Abstract
Five domestic cats were euthanased owing to confirmed or suspected Mycobacterium bovis infection. The initial source of infection remains unclear. Cat A was presented to a veterinary clinic in County Kildare, Ireland, with a discharging submandibular lesion. The infection appears to have been transmitted to four other cats through direct (cats B and C living in the same household as cat A) and non-direct (nosocomial spread during routine operations; cats D and E) contact over a 13.5-week period. Of the five cases, two (B and D) had post-mortem examinations in which gross changes consistent with tuberculosis were seen, moderate numbers of acid-fast bacteria (AFB) were seen on microscopy and M bovis (spoligotype SB0978) was confirmed on culture. Of the remaining three cats, one had a swab taken from its draining ovariohysterectomy wound, which revealed large numbers of AFB with morphology consistent with M bovis (cat E). Two cases were euthanased without diagnostic tests; however, their history and clinical presentations were highly suggestive of tuberculosis (cats A and C). To our knowledge, this is the first documented case of nosocomial spread of M bovis in cats.
Mycobacterial infections are a global health concern in humans and in other animals. 1 In cats, tuberculosis is most commonly caused by infection with Mycobacterium bovis or Mycobacterium microti (the vole bacillus), with disease due to Mycobacterium tuberculosis being very rare.2,3 A recent study in the UK found that 19% of cases of feline mycobacterial infections were caused by M microti and 15% by M bovis. 4 While feline tuberculosis is not common, it is probably more common than veterinary surgeons realise. A 2012 study found that histopathological changes consistent with mycobacterial infection were present in ~1% of all feline tissue samples submitted for histopathological examination in the UK. 5 Similar data is not available for Ireland.
Depending on the route of infection, cats with tuberculosis present with a range of clinical signs. Historically, when cats were infected with M bovis while drinking tuberculous cows’ milk, they presented with alimentary signs, intestinal tubercles and/or mesenteric lymphadenopathy.2,4,6,7 However, the most common presentation is now cutaneous, with respiratory and alimentary forms being seen less frequently. 4 The cutaneous lesions are usually firm, raised nodules, which may ulcerate, or form non-healing wounds with draining sinuses. Local or generalised lymphadenopathy may be present; in chronic cases, infection may spread to the lungs, cause dyspnoea and sometimes a cough, with an interstitial-to-bronchial pattern on radiography. 8 Where infection is obtained through inhalation, tubercles form in the lungs or hilar lymph nodes. Disseminated disease is rare, and can cause splenomegaly, hepatomegaly, pneumonia, pleural or pericardial effusions, generalised lymphadenopathy, fever and weight loss.3,4,6,9
The current epidemiology of feline tuberculosis is complex. Occasional alimentary cases may still result from drinking tuberculous cows’ milk. However, cutaneous lesions probably arise from infected bite wounds, as lesions typically involve the face, extremities, tail base or perineum – ‘fight and bite sites’. 7 Infection by this route is possible because wild mice and voles in the UK can carry M microti or M bovis,10–13 and hunting is a known risk factor for feline tuberculosis.4,14,15 M bovis is endemic in badgers in some areas of the UK and Ireland. 16 While cats and badgers rarely interact directly, there is the potential for cats to become infected via environmental contamination.
This report details a case series in which M bovis appears to have been transmitted through direct and non-direct contact, with nosocomial spread during routine operations.
Case series
Cat A
A 2-year-old male neutered domestic longhair cat (cat A) with outdoor access and hunting behaviour was presented to a clinic in County Kildare, Ireland, with discharging submandibular wounds assumed to be caused by an animal bite. Despite treatment with clavulanate amoxicillin (8.75 mg/kg PO q12h for 7 days; Noroclav, Norbrook) infection progressed over 3 weeks, and the cat developed anorexia, weight loss, lethargy, dehydration and generalised purulent lymphadenitis. The cat was not re-presented during this period. It was euthanased owing to financial constraints and no post-mortem examination (PME) was performed. See Figure 1 for the time line of infections in all cats included in the series (cats A–E).
Figure 1.
