal Medicine guidelines for blood pressure measurements. 10 A high-definition oscillometry device (HDO; S+B med VET) was used pre- and postoperatively. All cats were placed in a quiet room for 5–10 mins prior to SAP measurement. The red blood pressure cuff (C1, for animals <8 kg) from the HDO (S+B med VET) was used. Cats were in sternal recumbency in order to have five consecutive measurements taken with the cuff applied on the middle third of the antebrachium. The first measurement was discarded and the mean of the following four consecutive measurements was recorded and used for further analysis.
All cats received pre-anaesthetic medication with buprenorphine 20 µg/kg (Vetergesic; Alstoe), acepromazine 20 µg/kg (Calmivet 0.5%; Vétoquinol) and medetomidine 10 µg/kg (Sedator; Dechra) combined intramuscularly (IM). Intravenous (IV) catheterisation of a cephalic vein was performed 10–15 mins after the premedication injection was administered. Anaesthesia was induced with alfaxalone, 1–2 mg/kg, IV (Alfaxan; Vétoquinol), and endotracheal intubation performed 30–90 s after the application of 2–4 mg of lidocaine spray 2% (Intubeaze; Dechra) to the larynx. Anaesthesia was maintained with isoflurane (Isoflo; Abbott) vaporised in oxygen (200 ml/kg/min) delivered by a modified Ayre’s T-piece. Intravenous Hartmann’s solution (Vetivex 11; Dechra) was infused at 10 ml/kg/h.
End tidal carbon dioxide (EtCO2), respiratory rate (RR), heart rate (HR) and non-invasive SAP were monitored constantly during the anaesthesia using a multiparametric monitor, and recorded every 5 mins (Mindray PM-8000).
A complete oral examination was performed by one experienced veterinary surgeon who determined the dental treatment necessary in every case. If one or more dental extractions were required, the cat was included in the study and randomly assigned to one of two groups: the nerve block group or the control group receiving no nerve blocks. The cats in the nerve block group received the required nerve blocks for the dental extractions to be performed (left and/or right maxillary and/or inferior alveolar nerve blocks).
Maxillary and inferior alveolar nerve blocks were performed with 0.25 mg/kg of lidocaine 2% (2% w/v Lidocaine Injection; Braun) and 0.25 mg/kg of bupivacaine 0.5% (Marcain Polyamp; AstraZeneca) at each required site. A total of 0.01 ml/kg of lidocaine 2% and 0.05 ml/kg of bupivacaine 0.5% were administered per site of each nerve block, and the drugs were drawn up in the same syringe. The technique used to perform the nerve blocks has been widely described.1–3,11 For the maxillary nerve block, after aseptic preparation of the site, a 25 G needle was inserted percutaneously, perpendicular to the skin surface, just below the ventral border of the zygomatic arch at the level of a vertical line drawn from the lateral canthus of the eye. The needle was then directed medially and dorsally in the direction of the maxillary foramen in the pteryigopalatine fossa. Aspiration was performed to rule out intravenous puncture and, if resistance to injection was encountered, the needle was repositioned (slightly withdrawn). For the inferior alveolar nerve block a 25-G needle was inserted percutaneously, perpendicular to the skin surface, at the lower angle of the jaw, and advanced against the medial side of the mandible in the direction of the mandibular foramen. A finger was then inserted into the cat’s mouth and the correct positioning of the needle with the bevel adjacent to the mandibular foramen confirmed by palpation. Aspiration was again performed before injection. The study protocol did not interfere with normal clinical practice at our institution and did not prolong anaesthetic time.
The person monitoring the anaesthesia was unaware of group allocation. Anaesthetic depth was assessed by examination of jaw tone, globe position, palpebral reflex, and by evaluating changes in vital parameters (HR, RR, SAP and EtCO2) and physical reaction, such as the jaw trembling, in response to the dental procedures.
