Abstract
Novel treatment alternatives for feline ureteral obstruction(s) include placement of a double pigtail ureteral stent and a subcutaneous ureteral bypass (SUB) device. This study evaluated parameters for the prediction of hospitalization times, peri-operative survival, renal recovery and long-term survival in cats with benign ureteral obstructions after successful decompression with either a ureteral stent or SUB device. The medical records of 41 cats treated for benign ureteral obstruction(s) were retrospectively reviewed. Preoperative historical, biochemical and imaging parameters, along with intra- and postoperative biochemical parameters and complications were evaluated for predictors of hospitalization length, survival to discharge, 3-, 6- and 9-month post-procedure creatinine, and overall survival time. All patients had successful decompression of their renal pelvis. Hospitalization time was positively associated with presenting creatinine, perioperative complications, post-procedure creatinine and potassium, but was negatively associated with post-procedure sodium. No parameters were associated with survival to discharge. A higher creatinine at discharge was positively associated with a higher creatinine at follow-up. A decreased overall survival was associated with a higher presenting blood urea nitrogen, higher creatinine at hospital discharge and in overhydrated patients during hospitalization. Cats with International Renal Interest Society stage 1 and 2 kidney disease, versus stage 3 and 4, at 3 months and 6 months post-procedure, lived longer. Cats with ureteral obstruction(s) treated with a ureteral stent or SUB device had an overall good survival and no admitting parameter was associated with survival to discharge. No single parameter was associated with all outcomes in this study, making predicting patient survival and cost prior to ureteral decompression difficult.
Introduction
Feline ureteral obstructions are being recognized more frequently in recent years with the most common cause being an intraluminal obstruction secondary to ureteral calculi.1,2 Other causes include ureteral strictures, neoplasia, mucus plugs, dried solidified blood stones, fibrosis and surgical trauma.1,3–6 Historically, options for treatment of feline ureteral obstructions were restricted to either medical management, most commonly consisting of intravenous fluids, alpha blockade and diuretics, or surgical correction via a ureterotomy, ureteral resection and anastomosis, ureteronephrectomy or ureteroneocystostomy. 1 Medical treatment, while non-invasive, is not usually successful in resolving the obstruction (8–17%).4,7 On the contrary, surgical intervention is more effective, though it is associated with a high morbidity and mortality rate.2,4 The most common surgical complications include a uroabdomen from leakage at the surgical site, persistent or recurrent ureteral obstruction (from nephrolith migration, failure to remove all obstructive stones or surgical edema), or postsurgical stricture formation.1,2,4,7 The perioperative mortality rate has been reported to be 18–39% for cats with stone disease, depending on the procedure(s) being performed, and the expense is often high and variable.1,2,4
More recently the placement of feline double pigtail ureteral stents (Vet Stent, Infiniti Medical) or a subcutaneous ureteral bypass (SUB) device (Norfolk Vet) has allowed for novel treatment options for cats with ureteral obstructions that failed medical management or have conditions difficult to address with traditional surgery.5–7 These devices have the potential to relieve obstructions while theoretically avoiding some of the complications seen with traditional surgical interventions. This can provide an option for patients with complicated obstructions, such as those with multiple ureteroliths, proximal strictures, concurrent nephroliths or non-resectable tumors. 7 Currently, the use of these devices is the mainstay of treatment for feline ureteral obstructions in our practice.
Because treating feline ureteral obstructions can be difficult, time-consuming and expensive, predicting which cats will have a good short- and long-term outcome is important. Finding predictors of hospitalization times, survival to discharge and long-term renal recovery expectations would be ideal for both veterinarians and clients.
The purpose of this study was to evaluate pre-, peri- and postoperative parameters for the prediction of outcome and renal recovery in cats treated for a benign ureteral obstruction (stones, strictures, plugs). Outcome was defined as hospitalization time, survival to discharge, creatinine and associated International Renal Interest Society (IRIS) stage at 3, 6 and 9 months after the procedure, and overall survival time.
Materials and methods
Case selection
The medical records of all cats treated with a double pigtail ureteral stent or SUB device for either a benign partial or complete ureteral obstruction at the Animal Medical Center (AMC) from September 2009 to December 2010 were reviewed. Obstruction was initially diagnosed via abdominal ultrasound (AUS) based on concurrent hydroureter and hydronephrosis, and subsequently confirmed via antegrade pyelography immediately prior to stent or SUB placement in all cats. Antegrade pyeloureterography allowed characterization of either a complete or partial ureteral obstruction. Each patient was treated by the authors (AB and/or CW) both pre-, intra- and postoperatively, with a similar standardized algorithm. Cats were excluded if there was insufficient medical data for all required parameters, their obstruction was secondary to neoplasia or if there was less than 6 months postoperative follow-up available for those cats who were alive. Device placement was considered successful if short- and long-term renal ultrasound showed decompression and/or improvement in renal pelvis diameter compared with preoperative measurements (typically, stented pelvis 2–5 mm; SUB pelvis 2–7 mm). Animals with successful decompression were then compared for predictors of outcome.
A double pigtail ureteral stent (Vet Stent, Infiniti Medical) is a 2.5-French polyurethane catheter that extends from the affected renal pelvis, inside the ureteral lumen and into the urinary bladder, bypassing the ureteral obstruction and providing drainage of the renal collection system. A subcutaneous ureteral bypass device (SUB) (Norfolk Vet) is a bypass system that connects a locking loop pigtail nephrostomy tube to a cystostomy tube using a subcutaneous access port (Figure 1). 7 During this study period a ureteral stent was typically placed for ureterolith-induced obstruction(s) or if the dilated renal pelvis was <5 mm in diameter, and a SUB was typically placed for a stricture-induced obstruction or when stent placement was not possible.
