Abstract
Diagnosis of feline lungworm, Aelurostrongylus abstrusus, is typically achieved by identifying larvae in feces following concentration through flotation or using the Baermann technique. This work presents observations on the usefulness of an indirect immunofluorescence antibody assay for detection of antibodies to this parasite in the sera of infected cats. Using first-stage larvae of A abstrusus and sera from both experimentally and naturally infected cats, it was determined that the test was fairly sensitive and did not cross-react with serum from an Ancylostoma braziliense (hookworm)-infected cat.
Short Communication
The lungworm of cats, Aelurostrongylus abstrusus, inhabits the alveoli and terminal bronchioles causing coughing, chronic wasting, wheezing and dyspnea. In a prevalence survey completed in 2009, A abstrusus infections were reported across North America in both the USA and Canada. 1 In regions where Aelurostrongylus species is known to be present fecal flotations have revealed infection rates of 0.1–1.1% while Baermann fecal examinations revealed infection rates as high as 18.5%. 1 In a survey completed in shelter and foster cats in Upstate New York using centrifugal zinc sulfate and sugar flotations, A abstrusus larvae were found in 6.2% of fecal samples. 2
In 2009, a comparison of different methods found the Baermann technique the most sensitive test for the detection of A abstrusus when compared with bronchoalveolar lavage fluid analysis, necropsy, fecal sedimentation–flotation and histologic examination of lung tissue. 3 However, most veterinary clinics and many diagnostic laboratories do not routinely use this method to check for A abstrusus infection owing, in part, to the time required and still fairly low sensitivity of the Baermann technique. For this reason, it is likely that feline aelurostrongylosis remains under-diagnosed. As there has been previous success with immunofluorescent antibody tests (IFAT) for nematodes similar to A abstrusus, such as Angiostrongylus cantonensis,4,5 the purpose of this study was to develop an IFAT for the detection of A abstrusus-specific IgG antibodies in sera from cats.
Serum samples used in the assay were from several sources. One set of 5 samples was collected pre-infection and on postinfection days (PID) 8, 29, 141 and 176 (28 days post-treatment) from a specific pathogen-free cat experimentally infected with 51 A abstrusus third-stage larvae (EXP-Aa). This cat was housed in American Association for Laboratory Animal Science approved facilities at Cornell University under an approved Institutional Animal Care and Use Committee protocol. Two serum samples were collected from two naturally infected cats (NAT-Aa1 and NAT-Aa2) seen at the Cornell University Hospital for Animals as part of an on-going in-house spay–neuter program for shelter animals; these cats were found to be infected with A abstrusus by routine fecal analysis using centrifugal zinc–sulfate and sugar flotations. Control samples included pooled cat serum from 100 specific pathogen-free cats (NEG-Pool; Bethyl Laboratories) and phosphate buffered saline (PBS, pH 7.4, 0.02 M). Also, a single serum sample was collected PID 48 from a cat experimentally infected with a feline isolate of the hookworm Ancylostoma braziliense (EXP-Ab) from a concurrent study (also housed at Cornell University under an approved IACUC protocol). The experimentally infected cats (EXP-Aa and EXP-Ab) were verified to be positive for their respective infections by the presence of lungworm larvae or hookworm eggs in their feces. The A abstrusus-infected cat, though asymptomatic for its lungworm infection, had radiographic evidence of pulmonary pathology (Figure 1). The two experimentally infected cats were ultimately treated with moxidectin (Advantage Multi for Cats; Bayer Animal Health), verified to be free of parasites and placed in adoptive homes; the naturally infected cats were also dewormed before being returned to the shelter.
Figure 1.