At the end of the procedure meloxicam, 0.2 mg/kg (Metacam; Boehringer Ingelheim) and atipamezole, 25 µg/kg (Atipam; Dechra) were administered subcutaneously and intramuscularly, respectively, to all cats. On recovery from general anaesthesia, the cats were kept under a warmed air blanket (Bair Hugger Warming Unit Model 505; 3M) until rectal temperature reached a minimum temperature of 37°C. Postoperative analgesia was evaluated 30 mins, and 1, 2 and 4 h after isoflurane was disconnected, using the same pain score scale (Table 1) used before the dental procedure. At 30 mins, and 1 and 2 h, appetite was again not assessed, as cats could still not be fed at this point. However, SAP could be compared with the SAP obtained preoperatively, so the maximum pain score achieved at these points was 22. At 4 h, a quarter of a tin of food (a/d; Hill’s prescription) was offered and appetite assessed, and a maximum pain score of 24 could be obtained. The person pain scoring all cats (JA) was unaware of the treatment group. At any time point, rescue analgesia consisting of buprenorphine (20 μg/kg IM) was administered if pain scores were >7/22 or 8/24. These values were decided upon on the threshold value between mild and moderate pain if the pain scores were adjusted to a scale of 0 to 100, as suggested by the original pain score system. 9 The total final score could be adjusted to a scale of 0 to 100 by multiplying the total score by 4.16 (ie, total score × [100/24] = adjusted score). This procedure facilitates ranking pain intensity: where ⩽ 30 corresponds to mild pain, >30 and <70 is considered moderate pain, and ⩾ 70 means that the cat is in severe pain.
Statistical methods
A sample size calculation was performed based on the pain scores of a sample of cats undergoing dental extractions. Fourteen cats were required in each group to have an 80% power to detect a difference between mean pain scores of 2 with a significance level (a) of 0.05.
Pearson normality test and visual inspection of data distribution were used to check for normality. Student’s t-tests were used to compare the parametric variables (HR, RR, EtCO2, isoflurane settings and SAP). Pain scores and number of teeth extracted were analysed using the Mann–Whitney U-test and results were presented as mean ranks in order to compare the two groups. The median and interquartile range (IQR) for each group were also determined to allow the pain score results to be shown within the context of the pain score scale. Differences of P <0.05 were considered statistically significant. The software used for the statistical analysis was SPSS Statistics 20 for Windows.
Results
A total of 29 cats met the inclusion criteria for this project. Fifteen cats were included in the nerve block group (five neutered females and 10 neutered males) and 14 cats were included in the control group (seven neutered females and seven neutered males).
The results are presented as mean ± SD, unless otherwise stated. There were no significant differences between the two groups regarding age, weight and SAP before the dental procedure (Table 2). Pain scores obtained prior to the procedure were similar between groups (see Table 4).
Table 2.
Mean and SD for age, weight and systolic arterial blood pressure (SAP) before sedation, and median and range values for the number of teeth extracted in both groups
| Patient variable | Nerve blocks group, mean (± SD) | Control group (mean ± SD) | P |
|---|---|---|---|
| Age (months) | 112 (36) | 92 (42) | 0.89 |
| Weight (kg) | 4.1 (0.8) | 4.4 (0.9) | 0.64 |
| SAP before sedation (mmHg) | 152 (18) | 153 (21) | 0.67 |
| Number of teeth extracted, median (IQR) | 2 (2–3) | 1.5 (1.0–2.0) | 0.10 |
IQR = interquartile range
Table 4.
Mean rank, 25% interquartile range (IQR), median and 75% IQR, and P value of the Mann–Whitney U-test comparing the pain scores of the two groups at 30 mins, and 1, 2 and 4 h after isoflurane disconnection
| Group | Mean rank | Median (0.25% quartile–0.75% quartile) | P | |
|---|---|---|---|---|
| Pain score before the procedure | Nerve blocks group | 17.6 | 1 (1–2) | 0.066 |
| Control group | 12.2 | 1 (0–1) | ||
| Pain score after 30 mins | Nerve blocks group | 14.1 | 2 (1–4) | 0.536 |
| Control group | 16.0 | 3 (2–4) | ||
| Pain score after 1 h | Nerve blocks group | 12.4 | 1 (0–3) | 0.093 |
| Control group | 17.8 | 3 (1.8–3.3) | ||
| Pain score after 2 h | Nerve blocks group | 11.1 | 1 (0–2) | 0.009 |
| Control group | 19.2 | 3 (1.8–4.0) | ||
| Pain score after 4 h | Nerve blocks group | 10.9 | 2 (1–2) | 0.006 |
| Control group | 19.4 | 4 (2–6) |
Not all cats received the same number and type of nerve block, but the groups were found to be similar regarding the number of teeth extracted (Table 2). Five cats received one nerve block, seven cats received two nerve blocks, one cat received three nerve blocks and two cats received all four nerve blocks.
