Abstract
A 6-year-old neutered female cat was examined for chronic and progressive pelvic limb ataxia that progressed to non-ambulatory paraparesis over 1 month. Haematological and serum analyses were mainly within normal ranges. Thoracic and abdominal radiographs did not reveal any morphological abnormalities. Magnetic resonance imaging investigation of the thoraco-lumbar spine demonstrated a well-defined, extradural mass that extended into the epidural space from the L2 to L3 vertebral bodies and expanded in the L2 to L3 left intervertebral foramen. During surgery, a long, narrow, white parasite which was weakly adherent to the phlogistic epidural fat tissue was gently removed from the spinal canal. Histological examination of the pathological tissue supported a diagnosis of epidural steatitis surrounding a female adult Dirofilaria immitis. This is a novel case of natural D immitis infection with spinal localisation in a cat, well documented with magnetic resonance investigation, and cytological and histological examinations, introducing a novel differential diagnosis for extradural spinal masses in cats.
Case Report
The dog serves as a suitable host for Dirofilaria immitis; cats can also be infected, but are considered imperfect hosts for heartworms, and the infection prevalence is lower in felines than in dogs living in the same area. 1 In northern Italy, which is one of the largest endemic areas for canine dirofilariosis, positive antibody titre to D immitis was detected in 10/221 cats (4.7%), and the prevalence of feline heartworm disease was roughly estimated to be 10% of the prevalence in dogs. 2 In cats infected with D immitis that do not die suddenly without premonitory signs, the most common clinical signs stem from the development of a syndrome known as heartworm associated respiratory disease, although vomiting and neurological signs are also reported.3–5 The pathogenesis of vomiting is unknown, but neurological signs are attributed to aberrant migrations.6–8 Considering the relative prevalence rates of D immitis infections in dogs and cats, vomiting and neurological clinical signs occur more frequently in cats, and heartworms can be found in body cavities, systemic arteries and the central nervous system (CNS).9–11 Overall, seven cases of natural D immitis infections of the CNS have been described in cats; parasites were detected in the brain, the anterior cerebral artery, the lateral ventricle of brain, and in the cerebellar vermis. 7 However, while aberrant epidural localisation of D immitis has been described in dogs, it has not been previously reported in cat.12–15
A 6-year-old neutered female cat was examined for chronic and progressive pelvic limb ataxia that had progressed to non-ambulatory paraparesis over a period of 1 month. Physical examination of the pelvic limbs revealed bilateral hypomyotrophy and mild muscle tone loss. Hindlimb postural reactions were decreased to absent, and this was more pronounced on the left side. Spinal reflexes were normal in all four limbs. Cross-extensor reflex was present in the hind limbs. There were no alterations of forelimb postural reactions. Pain was detected in response to palpation of the cranial lumbar region (L1–L3).
Haematological and serum biological analysis were largely within reference intervals (RI), except haematocrit and red blood cell count, which were 49.6% (RI 24–45%) and 10.1 M/mm3 (RI 4.0–9.0 M/mm3), respectively. Coagulation time was also in the normal range: partial thromboplastin time was 16.1 s (RI 14–19 s) and activated partial thromboplastin time was 105 s (RI 75–105 s).
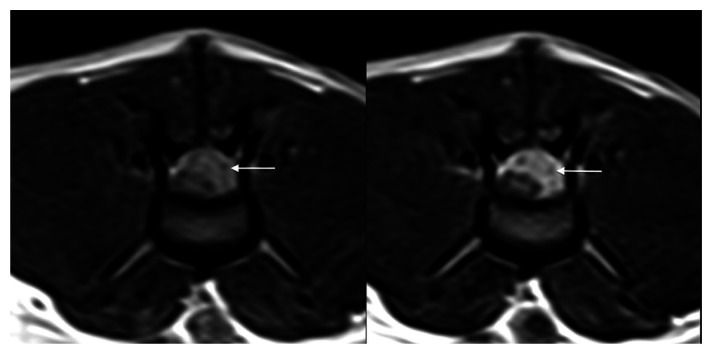
Thoracic and abdominal radiographs did not show any morphological abnormalities. Magnetic resonance imaging (MRI) of the thoraco-lumbar spine was performed with a low field (0.2 T) permanent magnet (Vet MRI; Esaote), including spin-echo (SE) T1-weighted (TR/TE/NEX, 800/26/1) and turbo SE (TSE) T2-weighted (TR/TE/NEX, 2770/100/1) sequences in the sagittal and transverse planes. We also performed a dorsal short TI inversion recovery (STIR) sequence (TR/TE/NEX, 1160/25/2) and SE T1-weighted sequences after intravenous injection of contrast medium (gadolinium–dimeglumine 0.5 mmol/ml; Omniscan, GE Healthcare). MRI revealed a well-defined, extradural mass in the epidural space from the L2 to L3 vertebral bodies that expanded in the L2–L3 left intervertebral foramen (Figure 1). The extradural mass was hyperintense on T2-weighted TSE and GE_STIR sequences, and hypointense on T1-weighted SE sequences. Lesion enhancement was evident after contrast medium injection. MRI suggested two differential diagnoses: infective/inflammatory granulomatous with unknown pathogenesis or extradural focal neoplasia (eg, lymphoma, haemangioma).