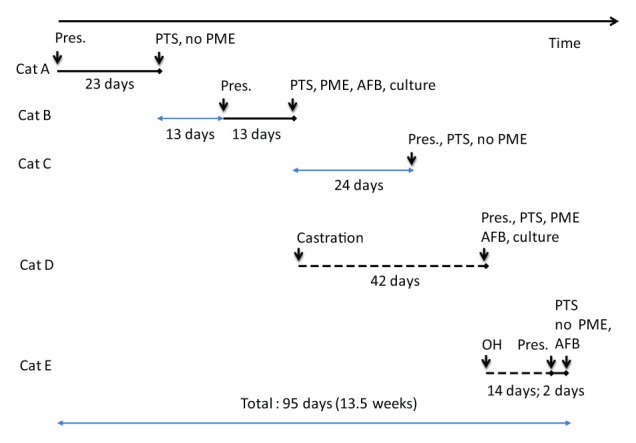
Timeline of infections in cats A–E. The solid black line indicates the known duration of clinical signs; the pale blue line indicates the duration between events; and the dashed black line the duration from elective procedure. Pres. = day of clinical presentation; PTS = euthanasia; PME = post-mortem examination; AFB = acid-fast bacteria seen on Ziehl–Neelsen staining; culture = specialist culture looking for mycobacteria; OH = ovariohysterectomy
Cat B
Thirteen days after cat A was euthanased, a second cat (cat B) from the same household was presented. This 3-year-old male neutered domestic shorthair (DSH) cat also had outdoor access and hunting behaviour. It presented with a similar submandibular wound. The underlying muscle was visible and the wound was discharging blood-stained purulent fluid (Figure 2). A blood sample was negative for feline immunodeficiency virus antibodies and feline leukaemia virus antigens (FASTest; Megacor). Owing to the history of cat A, this cat was treated more aggressively: cefovecin (8 mg/kg SC; Convenia, Bayer) and clindamycin (25 mg PO q12h, 5.5 mg/kg; Clindacyl, Vétoquinol) were administered. The wound was cleaned with dilute surgical scrub (chlorhexidine 4% w/v; Hibiscrub, Molnlycke Health Care Group) and the same surgical scrub was given to the owner to bathe the wound twice daily (while wearing surgical gloves). Surgical debridement was declined by the owner owing to financial constraints.
Figure 2.
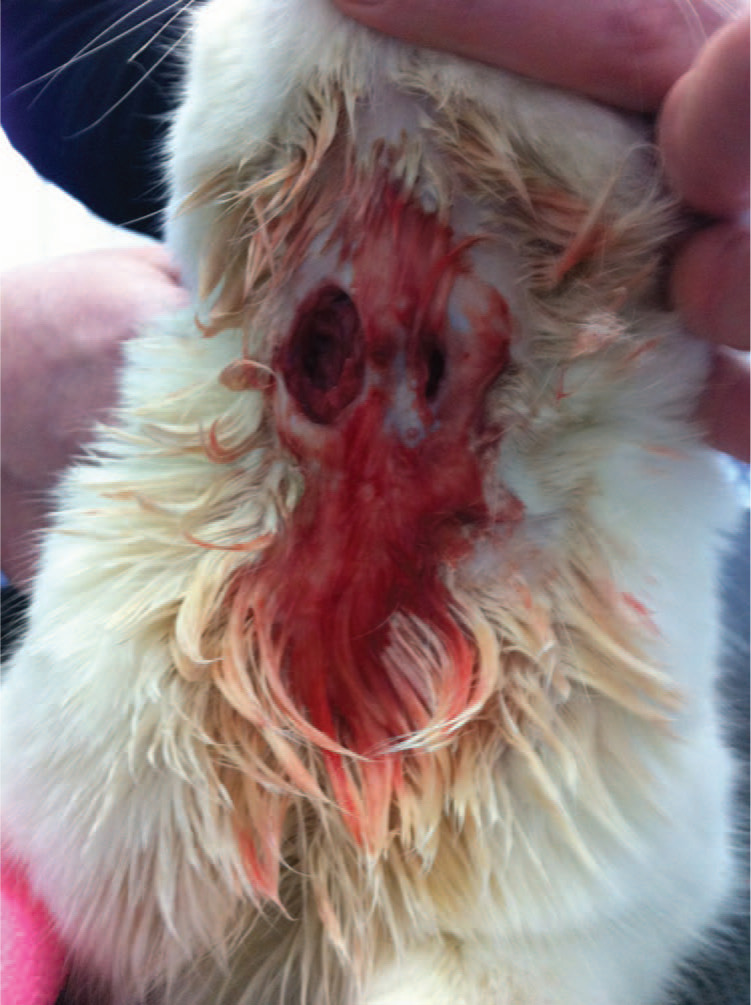
A 3-year-old male neutered domestic shorthair cat (cat B) with a submandibular wound through which the underlying muscle is visible and the wound is discharging blood-stained, purulent fluid
One week later, cat B’s wound showed little improvement and another wound had developed lateral to the first; both wounds were discharging serosanguineous fluid. Haematology revealed leucocytosis (30,600 cells/μl [reference interval {RI} 5.5–19,500 cells/μl]) and serum biochemistry revealed mild hypoalbuminaemia (21 g/dl [RI 27–45 g/dl]); serum calcium was not assessed. The wounds were clipped, cleaned and flushed with saline and dilute chlorhexidine solution. Hydrogel (Intrasite Gel; Smith & Nephew) was applied, and an absorbent dressing (Allevyn; Smith & Nephew) was secured over it with a section of nylon tights. The owner was asked to re-dress the wounds daily until revisit. Surgical debridement and culture and sensitivity were declined by the owner owing to financial constraints. Six days later, cat B presented with anorexia, weight loss and lethargy; the cat was dull, depressed and pyrexic. The wounds were heavily exudative and a mass was detected on abdominal palpation. The cat was euthanased and sent to the University Veterinary Hospital, Dublin, Ireland, for PME, which revealed enlarged retropharyngeal, popliteal and mesenteric lymph nodes, which, when cut, exuded purulent material. The lungs displayed scattered 1 mm, firm, white foci throughout each lobe extending from the pleural surface to within the pulmonary parenchyma. Histopathological examination of lymph nodes showed multifocal-to-coalescing areas of pyogranulomatous inflammation with centres of eosinophilic cellular debris surrounded by concentric layers of macrophages and neutrophils, and Ziehl–Neelsen (ZN) staining revealed abundant acid-fast bacteria (AFB) within macrophages and free within the extracellular space. The lung tissue showed multifocal-to-coalescing areas of alveolar wall necrosis with a marked inflammatory cell infiltrate, and abundant macrophages and neutrophils mixed with fibrin and cellular debris; ZN staining revealed numerous AFB within macrophages. The diagnosis was granulomatous necrotising pneumonia and pyogranulomatous lymphadenitis with intralesional AFB consistent with mycobacteriosis.