The RR, HR, SAP, EtCO2 and isoflurane settings obtained 5 mins before extractions started were compared with the values obtained 5 mins after all extractions had been finished. The difference between these two values was compared between groups with negative values indicating a decrease in values (Table 3). The mean differences were significantly different between groups for HR (P <0.0001), SAP (P = 0.02) and isoflurane vaporiser settings (P = 0.023). HR decreased by around 10 beats per minute (bpm) in the nerve block group, whereas in the control group it increased by around 8 bpm. SAP decreased by around 10 mmHg in the nerve block group, whereas in the control group it increased by 5 mmHg after the extractions. Isoflurane requirement decreased by 0.2% in the nerve block group, whereas no changes were found in the control group.
Table 3.
Mean and SD for the difference in physiological variables and isoflurane vaporiser settings between 5 mins before and 5 mins after all extractions for both groups (negative values indicate a decrease in values)
| Physiological variables and isoflurane settings difference | Nerve blocks group, mean (± SD) | Control group, mean (± SD) | P |
|---|---|---|---|
| Respiratory rate (rpm) difference | −3.4 (3.0) | −1 (3.6) | 0.06 |
| Heart rate (bpm) difference | −9.7 (10.6) | +7.6 (9.5) | <0.001 |
| SAP (mmHg) difference | −10.33 (18.44) | +5.21 (15.23) | 0.02 |
| EtCO2 (mmHg) difference | +2.2 (6.6) | −0.1 (5.7) | 0.33 |
| Isoflurane settings difference, median (IQR) | −0.2 (−0.5 to 0) | 0 (0-0) | 0.02 |
rpm = respirations per minute; bpm = beats per minute; EtCO2 = end tidal carbon dioxide; IQR = interquartile range
For the pain scores at 30 mins, and 1, 2 and 4 h after the isoflurane was discontinued, a difference in the mean ranks of groups was found to be significantly different at 2 and 4 h (Table 4). Median and IQR for pain scores are shown in Table 4 and Figure 1 for both groups. The rescue analgesia plan was not necessary for any of the patients enrolled in the study. No problems were encountered while performing the nerve blocks, and no adverse events were recorded.
Figure 1.
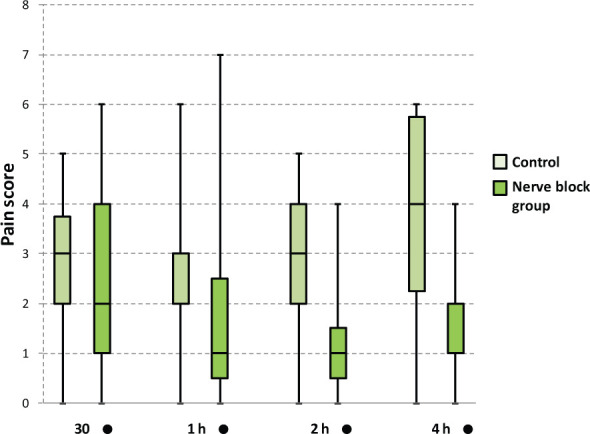
Box and whisker plot for the pain scores obtained by the two groups at 30 mins, and 1, 2 and 4 h after isoflurane disconnection. Pain score difference between groups was found to be statistically significant only at 2 and 4 h
Discussion
This study demonstrated that maxillary and inferior alveolar nerve blocks with lidocaine and bupivacaine allow for a decrease in intraoperative HR, SAP and isoflurane requirement when performed prior to dental extractions in cats. The nerve block group also demonstrated reduced postoperative pain scores at 2 and 4 h.
In the nerve block group both HR and SAP were found to be lower at the end of the extractions compared with 5 mins prior to them, despite a decrease in isoflurane settings. This may be due to an increase in analgesia and subsequent reductions in cardiovascular responses during dental extractions, thus limiting any rise in HR and blood pressure, while allowing for a reduction in isoflurane. However, systemic absorption of the local anaesthetics cannot be ruled out.