Figure 1.
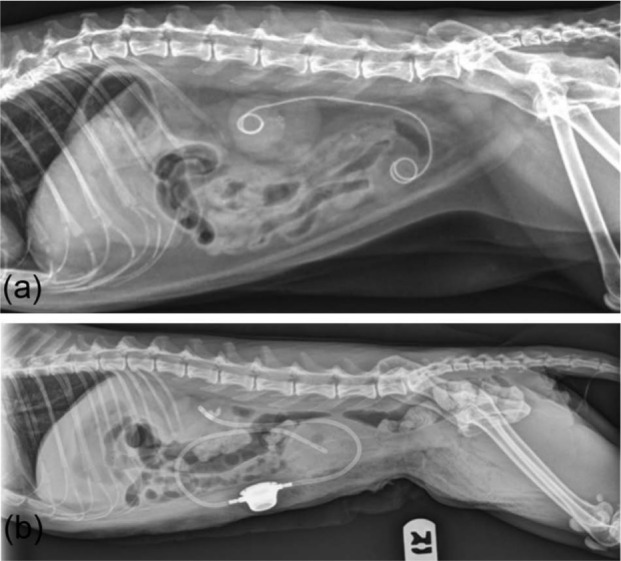
Lateral radiographs of cats treated with a ureteral stent (a) and subcutaneous ureteral bypass device (b)
Preoperative data obtained from the medical records included age; sex; breed; a known history of chronic kidney disease; a known history of a ureteral obstruction; if the ureteral obstruction was unilateral or bilateral; duration of obstruction (determined from the first imaging study documenting obstruction until presentation to the AMC); the need for preoperative intermittent hemodialysis (IHD) or nephrostomy tube placement; complete blood count and serum chemistry values at presentation; measurement of ultrasonographic renal pelvic dilation (in the transverse plane) of the affected kidney; ureteral dilation of the affected side; suspected cause of ureteral obstruction based on imaging; presence of abdominal effusion on imaging studies; presence of nephrolithiasis on abdominal radiographs (AXR) or AUS; and the presence or absence of chronic kidney changes on ultrasound images. Intraoperative data included the procedure performed (stent or SUB), if treatment was unilateral or bilateral, the cause of ureteral obstruction based on surgical inspection, anesthesia time, procedure time and intraoperative complications. Stricture classification was guided by clinical suspicion based on surgical palpation, ultrasound and radiograph findings, and the ureteropyelogram. Dried solidified blood stone identification was based on stone analysis or the identification of bloody stone debris during intraoperative assessment. Perioperative data (within 7 days of device placement) included complications, serum biochemical values obtained in the immediate postoperative period and daily until discharge, rate of urine production when a urinary catheter was placed, development of overhydration (pleural effusion, pulmonary edema, peripheral edema, chemosis, etc), the number of days of hospitalization and whether the patient survived to discharge. Postoperative data obtained included short-term (between 7 and 30 days after the procedure) and long-term (> 30 days after the procedure) complications, measurement of ultrasonic renal pelvic dilation (transverse) of the affected kidney(s), serum blood urea nitrogen (BUN) and creatinine at 3, 6 and 9 months after the procedure, and the overall survival time.
Statistical analysis
Pre- and perioperative biochemical and imaging parameters were evaluated and compared to determine parameters associated with survival to discharge, length of hospitalization, IRIS stage at 3, 6 and 9 months (stage 1 creatinine <1.6 mg/dl, stage 2 creatinine 1.6–2.8 mg/dl, stage 3 creatinine 2.9–5.0 mg/dl, and stage 4 creatinine >5.0 mg/dl), and overall survival. Owing to the small sample size, for the purposes of analyzing the IRIS stage, in all calculations except those involving complications, cats were divided into two groups: those with IRIS stage 1 and 2 chronic kidney disease, and those with IRIS stage 3 and 4 chronic kidney disease (sub-stages not evaluated).
The Mann–Whitney test comparing hospitalization time and creatinine concentrations at 3, 6 and 9 months postoperatively with groups with two categories was used [treatment with stent vs SUB, presence or absence of nephrolithiasis on AUS or AXR, AUS abdominal effusion, cystolithiasis or bladder sediment on AUS, presence of cystoliths on AXR, alive or dead status at discharge, presence or absence of complications (intraoperative, perioperative, short-term and long-term), if a mannitol continuous rate infusion (CRI) was used postoperatively, overhydration during hospitalization and treatment with intermittent hemodialysis]. The Kruskal–Wallis test was used to compare groups with three or more categories (left vs right vs bilateral treatment, and IRIS stage at 3, 6 and 9 months) and to compare continuous variables such as the number of days hospitalized after procedure, 3-, 6- and 9-month creatinine values, anesthesia time (min), procedure time (min), lowest and highest creatinine, sodium (Na), potassium (K) and lowest ml/kg/h urine.
Spearman correlation coefficients were used to assess the relationship between hospitalization time or 3-, 6- and 9-month creatinine values, and other continuous variables such as presenting creatinine; BUN, Na, K and packed cell volume; ipsilateral AUS renal pelvic size; ipsilateral AUS ureteral size; anesthesia time; procedure time; lowest and highest creatinine; Na; K; and lowest ml/kg/h urine. The Fisher’s exact test was used to determine the association between categorical variables.