Fresh samples of lymph nodes were submitted to the National Mycobacterial Reference Laboratory (NMRL) at the Central Veterinary Research Laboratory of the Department of Agriculture, Food and the Marine, Kildare, Ireland, for culture. The lymph nodes were homogenised in phosphate-buffered saline and the tissue suspension decontaminated with oxalic acid. After centrifugation and wash steps, a tissue pellet was prepared to inoculate Lowenstein–Jensen medium with pyruvate, Stonebrink medium with pyruvate and a Bactec MGIT 960 tube, containing OADC Growth Supplement and PANTA antimicrobial mixture. Cultures were incubated at 37°C. The liquid medium showed growth after 6 days of incubation, and AFB were present in smears prepared from this medium. Colonies were observed on solid medium on day 15 of incubation. Isolates were confirmed as M bovis and spoligotyping was performed as described by Kamerbeek et al, 17 but using a digoxigenin labelling and detection system. The spoligotype pattern was given the name assigned in the M bovis spoligotyping database, SB0978.
Pending the culture result, the owner was advised to monitor their other animals for wounds, lymphadenopathy, and respiratory or enteric disease. The owner was advised to disinfect all bowls, bedding and areas to which the cats had access. They were informed of the zoonotic implications and advised to seek medical advice.
Cat C
Twenty-four days after cat B was euthanased, the third and final cat (cat C) from that household, a predominantly indoor female neutered 1-year-old DSH cat, was presented with respiratory signs: respiration rate was 120 breaths per minute with bilaterally increased lung sounds and occasional coughing and sneezing. Tuberculosis was suspected. The cat was euthanased, but a PME was declined by the owner. The other animal in the house, a dog, remained healthy.
Cat D
The day cat B was euthanased, a 2-year-old male entire DSH outdoor cat (cat D) was hospitalised. The cat underwent elective castration prior to rehoming through the clinic. It returned 6 weeks later with non-healing scrotal wounds, associated purulent discharge (Figure 3), anorexia, weight loss and lethargy. Masses were palpable, extending from the wound to mid-abdomen. As tuberculosis was suspected, the cat was euthanased and sent for PME. A swab from the scrotal wound was sent to the Irish Equine Centre for cytology and ZN staining, which revealed large numbers of AFB. The PME revealed inspissated purulent material within the right and left vas deferens, extending to the cranial border of the pelvis on the right and the insertion of the left ureter to the bladder on the left, from where it extended retroperitoneally to within 2 cm caudal of the left kidney (Figure 4). Four variably sized (1–4 cm) firm masses were present within the omentum; on the cut surfaces these were pale yellow, wet and soft. The mesenteric lymph nodes were marginally enlarged. The lungs were gross normal. Histopathological examination of the omental masses revealed partially encapsulated masses composed of multiple granulomas surrounded by a moderate lymphocytic cell infiltrate. Mesenteric lymph nodes contained a large central focal granuloma and multifocal-to-coalescing areas of necrosis; ZN staining of omental masses and mesenteric lymph nodes revealed a few AFB within macrophages (Figure 5). Granulomatous inflammation was noted in the lung, with multifocal-to-coalescing areas of alveolar wall necrosis, and adjacent alveoli contained macrophages, fibrin and oedema; ZN staining showed AFB within macrophages. The diagnosis was granulomatous inflammation of the vas deferens and omentum, with granulomatous pneumonia and lymphadenitis, and intralesional AFB consistent with mycobacteriosis. A sample of mesenteric lymph node and omental mass was submitted to the NMRL for culture. AFB were recorded after 5 days of incubation in liquid medium, while growth on solid media was recorded after 12 days. The isolate was confirmed as M bovis, spoligotype SB0978.
Figure 3.