For the nerve blocks the maximum dose of lidocaine that could be administered was 1 mg/kg – and the same for bupivacaine (1 mg/kg) if both nerve blocks were performed bilaterally. It is always important to calculate the dose of each nerve block considering the maximum number of nerve blocks required in the patient in order to not exceed the maximum dose. Some systemic absorption may have occurred, but it is unlikely to have significantly contributed to the decrease in HR and blood pressure. Severe bradycardia, idioventricular rhythm and sinus arrest associated with severe hypotension has been described following an inferior alveolar nerve block with 1.16 mg/kg bupivacaine in a cat. 12 Intravascular injection in this case report cannot be ruled out, but aspiration of the needle prior to injection was negative for blood and the cardiovascular signs developed 5 mins after the nerve block was performed, suggesting a more delayed absorption. Intravenous toxic doses of lidocaine and bupivacaine in feline patients are 10 mg/kg and 4 mg/kg respectively. 1 Systemic toxicity from local anaesthetics, although rare, can occur, and the most common consequences are muscle twitching, seizure, depression, unconsciousness, coma and respiratory arrest, decreased cardiac output and systemic hypotension. 1 No such adverse events were seen in this study.
Vaporiser settings at the end of the procedure were lower than before the procedure in the group receiving nerve blocks, suggesting a reduction in anaesthetic requirements with the nerve blocks. However, end tidal isoflurane measurements would have provided more support for this, but were not available given the clinical nature of this study. Mosing et al 5 demonstrated that intra-operative isoflurane requirements were reduced in cats receiving brachial plexus nerve blocks and undergoing thoracic limb orthopaedic surgery.
Although statistically significant differences were observed in the change of vaporiser settings, these changes were small, and given the inaccuracy in measurements firm conclusions cannot be drawn.
The difference in vital parameters 5 mins prior to and 5 mins after the extractions was used to assess the change in depth of anaesthesia. It may have been better to assess changes in response to each tooth extraction, but it was felt that the overall comparison would be sufficient to demonstrate differences between the two groups. This method was used, as not all cats required the same number of teeth to be extracted. We recognise that this may have affected the results, as more teeth extracted can lead to more pain. However, the number of teeth extracted was found to be similar between groups.
Postoperatively, pain scores were lower in the nerve block group at 2 and 4 h after isoflurane disconnection. This suggests that the cats receiving nerve blocks were less painful at those time points. We found that independently of the group, the cats tended to have higher pain scores at the first two time points (30 mins and 1 h postoperatively). This may be because they were recovering from the general anaesthetic, were recumbent and not very responsive to stimuli.
Managing pain effectively requires looking for its signs and asking the right questions. 13 Because the majority of cats do not show obvious indications of being in pain, pain scales have been developed in order to allow a semi-quantitative evaluation of the degree of pain experienced.13,14 However, there is still no validated pain score scale for cats.9,15 Therefore, an adapted version of a composite pain score scale was used, which had previously been developed to assess acute postoperative pain in cats undergoing ovariohysterectomy. 9 This scale was designed and has been refined in order to become a validated pain score scale for cats. It encompasses psychomotor changes (posture, comfort, activity, mental status), responses to wound palpation and protection of the surgical area, physiological variables (blood pressure, appetite) and vocal expression of pain. In our study, this pain scale was adapted to the surgical site. Pain scoring the cats before the dental procedure was found to be helpful, not only to assess how painful each cat was on presentation, but also to record baseline SAP measurements. It was found that the psychomotor parameters scored (posture, comfort, activity and mental status) were strongly influenced by their temperament, and we found that very nervous and quiet cats were given higher pain scores.
The original plan was to assess appetite after discharge from the hospital. Owners and caregivers were initially contacted 2 days after the dental procedure, and information regarding the appetite of each cat after discharge was obtained. However, owing to poor response levels from the owners and the inherent bias inclusion from the subjective appreciation of each owner, it was decided to only assess appetite within the hospital environment.
Maxillary and inferior alveolar nerve blocks were performed by an anaesthesia specialist or a senior clinical training scholar in anaesthesia. There was no easy way to assess the effectiveness of each block, although the reduction in delivered isoflurane requirements during the procedure suggests that it was effective. The surgical conditions could have been assessed.