The analysis of long-term survival was accomplished using standard methods of survival analysis, ie, computing the Kaplan–Meier product limit curves, where the data were stratified by the different categorical variables described above. In cases where death had not yet occurred, the number of days until last follow-up was used to calculate survival time, and the case was considered censored. The groups were compared using the log-rank test. Median survival times were reported with their corresponding 95% confidence intervals, 8 using Greenwood’s formula to calculate the standard error. 9 Cox proportional hazards models were used to determine the relationship of the survival distribution to other continuous variables.
In order to ensure conservatism in the interpretation of the data due to many statistical tests being performed, we considered a result significant at the P <0.01 level of significance. All analyses were conducted using SAS version 9.2.
Results
Forty-one cats with a partial or complete ureteral obstruction(s) were treated using a double pigtail ureteral stent or a SUB device at the AMC from September 2009 to December 2010. One cat was excluded from the study owing to an incomplete medical record. Another cat was included in the study population twice owing to placement of a stent and a SUB in different ureters during separate hospitalizations within the study period; this cat was only counted once for overall survival statistics. In the final study analysis there was a total of 41 cats and 48 ureters. The patients included 16 neutered males and 25 spayed females with a median age of 9.6 years (range 2.6–15.4 years). Twenty-seven cats were treated with double pigtail ureteral stents and 14 with a SUB device. Thirty-four cats had one ureter treated and seven cats (17%) had bilateral treatment during the study period; of these bilateral treatments, six cats had bilateral stents placed and one cat had bilateral SUBs placed.
Twenty-three cats (56%) had a known history of chronic kidney disease of at least 6 months. Eighteen cats (44%) had a duration of documented ureteral obstruction of 1 week or less. Five cats (12%) had a duration of documented obstruction between 1 and 2 weeks. Four cats (10%) had a duration of documented obstruction between 2 weeks and 1 month, and 13 cats (32%) for longer than 1 month. One cat’s duration of obstruction was not documented in the record.
All cats had an AUS that revealed hydronephrosis and hydroureter before their procedure. The median pelvic dilation of the affected kidney was 11 mm (range 2–26 mm) and the median ipsilateral ureteral dilation was 3.1 mm (range 1–19 mm). Nineteen cats out of 33 (58%) in which renal images and/or a report evaluating nephroliths were available for review had documented nephrolithiasis. Twenty-eight cats had AXR taken before their procedure; 19 of those cats (68%) had radiographic evidence of nephrolithiasis.
Of the 48 obstructed ureters treated, 25 (52%) were associated with ureterolithiasis, 12 (25%) were associated with a suspected stricture, seven (15%) were associated with both ureterolithiasis and a suspected stricture, three (6%) were associated with numerous suspected dried solidified blood stones, and one (2%) was associated with a suspected stricture and dried solidified blood stones. Five cats (12%) had IHD before their procedure. One cat had an emergency nephrostomy tube placed at AMC during the study period.
The median BUN and creatinine concentration of cats with a ureteral obstruction at presentation was 80 mg/dl (range 25–267 mg/dl; reference interval RI 15–34 mg/dl) and 5.25 mg/dl, (range 1.4–20.3 mg/dl; RI 0.8–2.3 mg/dl), respectively. The median potassium at presentation was 4.9 mEq/l (range 3.1–8.8 mEq/l; RI 3.4–5.6 mEq/l).
No pre-operative historical, clinical, biochemical or imaging parameters were identified that were significantly associated with survival to discharge. Preoperative parameters significantly associated with hospitalization time included creatinine at presentation (rho = 0.53, P = 0.008) and BUN at presentation (rho = 0.54, P = 0.006). Pre-operative parameters that did not reach statistical significance for hospitalization time and survival to discharge are listed in Tables 1 and 2, respectively.
Table 1.
Parameters investigated for prediction of hospitalization time that did not achieve statistical significance
| Predictor | Correlation (rho) | P |
|---|---|---|
| Procedure type (stent vs SUB) | N/A | 0.1639 |
| Unilateral vs bilateral ureteral treatment | N/A | 0.0815 |
| Potassium at presentation | 0.40 | 0.0152 |
| Pelvic dilation of affected kidney | 0.22 | 0.1990 |
| Ureteral dilation of affected side | −0.29 | 0.1364 |
| Presence of nephrolithiasis on AUS | N/A | 0.4574 |
| Evidence of CKD on AUS | N/A | 0.3961 |
| Dialysis pre-procedure | N/A | 0.0174 |
| Intraoperative complications | N/A | 0.1465 |
| Fluid overload during hospitalization | N/A | 0.0349 |
SUB = subcutaneous ureteral bypass device; AUS = abdominal ultrasound; CKD = chronic kidney disease; N/A = not applicable; statistical significance = P <0.01
Table 2.
Parameters investigated for prediction of survival to discharge that did not achieve statistical significance
| Predictor | Cats that survived | Cats that died | P |
|---|---|---|---|
| Procedure type (stent vs SUB) | 24 stent 11 SUB |
3 stent 2 SUB |
1.00000 |
| Unilateral vs bilateral ureteral treatment | 28 unilateral 7 bilateral |
5 unilateral 0 bilateral |
0.1772 |
| Presenting creatinine concentration (median; mg/dl) | 4.7 | 7.6 | 0.24322 |
| Pelvic dilation of affected kidney (median; mm) | 11 | 11.75 | 0.67752 |
| Ureteral dilation of affected side (median; mm) | 3.25 | 2.10 | 0.28369 |
| Presence of nephrolithiasis on AUS | 17 yes 12 no |
2 yes 2 no |
1.00000 |
| Evidence of CKD on AUS | 11 yes 20 no |
1 yes 3 no |
1.00000 |
| Dialysis pre-procedure | 4 yes 31 no |
1 yes 4 no |
0.5066 |
| Intraoperative complications | 1 yes 34 no |
1 yes 4 no |
0.2372 |
| Perioperative complications | 4 yes 31 no |
3 yes 2 no |
0.0299 |
| Fluid overload during hospitalization | 5 yes 29 no |
3 yes 2 no |
0.0491 |
CKD = chronic kidney disease; AUS = abdominal ultrasound; N/A = not applicable; statistical significance = P <0.01
Three cats (7%) had intraoperative complications, consisting of ureteral guidewire puncture (n = 1), a bladder stay-suture tear (n = 1) and renal puncture with the guidewire (n = 1). Two of these complications were in cats treated with a ureteral stent and one with a SUB. These complications were relatively minor and did not interfere with procedure completion.