Figure 1.
Transverse T1-weighted, spin echo magnetic resonance imaging sequences at the level of L3 before (left) and after (right) contrast medium injection. A heterogeneous material (arrow) occupying more than half of the spinal canal was observed impinging on the epidural space on the left side of the spinal cord. The post-contrast medium injection image (right) shows a well-defined, heterogeneous enhancement
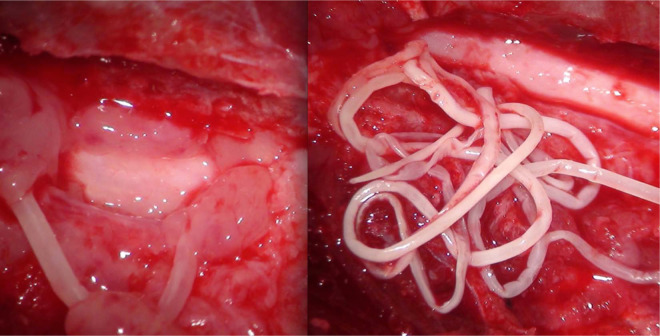
The patient underwent surgery (L2–L3–L4 left hemilaminectomy). A well-defined extradural mass comprised of fatty inflammatory tissue and a long, narrow, white parasite were detected. Both the mass and the parasite were gently removed from the spinal canal to decompress the spinal cord (Figure 2). Then, a serum sample was sent to laboratory for an enzyme-linked immunosorbent assay (Idexx Laboratories) for suspected dirofilariosis. The result was negative for D immitis.
Figure 2.
High-definition surgical microscope image acquired during the L2–L3–L4 left hemilaminectomy. Fatty inflammatory tissue was detected (left), and a long, narrow, white parasite (right) was observed weakly attached to the phlogistic epidural fat tissue. The whole mass was gently removed from the spinal canal to decompress the spinal cord
The extradural mass was completely resected and sent to the Department of Veterinary Science and Public Health of University of Pisa for histological examination, and the parasite collected during surgery was preserved in formalin and sent to the Department of Veterinary Science and Public Health of University of Milan for identification.
The epidural tissue was fixed in 10% buffered formalin solution, embedded in paraffin, cut into 45–μm sections, and stained with haematoxylin and eosin. Histological examination revealed the presence of a severe inflammatory reaction in the epidural adipose tissue (epidural steatitis) composed mainly of lymphocytes, macrophages and occasional eosinophils. Scattered haemorrhages were also observed.
The collected nematode was identified by molecular and morphological analyses. A central portion of the parasite body, about 1 cm long, was cut and processed for biomolecular analysis, and the remaining portions were preserved for morphological study with a light microscope (Axioskop 2; Zeiss). These portions of the parasite body were clarified in lactophenol before being studied microscopically and identified using the morphological keys of Lichtenfels et al 16 and Sonin. 17 The central body portion was placed in phosphate-buffered saline in a Petri dish for 4 h to remove the formalin. The sample was then placed overnight in a tube with 1 ml 20 mg/ml proteinase K. DNA extraction was performed using the DNeasy Blood and Tissue Kit (Qiagen). The extracted product was used in a multiplex species-specific polymerase chain reaction (PCR) assay for the simultaneous detection and discrimination of D immitis and D repens, which was performed in a Mastercycler Gradient thermal cycler (Eppendorf AG) using two sets of primers in the same mixture reaction, as described by Gioia et al. 18 The sample was tested in duplicate with negative and positive controls of DNA extracted from adult D immitis and Dirofilaria repens worms. Amplification products were run on 2.5% ethidium bromide agarose gels and visualised under ultraviolet light.
The parasite recovered in the paraparetic cat had a long, filiform, whitish body (195 mm long and 0.90 mm wide). Microscopically, the specimen showed a typical cuticle of filarial parasites, with transverse striations along the entire surface and a widely varying thickness from 7 to 35 μm. The anterior extremity of the parasite was recognisable owing to the presence of a small, lipless, rudimentary buccal capsule and a long, cylindrical oesophagus. The worm exhibited an obtuse posterior end with a sub-terminal anus, and the distance of this from the tip was 0.15 mm. The vulvar opening in the post-oesophagic position, and the distance from the anterior end was 0.73 mm.