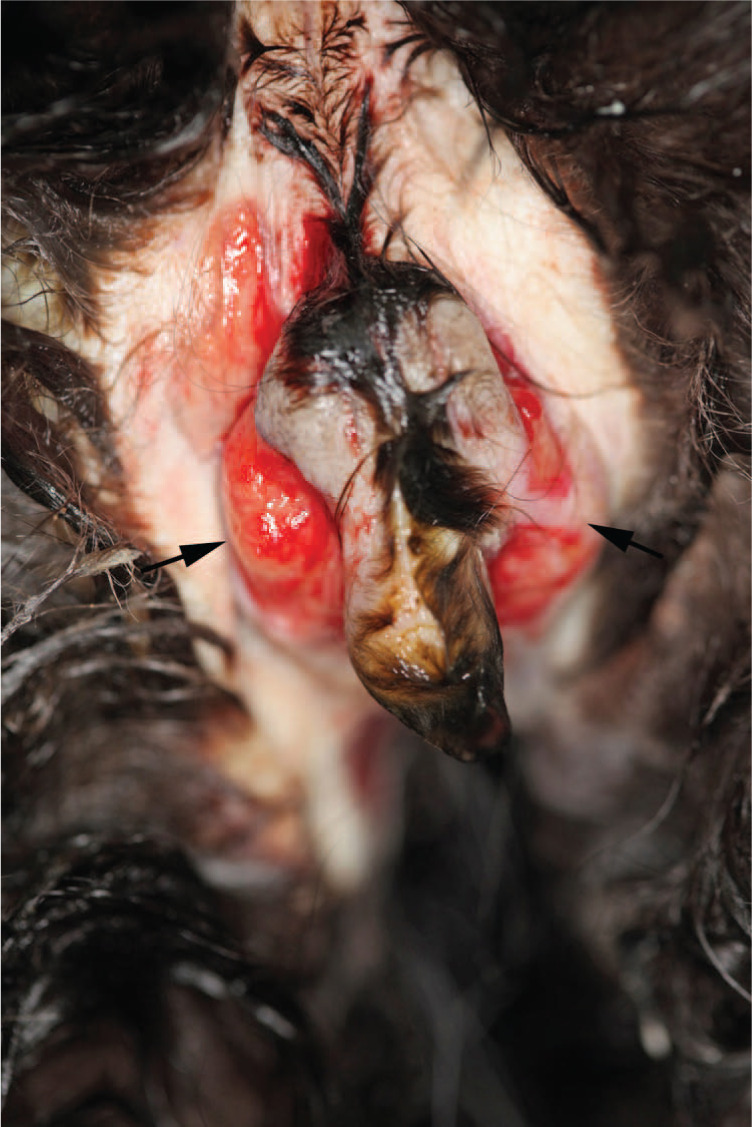
A 2-year-old male entire domestic shorthair cat 6 weeks after castration (cat D). Post-mortem examination revealed two surgical wounds (left and right) over the scrotum, which were expanded by purulent material, indicated by the black arrows
Figure 4.
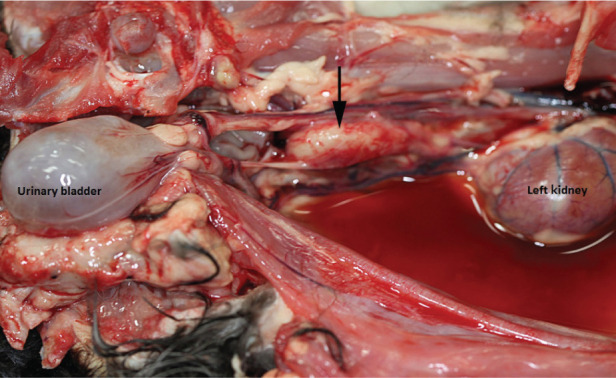
Post-mortem examination of cat D showing inspissated purulent material within the retroperitoneal space extending to 2 cm below the left kidney, as indicated by the black arrow
Figure 5.
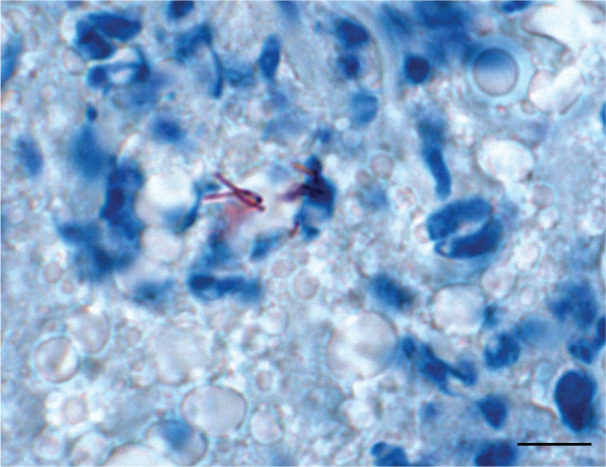
Histopathology of a mesenteric lymph node in cat D. Ziehl–Neelsen stain showing acid-fast bacteria within macrophages (granulomatous lymphadenitis). Bar =100 µm
Cat E
On the same day that cat D was euthanased, a 6-month-old entire strictly indoor female DSH cat (cat E) was admitted for ovariohysterectomy. Two days after the euthanasia of cat D, results for the swab of its serosanguineous fluid confirmed the presence of large numbers of AFB. As M bovis was suspected, concern was raised that cat E may have been exposed. As the owner of cat E had two other cats and a dog, she was advised to monitor cat E’s surgical wound, and cover it with an adhesive dressing (Primapore; Smith & Nephew) to minimise the risk of transmission. Five days after surgery the wound became mildly inflamed; a swab was taken for cytology, which was negative for AFB. The wound appeared to have healed and the sutures were removed 2 weeks after surgery. However, on the day after suture removal the wound broke down, and cat E was isolated. Two swabs were taken: one from the surface of the incision and one from the serosanguineous fluid. The results were received 24 h later: the surface swab was negative for AFB, while the fluid swab revealed many AFB with morphology consistent with mycobacteria. M bovis was presumed and cat E was euthanased the following day. PME was declined by the owner, and the other pets in the household remained healthy.