Seven cats (17%) had perioperative complications. Six of these cats were treated with a SUB; the most common complication was urine leakage secondary to nephrostomy tube leakage (two), nephropexy failure (one), nephrostomy tube migration (one) and leakage around the SUB port (one). Of these cats, 4/5 underwent a second anesthesia for revision of the device and all leakage stopped at this time. One cat with nephrostomy tube migration had a closed suction drain placed and the owners elected euthanasia owing to failure of creatinine improvement. One cat had a urethral catheter inadvertently cut during removal 2 days postoperatively, necessitating endoscopic retrieval. The remaining cat had occlusion of the SUB catheter with a blood clot and localized tissue plasminogen activator was infused through the SUB port, relieving the clot.
All cats had successful decompression of their ureter and renal pelvis based on serial ultrasound evaluations during hospitalization and at follow-up evaluations. Six cats (15%) had minimal short-term complications not requiring additional procedures; four of these cats were treated with ureteral stent and two with a SUB. All short-term complications involved lower urinary tract signs (stranguria or pollakiuria), and all signs improved with medical management (alpha-blockade or corticosteroids). Four cats (10%) had long-term complications associated with their procedure (all stented cats), including vesiculoureteral reflux (n = 1), stent migration (n = 1) and recurrent lower urinary tract signs (n = 2).
The median creatinine was 4.3 mg/dl post-procedure at the time of extubation (range 1.1–16.8 mg/dl), 3.9 mg/dl (range 1.1–16.1 mg/dl) 10–18 h post-procedure and 3.1 mg/dl (range 1.0–11.8 mg/dl) the day before discharge. The median K and Na 1-day post-procedure were 4.65 mEq/l (range 2.9–8.22 mEq/l) and 139 mEq/l (range 121–149.1 mEq/l; RI 145–158 mEq/l), respectively. Eight cats developed evidence of fluid overload during hospitalization (20%).
The median hospitalization time for all cats was 5 days [95% confidence interval (CI) 4–6]. Thirty-six of the 41 cats (88%) survived to discharge from the hospital. Five cats died during hospitalization owing to congestive heart failure (one), pancreatitis (two), euthanasia owing to lack of renal function improvement (one) and complications associated with renal transplantation when successful treatment of the ureteral obstruction did not improve the renal function (one). No cats died from persistent ureteral obstruction.
No intra- or postoperative parameters were significantly associated with survival to discharge (Table 2). Parameters associated with hospitalization time included the creatinine concentration immediately after the procedure (rho = 0.5, P = 0.0013), the creatinine concentration 1-day post-procedure (rho = 0.54, P = 0.0006), the potassium concentration 1-day post-procedure (rho = 0.33, P = 0.0005) and the presence of perioperative complications (P = 0.0069). Hyponatremia 1-day post-procedure was associated with a longer hospitalization time (rho = −0.56, P = 0.0005).
The median creatinine of cats postoperatively at 3 months was 2.4 mg/dl (range 1.6–10.0 mg/dl), at 6 months it was 2.4 mg/dl (range 1.4–10.4 mg/dl) and at 9 months it was 2.6 mg/dl (range 1.4–7.0 mg/dl).
The creatinine concentration before discharge was significantly positively associated with the creatinine at 3 (rho = 0.69, P = 0.0002), 6 (rho = 0.75, P <0.0001) and 9 (rho = 0.69, P = 0.0095) months after the procedure. The creatinine before discharge was significantly associated with IRIS stage at 3 months post-procedure (P = 0.0030), but was not significantly associated with IRIS stage at 6 (P = 0.0797) or 9 (P = 0.2532) months post-procedure. All other parameters evaluated were not statistically significant. Other non-statistically significant parameters are listed in Tables 3 and 4.
Table 3.
Parameters investigated for creatinine 3, 6 and 9 months post-procedure that did not achieve statistical significance
| Predictor | P (creatinine 3 months post-procedure) | P (creatinine 6 months post-procedure) | P (creatinine 9 months post-procedure) |
|---|---|---|---|
| Duration of ureteral obstruction | 0.4354 (rho = 0.15) | 0.4919 (rho = 0.14) | 0.5418 (rho = −0.18) |
| Pelvic dilation of affected kidney | 0.7113 (rho = −0.07) | 0.9817 (rho = 0.005) | 0.1294 (rho =0.49) |
| Ureteral dilation of affected side | 0.5834 (rho = −0.18) | 0.1789 (rho = −0.33) | 0.1500 (rho = 0.52) |
| Creatinine at presentation | 0.1553 (rho = 0.27) | 0.3126 (rho = 0.21) | 0.3302 (rho = 0.29) |
| Unilateral vs bilateral ureteral treatment | 0.4791 | 0.1925 | 0.9523 |
| Cause of obstruction | 0.1404 | 0.3449 | 0.8893 |
| Previous history of CKD | 0.2329 | 0.0832 | 0.0744 |
| Presence of nephrolithiasis on AUS | 0.1581 | 0.0684 | 0.1055 |
| Evidence of CKD on AUS | 0.2056 | 0.3308 | 0.5044 |
| Intraoperative complications | 0.4583 | 0.7968 | N/A |
| Perioperative complications | 0.2301 | 0.6704 | 0.1366 |
| Short-term complications | 0.8965 | 0.9501 | 0.2693 |
| Long-term complications | 0.2873 | 0.6564 | 0.6709 |
| Fluid overload during hospitalization | 0.4212 | 0.8849 | N/A |
| Dialysis pre-procedure | 0.4757 | 0.4623 | 0.7659 |
CKD = chronic kidney disease; AUS = abdominal ultrasound; N/A = not applicable; statistical significance = P <0.01
Table 4.