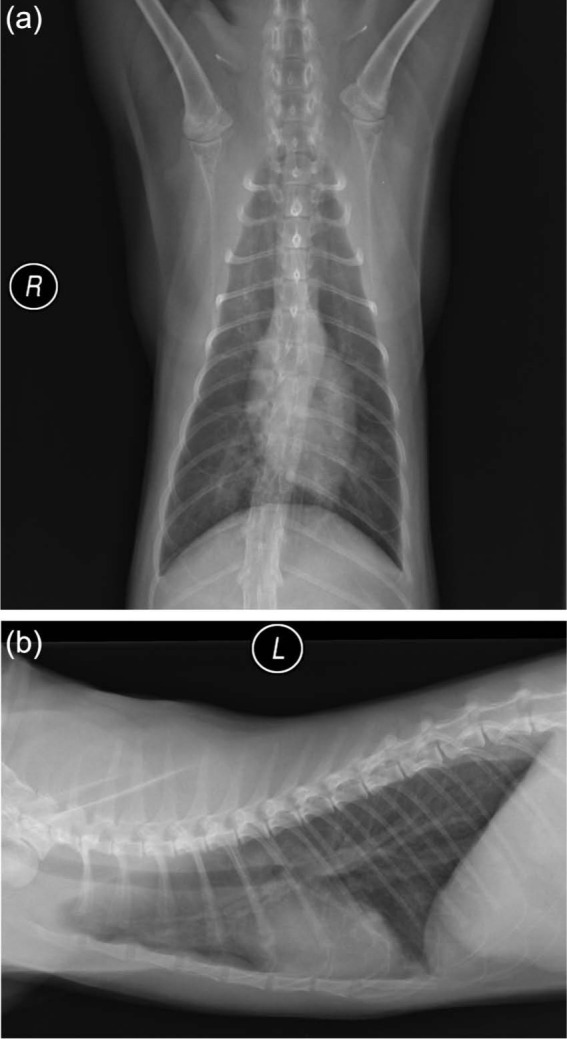
Ventrodorsal (a) and lateral (b) thoracic radiographs of a cat (EXP-Aa) taken 148 days after experimental infection with 51 Aelurostrongylus abstrusus larvae. The radiographs reveal a patchy, moderately increased soft tissue opacity in the lungs with a predominantly bronchocentric distribution. R = right; L = left lateral
For the indirect IFAT, first-stage larvae from the feces of experimentally or naturally infected cats were collected using a Baermann funnel, and for each serum sample or PBS approximately 100 larvae were placed in a 1.5 ml microfuge tube containing 100 µl of a 1:8 dilution of serum in PBS. Tubes with larvae were incubated for 1 h at 37°C, centrifuged at 6000 g for 1 min, the supernatant removed and larvae washed three times in PBS with centrifugation. Then, 100 µl of a 1:15 dilution in PBS of fluorescein-isothiocyanate (FITC)-conjugated goat anti-cat IgG (MP Biomedicals) was added to the larvae in each tube. In the first experiment, the final dilution of the FITC antibody was 1:30; in the second and third experiments, the final dilution was 1:60. Larvae were again incubated for an hour at 37°C followed by three PBS washes. The larvae were then divided evenly into three tubes, with each new tube being assigned a new sample number to mask the original sample’s identity.
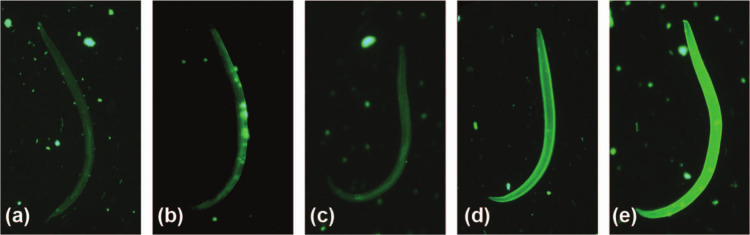
The fluorescence of larvae was scored by individuals masked as to test conditions. Aliquots (10 µl) from the tubes containing larvae were placed on slides under a coverslip and examined with a fluorescence microscope (Olympus BX41 with the U-TV1X-2 camera). The larvae seen on each slide were scored on a scale from 0 for no fluorescence to 5 for brightest fluorescence (Figure 2). In experiment one, only the first two larvae from each of three aliquots were scored, but, in the two successive experiments, all larvae seen on the slide were scored for fluorescence. Graphs and statistical comparisons [analysis of variance (ANOVA) with Tukey’s and Dunnett’s comparisons as appropriate] were performed using Minitab v 16.2.2.