Discussion
To our knowledge, this is the first case series of nosocomial transmission of M bovis in domestic cats. Previously, there was a single report of a potentially practice-related cluster in New Zealand; however, the authors were not able to suggest how nosocomial infection may have occurred. 18 Our study demonstrates that this important, re-emerging and potentially zoonotic infection can be transmitted in the clinic environment, and it acts as a warning to all veterinary staff when dealing with non-healing wounds in cats: when a wound is not responding to treatment as expected it should be investigated further with cytology, Gram and ZN staining, culture and/or molecular diagnostics (where possible).
Direct and indirect infections are represented here. Cat A had discharging submandibular wounds, which are common in cats with tuberculosis, and are believed to have resulted from being bitten by infected prey species, probably mice and voles. 4 While M bovis can be found in small rodents, it is not believed to be endemic in these species;12,13 they are probably infected when foraging in land contaminated by tuberculous cattle or badgers. Reactor cattle have been detected near to where the cats lived (J Eves, personal communication), 19 and the spoligotype found in the cats, while rare, has been previously documented in three badgers 20 and occasional cattle from this region (unpublished observations). Cat B presented with similar clinical signs, 13 days after cat A was euthanased. It is not clear whether this cat was infected by cat A (they lived together), or from a similar hunting incident to cat A. The similarity of the lesions makes a hunting incident more likely. Cat C was not a hunter, but it did sleep with cats A and B, and its disease was respiratory, so it is most likely that it was infected directly by cat A and/or cat B. Cats D and E had nosocomial infections from their surgical wounds.
When cats A and B were presented, tuberculosis was not initially considered in the differential diagnoses. The wounds of cat A appeared to be from a bite, which is not unusual in an outdoor male cat. The same is true for cat B, and while PME suggested tuberculosis, it was advised that confirmation by culture could take up to 6 weeks. Unfortunately, barrier nursing was not implemented; if it had been, infection may have been contained to the first three cats, and nosocomial infection prevented.
We can speculate on how the nosocomial infections arose. Infection is unlikely to have come from kennels or consultation/surgery tables, as they were disinfected with 10% Trigene solution (halogenated tertiary amine; Medichem International), which (like 3% iodine preparations and 60–90% alcohol) has excellent activity against mycobacteria, for example Mycobacterium avium and Mycobacterium terrae. 21 However, while it is no longer approved by the Department for Environment, Food and Rural Affairs for use with Tuberculosis Orders 22 and neither is its replacement Anigene (halogenated tertiary amine; Medichem International), they are mycobactericidal at dilutions of 1:10 (10% v/v) and are used by the Irish Government for disinfections of cattle buildings in which M bovis is suspected.
A potential source of infection could have been the staff or their uniforms. Their uniforms could have been contaminated when holding the cats with draining lesions for euthanasia; the contaminated area could then have come in contact with another cat’s moist surgical wound when it was held post-surgery. However, perhaps the most likely source of infection would have been staff hands as, although they were washed between patients, 4% chlorhexidine solution was used (it was also used as the surgical scrub solution) and this has only fair activity against mycobacteria. 23 Gloves were used while dealing with draining wounds; however, they were not routinely used for procedures such as euthanasia or preoperative skin preparation. It was not clear that a cluster was forming until the swab results were received from cat E. Initially, the infection was thought to be isolated to the three cats from the same household. When it became clear that cat D had become infected in the clinic, cat E’s surgery had already been performed. This was when precautions were taken to minimise the risk to other animals. These precautions were successful. The only two animals that were treated surgically on the days of cat B’s and cat D’s euthanasia were the two in this case series; if there had been any others, prophylactic treatment may have been advised.
The cats presented with clinical signs similar to those previously reported.2,4,6,9 Cats A and B had draining submandibular lesions, which progressed to systemic disease, with cat B developing generalised lymphadenopathy and pneumonia. Cat C also had pneumonia. Cats D and E developed focal lesions at their surgical sites, with cat D developing generalised lymphadenopathy and pneumonia. However, the short incubation periods were unexpected: 42 days for cat D, and just 14 days for cat E. The speed of disease progression was also unexpected; it only took 23 days for cat A to progress to the point of euthanasia and only 13 days in cat B. By the time cat D presented with scrotal lesions, just 42 days after castration, it already had disseminated abdominal lesions and pneumonia.