Parameters investigated for prediction of International Renal Interest Society (IRIS) stage at 3, 6 and 9 months post-procedure that did not achieve statistical significance
| Predictor | P (IRIS stage 3 months post-procedure) | P (IRIS stage 6 months post-procedure) | P (IRIS stage 9 months post-procedure) |
|---|---|---|---|
| Duration of ureteral obstruction | 0.3199 | 0.2541 | 0.3221 |
| Pelvic dilation of affected kidney | 0.6554 | 0.6192 | 0.2850 |
| Ureteral dilation of affected side | 0.8082 | 0.8669 | 0.1159 |
| Creatinine concentration at presentation | 0.3539 | 0.1571 | 0.4918 |
| Unilateral vs bilateral ureteral treatment | 0.5553 | 0.7231 | 0.4289 |
| Cause of obstruction | 0.9233 | 0.7996 | 0.5746 |
| Previous history of CKD | 0.2113 | 0.3222 | 0.5790 |
| Presence of nephrolithiasis on AUS | 0.2425 | 0.4352 | 1.00000 |
| Evidence of CKD on AUS | 0.2794 | 0.7673 | 0.7273 |
| Intraoperative complications | 0.3488 | 1.00000 | N/A |
| Perioperative complications | 0.7844 | 0.6034 | 0.1923 |
| Short-term complications | 1.00000 | 0.6584 | 0.4860 |
| Long-term complications | 0.4276 | 0.4724 | 1.00000 |
| Dialysis pre-procedure | 1.00000 | 1.00000 | 1.00000 |
CKD = chronic kidney disease; AUS = abdominal ultrasound; N/A = not applicable; statistical significance = P <0.01
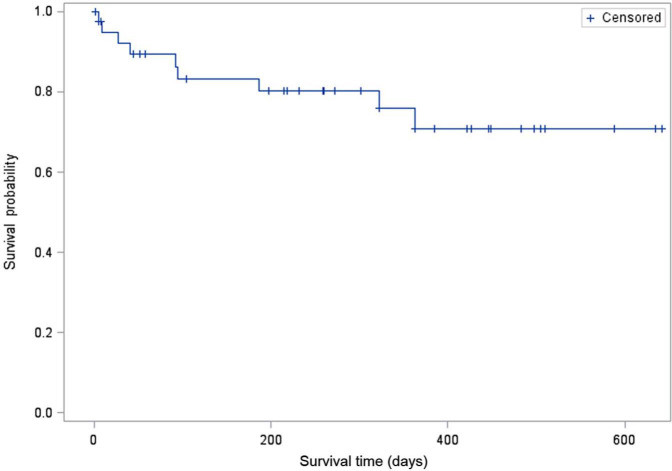
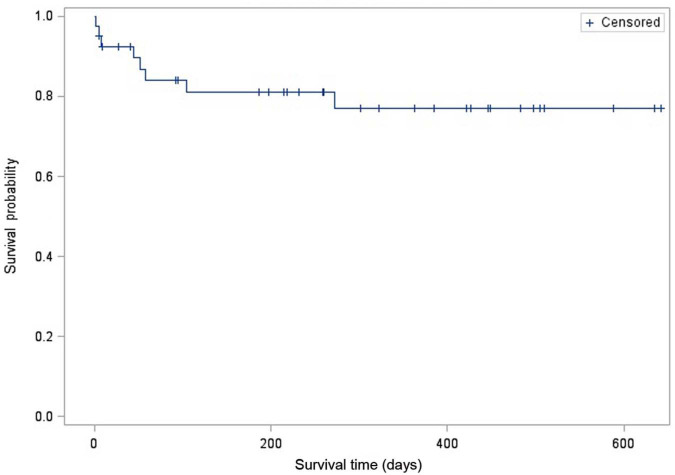
Twenty-three cats (58%) were alive at the end of the study period. Sixteen cats were euthanased and one cat died during the study period. Twelve cats were discharged from the hospital after their procedure and five died perioperatively prior to discharge, as discussed earlier. Of those 12 cats that died or were euthanased after discharge, seven died from progressive clinical signs associated with chronic kidney disease (18%), two from neoplasia (5%), one from refractory seizures (3%), one from pancreatitis (3%) and one weakness of unknown etiology (3%). All recovered from their ureteral surgery prior to the development of these ailments. No cat died as a result of a known recurrent or persistent ureteral obstruction. Owing to a less than 50% overall mortality, a median survival time for cats during the study period was not reached. When cats were evaluated based on their cause of death (definitely or likely renal versus unlikely and definitely not renal), the median survival times for both groups were still not met (Figures 2 and 3).
Figure 2.