When the study was designed we never considered the possibility of injecting saline at the point where the nerve blocks would be performed in the cats of the control group as it is not considered clinical practice and would not be allowed unless under Home Office regulations in the UK. Furthermore, the use of a control group using a an injection of saline was not considered necessary as the assessor was unaware of treatment and there was no evidence of injection in the cats that received nerve blocks and this procedure would be associated with trauma and potentially pain as opposed to a true control.
Regarding the ethical implications of including a control group, it was considered that these cats represent what is performed on a regular basis in first-opinion practice. The highest pain score obtained in the two groups was 7/22, which shows good pain control even in the control group. Both groups received systemic analgesia provided by buprenorphine, medetomidine and meloxicam. However, the study still demonstrated the beneficial effects of performing nerve blocks.
Conclusions
Maxillary and inferior alveolar nerve blocks with lidocaine and bupivacaine, prior to dental extractions, resulted in a reduction in heart rate and blood pressure, while allowing for a reduction in isoflurane, suggesting a reduction in nociceptive input. Furthermore, cats receiving nerve blocks had lower postoperative pain scores compared with the control group, even when routine systemic analgesia was used.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Accepted: 7 April 2014
This study was presented as an abstract at the British Small Animal Veterinary Association (BSAVA) congress in April 2013, Birmingham, UK
References
- 1. Reuss-Lamky H. Administering dental nerve blocks. J Am Anim Hosp Assoc 2007; 43: 298–305. [DOI] [PubMed] [Google Scholar]
- 2. Rochette J. Regional anesthesia and analgesia for oral and dental procedures. Vet Clin North Am Small Anim Pract 2005; 35: 1041–1058. [DOI] [PubMed] [Google Scholar]
- 3. Woodward TM. Pain management and regional anesthesia for the dental patient. Top Comp Anim Med 2008; 23: 106–114. [DOI] [PubMed] [Google Scholar]
- 4. Robertson SA. Assessment and management of acute pain in cats. J Vet Emerg Crit Care 2005; 15: 261–272. [Google Scholar]
- 5. Mosing M, Reich H, Moens Y. Clinical evaluation of the anaesthetic sparing effect of brachial plexus block in cats. Vet Anaesth Analg 2010; 37: 154–161. [DOI] [PubMed] [Google Scholar]
- 6. Tobias KM, Harvey RC, Byarlay JM. A comparison of four methods of analgesia in cats following ovariohysterectomy. Vet Anaesth Analg 2006; 33: 390–398. [DOI] [PubMed] [Google Scholar]
- 7. Gross ME, Pope ER, Jarboe JM, et al. Regional anesthesia of the infraorbital and inferior alveolar nerves during noninvasive tooth pulp stimulation in halothane-anesthetized cats. Am J Vet Res 2000; 61: 1245–1247. [DOI] [PubMed] [Google Scholar]
- 8. Bednarski R, Grimm K, Harvey R, et al. AAHA anesthesia guidelines for dogs and cats. J Am Anim Hosp Assoc 2011; 47: 377–385. [DOI] [PubMed] [Google Scholar]
- 9. Brondani JT, Luna SPL, Padovani CR. Refinement and initial validation of a multidimensional composite scale for use in assessing acute postoperative pain in cats. Am J Vet Res 2011; 72: 174–183. [DOI] [PubMed] [Google Scholar]
- 10. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007; 21: 542–558. [DOI] [PubMed] [Google Scholar]
- 11. O’Morrow C. Advanced dental local nerve block anaesthesia. Can Vet J 2010; 51: 1411–1415. [PMC free article] [PubMed] [Google Scholar]
- 12. Aprea F, Vettorato E, Corletto F. Severe cardiovascular depression in a cat following a mandibular nerve block with bupivacaine. Vet Anaesth Analg 2011; 38: 614–618. [DOI] [PubMed] [Google Scholar]
- 13. Hellyer P, Rodan I, Brunt J, et al. AAHA/AAFP pain management guidelines for dogs and cats. J Am Anim Hosp Assoc 2007; 43: 235–248. [DOI] [PubMed] [Google Scholar]
- 14. Wright BD. Clinical pain management techniques for cats. Clin Tech Small Anim Pract 2002; 17: 151–157. [DOI] [PubMed] [Google Scholar]
- 15. Robertson SA. Managing pain in feline patients. Vet Clin North Am Small Anim Pract 2008; 38: 1267–1290. [DOI] [PubMed] [Google Scholar]