Tuberculosis is typically considered a slowly developing disease, with humans developing disease over months to years, depending on their age, immune status, gender, the amount and genotype of mycobacteria they are exposed to, whether they are extra-pulmonary cases (which develop more rapidly than pulmonary cases) and whether they are secondary cases, that is, cases in recent contact with a clinical case. 24 One study found the median incubation period of secondary cases to be 1.26 years. 24 While primary infection causes disease in only 10% of humans, most cases develop symptoms within 2 years. The rest develop latent infections, although 10% of these will later become clinical, presenting with incubation periods of many years. 25
Few studies have been published on the incubation period and/or progression of feline tuberculosis. Rapid progression is not typical of field cases, which usually take weeks or months to progress.8,14,26 However, in 1958 experimental subcutaneous infection with M bovis produced incubation periods of 28–42 days, 2 and in 1983 an Australian paper reported on a naturally occurring colony outbreak of M bovis where a cat developed pneumonia 44 days after being exposed to a cat with draining submandibular lesions and progressed to death in only 8 days. 27 The speed of incubation and disease progression in the current study is therefore consistent with these primary infections where cats were infected with high doses of mycobacteria; this is supported by finding large numbers of mycobacteria in the discharge and tissue samples of the cats in this series and the rapid growth of mycobacteria in culture from cat B (only 6 days). Some strains of M bovis are more pathogenic than others; this has been seen in laboratory mice, and may be related to them arising from different hosts. 28 The strain (spoligotype) of M bovis found in the cats in this series is unusual. While it has been found in three badgers and occasional cattle from the same area (unpublished observations), 20 it is not known what other species may also have been infected, or how the first cats were infected. It has been suggested that they might have been infected by feral mink as cats A–C lived near to a river where mink are regularly seen (unpublished observations), and the neck lesions resembled mustelid bites (Figure 2). Francis showed that intraperitoneal infection of cats with M bovis had an incubation period of just 11–36 days; 2 this explains why cat E developed disease so rapidly – it was infected via the fresh spay wound.
Diagnosis of feline tuberculosis is challenging. When a cat presents with a discharging or suspicious wound, tuberculosis should be considered as a differential diagnosis. Ideally, barrier nursing should be implemented. Discharge and/or tissue samples should be taken for cytology/histopathology and ZN staining; finding AFB with consistent morphology and evidence of (pyo-)granulomatous change is highly suggestive of mycobacterial infection.29,30 However, culture and/or molecular tests (eg, polymerase chain reaction-based diagnostics) are needed to reveal the species of mycobacteria involved. Unfortunately, M bovis can take up to 6 weeks to culture or may give a false-negative result, 14 particularly in paucibacillary samples.31,32 Specific tests, such as serology and intradermal testing, have generally proved unhelpful,9,33–35 although the interferon-gamma (IFN-ϒ) assay and other immunoassays are showing promise in detecting and differentiating cats infected with M bovis, M microti and M avium,36–39 and the IFN-ϒ assay is now commercially available in the UK for both cats and dogs (Biobest Laboratories). As M bovis can survive for up to 2 months in appropriate conditions, 40 it is important to recognise potential cases and make the diagnosis; if not, environmental contamination can occur. This is of particular importance in a veterinary hospital. If cleaning procedures are not adequate, there is risk of exposing multiple patients and staff to infection and, as this case series shows, M bovis can be readily transmitted between animals in a clinic environment.
Treating a cat with tuberculosis is challenging and contentious. 26 Treatment is not advisable if there are extensive draining lesions, or if respiratory or generalised disease are present. These cases are less likely to respond to treatment, and represent increased transmission risks. 9 Other considerations must also be made, such as lack of approved drugs, possible drug toxicity, restricted finances and compliance issues, as treatment typically involves giving two or three drugs for up to 6 months. 3 In addition, in the UK, M bovis is notifiable in all mammals, 41 so the Animal Health and Veterinary Laboratories Agency must be contacted; in Ireland, it is only notifiable in ruminants. 42 As the cats in this study all had significant pathology, euthanasia was the appropriate response. Prophylactic treatment of the potentially exposed cats could, perhaps, have been undertaken, as this may have prevented clinical disease. However, prophylactic treatment has not been previously documented in cats, and while long-term (6–12 months) single-drug (isoniazid at 10 mg/kg PO q24h) regimens have been suggested for dogs, it is not known whether this would be effective in cats.
Cats with tuberculosis pose a low, but potential, zoonotic risk. 3 There is only one published report of a human gaining an infectious challenge sufficient to cause a positive Mantoux test reaction; the person had been in close contact with five tuberculous cats and one tuberculous brush-tailed possum. 27 However, there have recently been a number of M bovis tuberculosis clusters in cats and dogs in the UK, where owners have become infected and needed treatment (unpublished observations). In-contact individuals (such as owners and veterinary staff) should observe strict hygiene in relation to animal handling and disinfection, and attend their doctors for advice if they receive significant exposure. All staff and pet owners involved in this case contacted their general practitioners; all of the veterinary staff had received the Bacillus Calmette–Guérin vaccination and were over the age of 16 years. Prophylactic treatment was not recommended by any of the doctors.
Conclusions
This report shows that veterinary surgeons need to recognise that cats can become infected with M bovis and that, if the disease is not recognised, nosocomial spread can occur.