Kaplan–Meier survival curve of cats that died owing to definite renal or likely renal causes (n = 9). A median survival time was not reached
Figure 3.
Kaplan–Meier survival curve of cats that died owing to unlikely renal or definitely not renal causes (n = 8). A median survival time was not reached
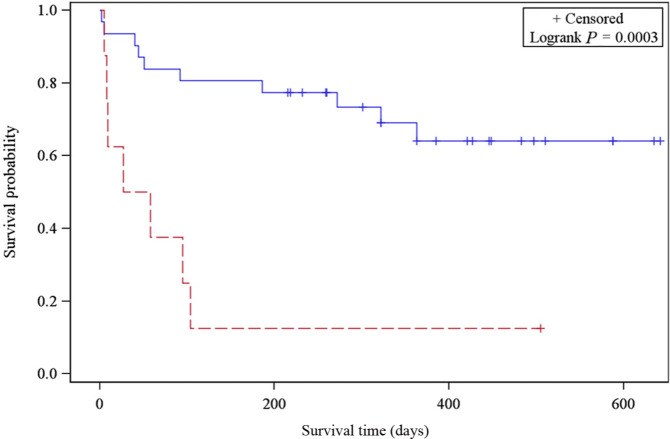
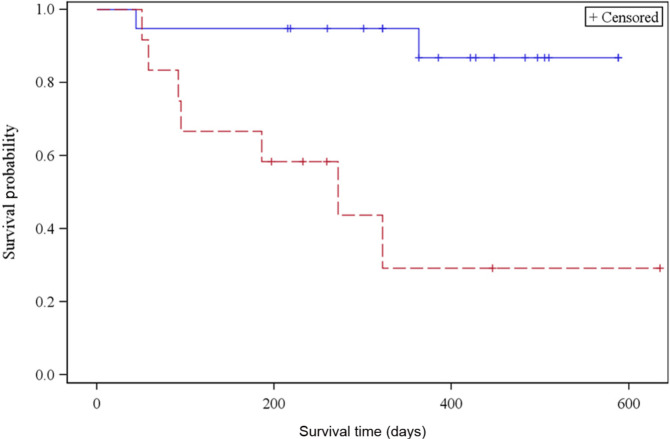
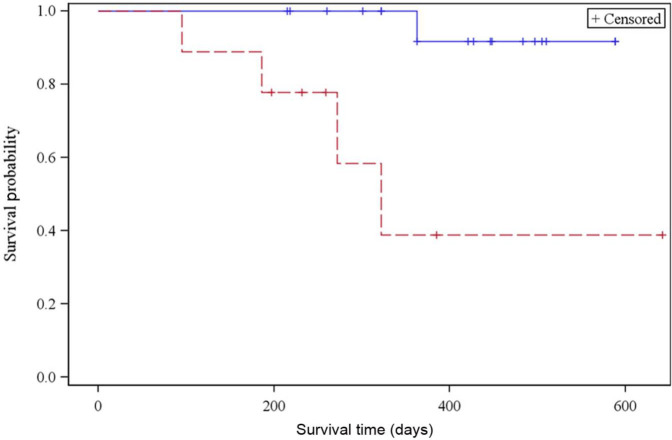
Predictors significantly associated with a decreased overall survival included creatinine concentration before hospital discharge [hazard ratio (HR) = 1.54, P <0.0001], presenting BUN (HR = 1.01, P = 0.0015) and overhydration during hospitalization (P = 0.0003, Figure 4). In addition, IRIS stage at 3 and 6 months post-procedure was associated with overall survival. Cats with IRIS stage 1 or 2 chronic kidney disease at 3 months post-procedure lived longer (median survival not reached) than cats with IRIS stage 3 and 4 chronic kidney disease (median survival time: 272 days; 95% CI: 58 d, indeterminate, P = 0.0014; Figure 5). Cats with IRIS stage 1 and 2 CKD also had significantly increased overall survival at 6 months post-procedure (P = 0.0024; Figure 6). Other parameters evaluated that did not reach statistical significance are listed in Table 5.
Figure 4.
Kaplan-–Meier survival curve of cats that did (dotted line) and did not (straight line) have fluid overload during hospitalization for treatment of ureteral obstruction
Figure 5.
Kaplan–Meier survival curve of cats with International Renal Interest Society (IRIS) stage 1 and 2 chronic kidney disease (CKD) (straight line) vs IRIS stage 3 and 4 CKD (dotted line) 3 months post-procedure
Figure 6.
Kaplan–Meier survival curve of cats with IRIS stage 1 and 2 CKD (straight line) vs IRIS stage 3 and 4 CKD (dotted line) 6 months post-procedure. CKD = chronic kidney disease; IRIS = International Renal Interest Society
Table 5.