Figure 2.
Representative larval images depicting the fluorescence intensity scale used in the indirect immunofluorescent antibody test [serum sample or phosphate buffered saline (PBS) treatment of the depicted larva is specified]. (a) Score of 1 (minimal fluorescence), larva incubated with serum from a cat experimentally infected with Ancylostoma braziliense (EXP-Ab); (b) score of 2, larva incubated with PBS; (c) score of 3, larva incubated with serum from a cat naturally infected with Aelurostrongylus abstrusus (NAT-Aa2); (d) score of 4, larva incubated with serum from a cat naturally infected with A abstrusus (NAT-Aa1); (e) score of 5, larva incubated with serum from a cat experimentally infected with A abstrusus (EXP-Aa). Score of 0 (ie, no fluorescence) is not represented here as the larva would not be visible using the fluorescein-isothiocyanate filter
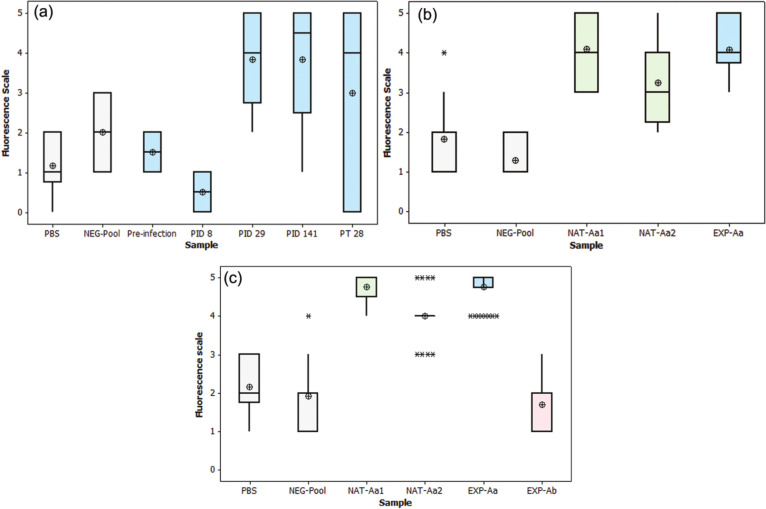
The results from three experiments are presented. In the first, fluorescence of larvae incubated with serum from an A abstrusus-infected cat (EXP-Aa) was compared with two negative controls: the first controlled for background fluorescence in the absence of antibody by incubating larvae in PBS only, and the second controlled for potential non-specific antibody binding through incubation with non-infected cat serum (NEG-Pool). Larvae incubated in sera from EXP-Aa had greater fluorescence than those from the PBS control or NEG-Pool serum. An ANOVA with Dunnett’s comparisons showed that fluorescence of larvae incubated in sera from EXP-Aa collected PID 29 and 141 was significantly increased (P <0.05; Figure 3a) compared with those incubated in either PBS or NEG-Pool; sera collected PID 8 and 176 (28 days post-treatment) were not significantly different from the two controls. In the second experiment (Figure 3b), larvae incubated in sera from NAT-Aa1, NAT-Aa2 and EXP-Aa (PID 141) were found by ANOVA with Tukey’s multiple comparisons at P <0.05 to have significantly higher levels of fluorescence than larvae treated with NEG-Pool or PBS. The third experiment assessed the potential for cross reactivity of this test with antibodies from the cat EXP-Ab infected with A braziliense. In this assay, larvae incubated with sera from NAT-Aa1, NAT-Aa2 and EXP-Aa on PID 141 were significantly more fluorescent than were larvae incubated with sera from EXP-Ab, Neg-Pool or PBS (ANOVA with Tukey’s multiple comparisons, P <0.05) (Figure 3c).