Acknowledgments
We wish to thank the owners of the animals, and all of the veterinary surgeons and nurses of the veterinary clinics involved; Mr Brian Cloak, senior pathology technician, University College Dublin, for taking the photographs; Professor Sean Callanan MVB, PhD, MRCVS, MRC Path., Cert VR, Dip ECVP; Dr Nola Leonard MVB PhD MRCVS; Mr John Eves MVB, District Veterinary Office; Dr Kevin Kenny, National Mycobacterial Reference Laboratory (NMRL) at the Central Veterinary Research Laboratory of the Department of Agriculture; Jack McGuirk, TB Section, Backweston Laboratory, Department of Agriculture and Food; Professor Thomas Buckley MSC, FIBMS, FAMLS, Head of Microbiology, Irish Equine Centre; and Dr Ursula Fogarty MVB, PhD, MRCVS Dip.Stat, Irish Equine Centre.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case series.
Accepted: 6 March 2014
References
- 1. Lobue PA, Enarson DA, Thoen CO. Tuberculosis in humans and animals: an overview. Int J Tuberc Lung Dis 2010; 14: 1075–1078. [PubMed] [Google Scholar]
- 2. Francis J. Tuberculosis in animals and man: a study in comparative pathology. London: Cassell and Company, 1958. [Google Scholar]
- 3. Gunn-Moore DA. Mycobacterial infections in cats and dogs. In: Ettinger S, Feldman E. (eds). Textbook of veterinary internal medicine. 7th ed. Philadelphia: WB Saunders, 2010, pp 875–881. [Google Scholar]
- 4. Gunn-Moore DA, McFarland S, Brewer JI, et al. Mycobacterial disease in cats in Great Britain: I. Culture results, geographical distribution and clinical presentation of 339 cases. J Feline Med Surg 2011; 13: 934–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gunn-Moore DA, Gaunt C, Shaw DJ. Incidence of mycobacterial infections in cats in Great Britain: estimate from feline tissue samples submitted to diagnostic laboratories. Transbound Emerg Dis 2013; 60: 338–344. [DOI] [PubMed] [Google Scholar]
- 6. Jennings AR. The distribution of tuberculous lesions in the dog and cat, with reference to pathogenesis. Vet Rec 1949; 27: 380–385. [Google Scholar]
- 7. Gunn-Moore DA, Dean R, Shaw S. Mycobacterial infections in cats. In Practice 2010; 32: 444–452. [Google Scholar]
- 8. Bennett A, Lalour S, Swartz T, et al. Radiographic changes in cats with mycobacterial infections. J Feline Med Surg 2011; 13: 718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greene CE, Gunn-Moore DA. Mycobacterial infections. In: Greene CE. (ed). Infectious diseases of the dog and cat. 4th ed. Philadelphia: WB Saunders, 2012, pp 495–510. [Google Scholar]
- 10. Cavanagh R, Begon M, Bennett M, et al. Mycobacterium microti infection (vole tuberculosis) in wild rodent populations. J Clin Microbiol 2002; 40: 3281–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burthe S, Bennett M, Kipar A, et al. Tuberculosis (Mycobacterium microti) in wild field vole populations. Parasitology 2008; 135: 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Delahay RJ, De Leeuw AN, Barlow AM, et al. The status of Mycobacterium bovis infection in UK wild mammals: a review. Vet J 2002; 164: 90–105. [DOI] [PubMed] [Google Scholar]
- 13. Delahay RJ, Smith GC, Barlow AM, et al. Bovine tuberculosis infection in wild mammals in the South-West region of England: a survey of prevalence and a semi-quantitative assessment of the relative risks to cattle. Vet J 2007; 173: 287–301. [DOI] [PubMed] [Google Scholar]
- 14. Smith NH, Crawshaw T, Parry J, et al. Mycobacterium microti: more diverse than previously thought. J Clin Microbiol 2009; 47: 2551–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gunn-Moore DA, Jenkins PA, Lucke VM. Feline tuberculosis: a literature review and discussion of 19 cases caused by an unusual mycobacterial variant. Vet Rec 1996; 138: 53–58. [DOI] [PubMed] [Google Scholar]
- 16. Gallagher J, Clifton-Hadley RS. Tuberculosis in badgers; a review of the disease and its significance for other animals. Res Vet Sci 2000; 69: 203–217. [DOI] [PubMed] [Google Scholar]
- 17. Kamerbeek J, Schouls L, Kolk A, et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol 1997; 35: 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Lisle GW, Collins DM, Loveday AS, et al. A report of tuberculosis in cats in New Zealand, and the examination of strains of Mycobacterium bovis by DNA restriction endonuclease analysis. N Z Vet J 1990; 38: 10–13. [DOI] [PubMed] [Google Scholar]
- 19. Abernethy DA, Upton P, Higgins IM, et al. Bovine tuberculosis trends in the UK and the Republic of Ireland, 1995–2010. Vet Rec 2013; 172: 312. [DOI] [PubMed] [Google Scholar]
- 20. Furphy C, Costello E, Murphy D, et al. DNA typing of Mycobacterium bovis isolates from badgers (Meles meles) culled from areas in Ireland with different levels of tuberculosis prevalence. Vet Med Int 2012; 2012: 742478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boyce JM, Pittet D, et al. Guideline for hand hygiene in health care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm Rep 2002; 51: 1–45. [PubMed] [Google Scholar]
- 22. Department for Environment, Food and Rural Affairs. Disinfectants Approved for use in England, Scotland and Wales. http://disinfectants.defra.gov.uk (accessed 17 March 2014).