Parameters investigated for prediction of overall outcome that did not achieve statistical significance
| Predictor | Hazard ratio | P |
|---|---|---|
| Creatinine concentration at presentation | 1.11 | 0.0251 |
| Potassium concentration at presentation | 1.43 | 0.04676 |
| Potassium concentration before hospital discharge | 2.44 | 0.0444 |
| Length of hospitalization | 1.16 | 0.039 |
| Procedure type (stent vs SUB) | N/A | 0.8524 |
| Unilateral vs bilateral ureteral treatment | N/A | 0.3619 |
| Duration of ureteral obstruction | 1.034 | 0.7430 |
| Cause of obstruction | N/A | 0.9674 |
| Previous history of CKD | N/A | 0.7184 |
| Pelvic dilation of affected kidney | 0.960 | 0.3646 |
| Ureteral dilation of affected side | 1.009 | P=0.9272 |
| Presence of nephrolithiasis on AUS | N/A | 0.3009 |
| Evidence of CKD on AUS | N/A | 0.8132 |
| Intraoperative complications | N/A | 0.6227 |
| Perioperative complications | N/A | 0.1808 |
| Short-term complications | N/A | 0.8611 |
| Long-term complications | N/A | 0.1600 |
| Dialysis pre-procedure | N/A | 0.8995 |
SUB = subcutaneous ureteral bypass device; CKD = chronic kidney disease; AUS = abdominal ultrasound; N/A = not applicable; statistical significance = P <0.01
Discussion
Cats with ureteral obstruction(s) that were treated with a ureteral stent or SUB device did not have any preoperative imaging, biochemical or historical parameters that predicted survival to discharge, and only BUN at presentation predicted long-term survival. This suggests that predicting patient outcome and ultimate cost, prior to ureteral decompression, is difficult. As this study evaluated a large number of different predictors we elected to use a cut-off of P <0.01 for our level of significance to attempt to minimize the number of false-positive results.
Previous studies have evaluated cats with ureteral obstruction secondary to ureterolithiasis treated with medical and surgical management. One study by Kyles et al 4 found increased morbidity and mortality in patients that received IHD or emergency nephrostomy tube placement. A more recent study failed to identify any predictors of mortality in a group of cats treated for ureteral obstruction after a ureterotomy. 2 The present study did not identify IHD as a significant negative prognosticator for any outcome evaluated; however, it was associated with a longer hospitalization time (P = 0.0174), which would correlate to a more expensive hospitalization. Owing to the low number of patients in this study that had IHD before their procedure (five cats, 12%), generalizations cannot be made. Cats with obstruction associated with more severe pelvic dilations or ureteral obstructions associated with significant electrolyte abnormalities, such as hyperkalemia, may have been more likely to require IHD for stabilization before their procedure, resulting in longer hospital times without affecting overall survival. Alternately, if an experienced surgical team is not immediately available, IHD may provide stabilization until a definitive procedure can be scheduled. As only one cat received emergency nephrostomy tube placement at AMC in this study we were unable to evaluate this as a predictor of outcome.
Creatinine concentration at various time-points was predictive of hospitalization time, IRIS stage at 3, 6 and 9 months post-procedure, and overall survival time. A higher creatinine concentration at presentation correlated with a longer hospital time, but did not significantly affect survival to discharge, postoperative creatinine concentrations or overall survival times; however, there was an association that approached significance between a higher creatinine at presentation and decreased overall survival (P = 0.0251). This may indicate that cats with a higher creatinine at presentation had a more severe obstruction and required longer hospitalization for postoperative diuresis, but had an equal chance of recovery long-term. Though these cats may have required longer initial management, they still were able to achieve renal recovery and outcome similar to cats with lower presenting creatinine concentration. Alternatively, the association of a higher creatinine at presentation and decreased overall survival, although not statistically significant, could indicate that cats with more severe obstructions may have suffered permanent renal damage resulting in decreased overall survival. However, there was no association between presenting creatinine and postoperative creatinine and associated IRIS stage, making the significance of the presenting creatinine unclear. These inconsistent findings may result from a small number of patients and warrant future investigation. Cats with higher creatinine concentrations before hospital discharge (median 5 days postoperatively) continued to have high creatinine concentrations at 3, 6 and 9 months post-procedure, as well as a decreased overall survival time.
Previous literature has shown that full renal recovery can take up to 4 months following resolution of ureteral obstruction in dogs.7,10,11 This was also seen in the cats of this study. Though the creatinine at the time of discharge seemed to approximate their long-term baseline creatinine, over the following few months improvements were still seen in many cats. In addition, the finding that creatinine before discharge, and IRIS stage at 3 and 6 months post-procedure, were associated with overall outcome is consistent with previous studies which have shown that baseline creatinine concentrations and IRIS stage are predictive of overall survival. 12
Fluid overload was significantly associated with overall outcome and was nearly significant for hospitalization time (P = 0.0349) and survival to hospital discharge (P = 0.0491). Fluid overload is a major concern in treating cats with ureteral obstructions. Because many cats may develop a brisk postobstructive diuresis, aggressive fluid therapy may be indicated. 7 While not significantly associated with long-term renal recovery in this study, fluid overload can potentially affect short (survival to discharge) and long-term (overall) survival. Cats that showed signs of fluid overload could be those with underlying cardiac disease, and this can then result in congestive heart failure postoperatively during fluid therapy. Care should be taken in managing patients with aggressive fluid therapy to avoid this condition. We routinely follow body weight, heart rate, heart rhythm, respiratory rates, respiratory efforts, oxygen saturation and central venous pressures postoperatively, and are very cautious to prevent overhydration during hospitalization.
Perioperative complications affected hospitalization time in this study, and were associated with a decreased survival to discharge (P = 0.0299). Although the perioperative complication rate (17%) was similar to that previously reported during traditional surgical intervention,2,4,7 the majority of these complications occurred in cats with a SUB device placed (6/7 cats), and were easily fixed when needed, compared with traditional surgery. Since the time of carrying out this study we have modified the SUB device with a renal capsular cuff, and, in an additional 60 cases where the new SUB device was placed, there has not been any documentation of nephrostomy leakage (A Berent, personal communication).
The intraoperative and short- and long-term complications were relatively minor in this study. They were not associated with increased overall mortality and the majority were addressed on an outpatient basis. Importantly, a second episode of ureteral obstruction was not seen as a short or long-term complication in this study, with all renal units being immediately decompressed successfully with both interventional techniques. This is a large concern with traditional surgical techniques, in which recurrent ureterolithiasis has been documented in up to 40% of cats. 4 This study period was short; thus, drawing conclusions about long-term complications beyond the time frame evaluated is difficult.