Figure 3.
Box and whisker plots of larval fluorescence after incubation with sera or phosphate buffered saline (PBS). The horizontal line within each box represents the median of the six readings (absent if there are only two integer readings), and the ⊕ symbol indicates the mean. Negative controls consisted of PBS and pooled serum from 100 specific pathogen-free cats (NEG-Pool). Fluorescence resulting from incubation of larvae with (a) serum from a cat experimentally infected with Aelurostrongylus abstrusus, collected before infection, on post-infection days (PID) 8, 29 and 141, and on post-treatment (PT) day 28 (PID 176); (b) serum from the experimentally infected cat (Exp-Aa) collected PID 141 and from two naturally infected cats (NAT-Aa1 and NAT-Aa2); (c) serum from NAT-Aa1, NAT-Aa2 and EXP-Aa (PID 141), and from a cat experimentally infected with the hookworm Ancylostoma braziliense (EXP-Ab)
This is only the second report, of which we are aware, where IFAT has been used to examine the blood of infected cats for antibodies to A abstrusus. A previous study examined the ability of the sera from naturally infected cats to react with third-stage larvae recovered from snails and fixed in PBS containing 10% acetone. 6 In that assay, sera collected at euthanasia from 70 cats were examined; 24 of these cats were known to have active A abstrusus infections (four of these had been experimentally infected 3 or 4 weeks before euthanasia). All 24 A abstrusus-infected cats gave a positive reaction in the assay. There were 13 other cats that gave positive reactions, 10 of which had pulmonary lesions, such as medial hypertrophy of the pulmonary arteries, that could have been caused by A abstrusus infections. Sera from 14 cats with Toxocara cati, four cats with Taenia taeniaeformis and one cat with both of these parasites did not react in the assay, nor did sera from 14 non-parasitized cats. The assay described herein does require a cat as a source of first-stage larvae, but does not require growing third-stage larvae in snails. Future work could determine if the assay would work with fixed or frozen larvae to allow for storage of this diagnostic reagent.
For the purpose of detecting antibodies in the serum or plasma of cats, another approach would be to develop an enzyme-linked immunosorbent assay (ELISA) using excretory–secretory fluids or somatic antigens from the larvae. There have been considerable strides made in the detection of Angiostrongylus vasorum, a relative of A abstrusus that lives in the pulmonary vasculature of dogs, using the ELISA methodology. 7 With A vasorum, recent comparisons of serum ELISAs have been reported for adult excretory–secretory, first-stage larval somatic, and adult somatic antigens, the latter including a crude antigen and two others that were purified using different monoclonal antibodies to A vasorum. 7 The ELISAs with all five antigens had similarly high sensitivities (88-100%) when used with sera from experimentally infected dogs; however, when using sera from naturally infected dogs, the sensitivity of the first-stage somatic antigen dropped to 42.9% (range 21.8-66.0%) while the adult antigens had sensitivities of 76.2–85.7%. All tests were similarly specific when using sera from dogs with experimental Ancylostoma caninum infections as a negative control. However, when the assays were compared using canine sera from verified natural infections, cross reactions occurred with the non-antibody-purified antigens, with the first-stage somatic antigen having false-positive results in 2/9 Crenosoma vulpis-, 2/20 Dirofilaria immitis-, 4/5 Dirofilaria repens- and 1/6 Eucoleus aerophilus-infected dogs. Similar cross reactions were noted in testing a serum ELISA for feline heartworm infection. 8 This ELISA was highly sensitive in experimentally infected cats (positive in 80% and 100% of cats, respectively, 2 months and 4–9 months post-infection), but, again, an examination of sera from cats experimentally infected with other agents revealed some cross reaction with the following: T taeniaeformis (16/92, 17.4%), T cati (5/28, 17.9%), and Ancylostoma tubaeforme (1/20, 5%). Thus, it may be worth applying ELISA technology to the A abstrusus system, but it will need to be carefully examined for potential cross reactivity using sera from cats infected with other parasites.