- 23. Boyce JM, Pittet D. and Healthcare Infection Control Practices Advisory Committee. Guideline for hand hygiene in health care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am J Infect Control 2002; 30: S1–S46. [DOI] [PubMed] [Google Scholar]
- 24. Borgdorff MW, Sebek M, Geskus RB, et al. The incubation period distribution of tuberculosis estimated with a molecular epidemiological approach. Int J Epidemiol 2011; 40: 964–970. [DOI] [PubMed] [Google Scholar]
- 25. Tufariello JM, Chan J, Flynn JL. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect Dis 2003; 3: 578–590. [DOI] [PubMed] [Google Scholar]
- 26. Gunn-Moore DA, McFarland S, Schock A, et al. Mycobacterial disease in cats in Great Britain: II. Histopathology of 225 cases, and treatment and outcome of 184 cases. J Feline Med Surg 2011; 13: 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Isaac J, Whitehead J, Adams JW, et al. An outbreak of Mycobacterium bovis infection in cats in an animal house. Aust Vet J 1983; 60: 243–245. [DOI] [PubMed] [Google Scholar]
- 28. Aguilar León D, Zumárraga MJ, Jiménez Oropeza R, et al. Mycobacterium bovis with different genotypes and from different hosts induce dissimilar immunopathological lesions in a mouse model of tuberculosis. Clin Exp Immunol 2009; 157: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carter GR, Wise DJ. Veterinary bacteriology and mycology. Ames: Iowa State Press, 2004. [Google Scholar]
- 30. Ginn PE, Mansell J, Rakich PM. Mycobacterial infections. In: Maxie G. (ed). Jubb, Kennedy and Palmer’s pathology of domestic animals. 5th ed. Edinburgh: Saunders Elsevier, 2007, pp 687–691. [Google Scholar]
- 31. Aranaz A, Liébana E, Pickering X, et al. Use of polymerase chain reaction in the diagnosis of tuberculosis in cats and dogs. Vet Rec 1996; 138: 276–280. [DOI] [PubMed] [Google Scholar]
- 32. Mishra A, Singhal A, Chauhan DS, et al. Direct detection and identification of Mycobacterium tuberculosis and Mycobacterium bovis in bovine samples by a novel nested PCR assay: correlation with conventional techniques. J Clin Microbiol 2005; 43: 5670–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaneene JB, Bruning-Fann CS, Dunn J, et al. Epidemiologic investigation of Mycobacterium bovis in a population of cats. Am J Vet Res 2002; 63: 1507–1511. [DOI] [PubMed] [Google Scholar]
- 34. Hawthorne VM, Lauder IM. Tuberculosis in man, dog and cat. Am Rev Resp Dis 1962; 85: 858–869. [DOI] [PubMed] [Google Scholar]
- 35. Snider WR, Cohen D, Reif JS, et al. Tuberculosis in canine and feline populations. Study of high risk populations in Pennsylvania, 1966–1968. Am Rev Respir Dis 1971; 104: 866–876. [DOI] [PubMed] [Google Scholar]
- 36. Fenton KA, Fitzgerald SD, Kaneene JB, et al. Comparison of three immunodiagnostic assays for antemortem detection of Mycobacterium bovis stimulation in domestic cats. J Vet Diagn Invest 2010; 22: 724–729. [DOI] [PubMed] [Google Scholar]
- 37. Rhodes SG, Gruffydd-Jones TJ, Gunn-Moore DA, et al. An interferon-gamma test for feline tuberculosis. Vet Rec 2008, 162: 453–455. [DOI] [PubMed] [Google Scholar]
- 38. Rhodes SG, Gruffydd-Jones TJ, Gunn-Moore DA, et al. Adaptation of IFN-gamma ELISA and ELISPOT for the diagnosis of feline tuberculosis. Vet Immunol Immunopathol 2008; 124: 379–384. [DOI] [PubMed] [Google Scholar]
- 39. Rhodes SG, Gunn-Moore DA, Boschiroli L, et al. Comparative study of IFNγ and antibody tests for feline tuberculosis. Vet Immunol Immunopathol 2011; 144: 129–134. [DOI] [PubMed] [Google Scholar]
- 40. Fine AE, Bolin CA, Gardiner JC, et al. A study of the persistence of Mycobacterium bovis in the environment under natural weather conditions in Michigan, USA. Vet Med Int 2011; 2011: 765430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. DEFRA. Bovine tuberculosis in domestic pets. http://www.defra.gov.uk/ahvla-en/files/AG-TBYP-01.pdf (2013, accessed 17 March 2014).
- 42. DEFRA. List of Notifiable Diseases. http://www.agriculture.gov.ie/animalhealthwelfare/diseasecontrol/listofnotifiablediseases/ (2013, accessed 14 March 2014).