Although not statistically significant, there were a number of parameters evaluated in this study that did not affect outcome, but are considered to be clinically relevant. Creatinine concentrations postoperatively and overall outcome were not statistically different between cats with different causes of obstruction. Cats with evidence of ureteral obstruction of a longer duration or a larger renal pelvic dilation at presentation did not have statistically worse renal recovery or overall survival than cats with a shorter duration of obstruction or smaller pelvic dilation. Duration of obstruction was likely underestimated in cats in this study as duration of obstruction was determined based on confirmation of obstruction (hydroureter and hydronephrosis) by AUS. Cats may have been partially or completely obstructed prior to imaging studies without clinical signs or notable azotemia, especially if the obstruction was unilateral and no pre-existing renal disease was present. Cats treated for bilateral ureteral obstruction did not have longer hospitalization times, significantly different renal recovery, or worsened short or long-term survival compared with those treated unilaterally.
Ultimately, few patients died of renal-associated causes and no patients died of recurrent ureteral obstruction. When patients were evaluated based on survival with a renal cause of death, the median survival was not met.
There were a number of limitations to this study, including the retrospective design, the short inclusion time period and small case numbers. This study included two different treatment techniques for feline ureteral obstruction, regardless of both providing successful ureteral decompression. Because of this variable, outcomes may have varied with each technique, potentially affecting the results. Patient bloodwork was variably run on different laboratory machines within the hospital, which may have affected results. In addition, the majority of our study population was alive at the end of the study period (58%). This study analyzed a large number of variables that may have caused some false-positive results. Owing to this concern, as stated previously, we elected to use P <0.01 as our level of significance instead of the more traditional level of P <0.05. Additionally, this study was not aimed at comparing the various devices used for traditional surgical intervention, but instead to look at various parameters to predict outcome for owners after successful decompression so expectations can be appropriate.
Conclusions
This study evaluated clinical, biochemical and imaging parameters to predict hospitalization time, survival to discharge, renal recovery and overall survival in cats with ureteral obstructions treated with a ureteral stent or SUB device. While our data revealed a number of individual parameters that affected separate outcomes, there was not a single parameter that predicted all outcomes analyzed. Overall, patients treated for ureteral obstruction(s) with a stent or a SUB device had a high likelihood of survival to discharge and good long-term survival. Future studies with larger case numbers and longer follow-up times are indicated to further investigate these findings.
Footnotes
Information from this study was presented as an oral abstract at ACVIM 2012 in New Orleans, LA, USA
Funding: This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Drs Berent and Weisse are advisors for both Infiniti Medical, LLC, and Norfolk Vet Products, from whom both the ureteral stent and subcutaneous ureteral bypass device are distributed, respectively.
Accepted: 4 April 2013
References
- 1. Hardie EM, Kyles AE. Management of ureteral obstruction. Vet Clin North Am Small Anim Pract 2004; 34: 989–1010. [DOI] [PubMed] [Google Scholar]
- 2. Roberts SF, Aronson LR, Brown DC. Postoperative mortality in cats after ureterolithotomy. Vet Surg 2011; 40: 438–443 [DOI] [PubMed] [Google Scholar]
- 3. Kyles AE, Hardie EM, Wooden BG, et al. Clinical, clinicopathologic, radiographic and ultrasonographic abnormalities in cats with ureteral calculi: 163 cases (1984–2002). J Am Vet Med Assoc 2005; 226: 932–936. [DOI] [PubMed] [Google Scholar]
- 4. Kyles AE, Hardie EM, Wooden BG, et al. Management and outcome of cats with ureteral calculi: 153 cases (1984–2002). J Am Vet Assoc 2005; 226: 937–944. [DOI] [PubMed] [Google Scholar]
- 5. Berent AC. Ureteral interventions: A minimally invasive approach to diagnosis and treatment of ureteral disease. In: Proceedings of the American College of Veterinary Internal Medicine, San Antonio, TX, USA, 4–7 June 2008. [Google Scholar]
- 6. Zaid MS, Berent AC, Weisse C, et al. Feline ureteral strictures: 10 cases (2007–2009). J Vet Intern Med 2011; 25: 222–229. [DOI] [PubMed] [Google Scholar]
- 7. Berent AC. Ureteral obstructions in dogs and cats: A review of traditional and new interventional diagnostics and therapeutic options. J Vet Emerg Crit Care 2011; 21: 86–103. [DOI] [PubMed] [Google Scholar]
- 8. Lee ET. Statistical methods for survival data analysis. 2nd ed. New York: John Wiley, 1992. [Google Scholar]
- 9. Greenwood M. The errors of sampling of the survivorship table, vol 33 of reports on public health and medical subjects. London: Her Majesty’s Stationery Office, 1926, pp 23–25. [Google Scholar]
- 10. Douglas R, Wilson MD. Renal function during and following obstruction. Annu Rev Med 1997; 28: 329–339. [DOI] [PubMed] [Google Scholar]
- 11. Wen JG, Frøkiær J, Jorgensen TM, et al. Obstructive nephropathy: an update of the experiment research. Urol Res 1999; 27: 29–39. [DOI] [PubMed] [Google Scholar]
- 12. Boyd LM, Langston C, Thompson K, et al. Survival in cats with naturally occurring chronic kidney disease (2000–2002). J Vet Intern Med 2008; 22: 1111–1117. [DOI] [PubMed] [Google Scholar]