Assays that could supersede the need for an immunologic test might be one of the many current molecular methods. A nested polymerase chain reaction (PCR) performed on DNA extracted from pharyngeal swabs found that 6/50 cats suspected of having A abstrusus infection were positive by PCR as well as the Baermann assay; five cats were positive by swab PCR but negative by Baermann assay. 9 A real-time PCR for the detection of A vasorum was compared with diagnosis by serum ELISA with a soluble adult female antigen and by Baermann assay on 148 dogs with suspected A vasorum infection. 10 Of 31 animals positive in at least one of these three assays, the greatest number of infected animals was detected by ELISA (24), followed by Baermann (20) and blood PCR (18). There were 28 dogs that were positive by either blood PCR or ELISA, and 28 that were positive by the combination of ELISA and Baermann results.
Conclusions
Under the conditions of this preliminary investigation, it was shown that an IFAT may be useful for the detection of A abstrusus antibodies. It was also shown that no cross reaction was detected using serum from a cat infected with the hookworm A braziliense. If the IFAT was to be used more broadly, it would require validation using sera from cats with other helminth infections.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this short communication.
The authors do not have any potential conflicts of interest to declare.
Accepted: 15 April 2013
References
- 1. Conboy G. Helminth parasites of the canine and feline respiratory tract. Vet Clin North Am Small Anim Pract 2009; 39: 1109–1126. [DOI] [PubMed] [Google Scholar]
- 2. Lucio-Forster A, Bowman DD. Prevalence of fecal-borne parasites detected by centrifugal flotation in feline samples from two shelters in upstate New York. J Feline Med Surg 2011; 13: 300–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lacorcia L, Gasser RB, Anderson GA, Beveridge I. Comparison of bronchoalveolar lavage fluid examination and other diagnostic techniques with the Baermann technique for detection of naturally occurring Aelurostrongylus abstrusus infection in cats. J Am Vet Med Assoc 2009; 235: 43–49. [DOI] [PubMed] [Google Scholar]
- 4. Ishii A, Kamiya M. Indirect fluorescent antibody test in experimental infection of Angiostrongylus cantonensis in rats. Jap J Exp Med 1973; 43: 17–23. [PubMed] [Google Scholar]
- 5. Liang HK, Jin XX, Feng ZM, et al. Detection of serum antibodies in rats infected with Angiostrongylus cantonensis by the indirect fluorescent antibody test. Chin J Parasit Parasit Dis 1993; 11: 286–287. [PubMed] [Google Scholar]
- 6. Hamilton JM, Roberts RJ. Immunofluorescence as a diagnostic procedure in lungworm disease of the cat. Vet Rec 1968; 83: 401–403. [DOI] [PubMed] [Google Scholar]
- 7. Schucan A, Schnyder M, Tanner I, et al. Detection of specific antibodies in dogs infected with Angiostrongylus vasorum. Vet Parasitol 2012; 185: 216–224. [DOI] [PubMed] [Google Scholar]
- 8. McCall JW, Supakorndej JW, Ryan W, Soll MD. Utility of an ELISA-based antibody test for detection of heartworm infection in cats. In: Soll MD, Knight DH. (eds). Proceedings of the heartworm symposium ‘95, Auburn, AL, USA, 31 March–2 April 1995, pp 127–133. Batavia: American Heartworm Society. [Google Scholar]
- 9. Traversa D, Iorios R, Otranto D. Diagnostic and clinical implications of a nested PCR specific for ribosomal DNA of the feline lungworm Aelurostrongylus abstrusus (Nematoda, Strongylida). J Clin Microbiol 2008; 46: 1811–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jeffries R, Morgan ER, Helm J, et al. Improved detection of canine Angiostrongylus vasorum infection using real-time PCR and indirect ELISA. Parasitol Res 2011; 109: 1577–1583. [DOI] [PubMed] [Google Scholar]