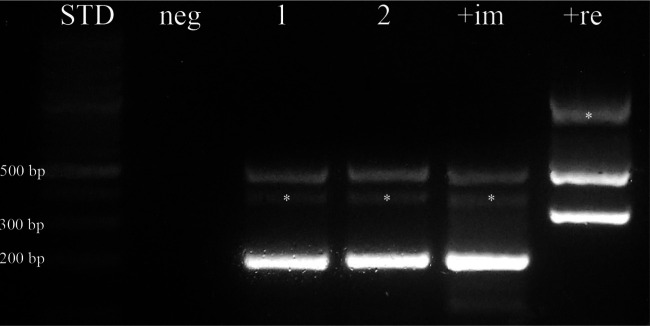
The morphological features of the specimen were consistent with an adult filarial female belonging to the D immitis species. This result was confirmed by agarose gel separation after multiplex PCR analysis, which showed the expected amplification products of 204 and 327 base pairs, respectively, which matched the D immitis and D repens positive controls. Furthermore, the processed sample tested in duplicate showed an unambiguous amplification product corresponding to the pattern associated with D immitis (Figure 3).
Figure 3.
Multiplex polymerase chain reaction amplification of DNA after agarose gel separation. Lanes 1 and 2: sample, tested in duplicate; lane +im: positive Dirofilaria immitis control; lane +re: positive Dirofilaria repens control; lane neg: negative control; (*) non-specific amplification products. The ~500-base pair (bp) fragment observed in both the tested samples and positive controls is the result of the amplification of a 12s rDNA region highly conserved among the nematode species. The 204-bp D immitis-specific fragment is only visible in lanes 1, 2 and +im. The 327-bp D repens-specific fragment is only found in the +re lane
Postoperatively, the patient received 0.25 mg/kg prednisolone (Vetsolone; Bayer) q12h for 3 days, and then 0.25 mg/kg once every 2 days for the next week. Analgesic (2 mg/kg of tramadole, Altadol: Formevet) and antimicrobial therapy (20 mg/kg q12h of cefalexine, ICF-Vet; ICF) were also administered for 5 and 10 days, respectively. The patient had recovered completely without any neurological deficit at 2-month follow-up.
Based on a review of the literature related to these findings, we can formulate a hypothesis regarding aberrant CNS localisation of D immitis. Firstly, filarial nematodes seem develop to sub-adults and adults in typical sites (right ventricle and pulmonary artery) before migrating to the CNS; in 5/8 cats, the parasites were found both in these organs and the CNS.7,19–22 Furthermore, in cats with CNS infection, two gravid females were found, whereas a male was in a pulmonary vein, suggesting that females migrate to the CNS after mating. 21 In addition, if the nematode is trapped in a small vessel, it could extrude out of the vessel and migrate into the CNS.19,23 In fact, Miura et al 22 demonstrated that D immitis is able to migrate from a ventricle to another part of the brain. Moreover, the cat CNS could be a site where heartworms could easily settle; McCall et al 24 found nematodes in the CNS of 2/10 cats experimentally inoculated with 100 larvae (L3).
Additionally, none of the CNS infections in cats were in the spinal cord; D immitis in dogs can involve neurological signs and spinal cord damage. 25 This may be owing to the smaller size of cat spinal vessels preventing filarial nematode migration.
Conclusions
The case described here is the first record of natural spinal infection in a cat due to D immitis. However, the collection of an adult specimen from the infected cat seems to suggest that the neurological signs in feline CNS D immitis infections are likely due to adult or sub-adult nematodes that primarily cause vascular damage, rather than the migration of larval stages in brain proposed by some authors. 26
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors for the preparation of this case report.
The authors do not have any potential conflicts of interest to declare.
Accepted: 14 May 2013
References
- 1. Knight DH, Lok JB. Seasonality of heartworm infection and implications for chemoprophylaxis. Clin Tech Small Anim Pract 1998; 13: 77–82. [DOI] [PubMed] [Google Scholar]
- 2. Venco L, Genchi M, Genchi C, et al. Can heartworm prevalence in dogs be used as provisional data for assessing the prevalence of the infection in cats? Vet Parasitol 2011; 176: 300–303. [DOI] [PubMed] [Google Scholar]
- 3. Atkins CE, De Francesco TC, Coats JR, et al. Heartworm infection in cats: 50 cases. J Am Vet Med Assoc 2000; 217: 355–358. [DOI] [PubMed] [Google Scholar]
- 4. García-Guash L, Caro-Vadillo A, Manubens-Grau J, et al. Evaluation of pulmonary function variables by using plethysmography in cats with respiratory disease associated to Dirofilaria immitis. Vet Parasitol 2012; 187: 254–258. [DOI] [PubMed] [Google Scholar]
- 5. Lee ACY, Atkins CE. Understanding feline heartworm infection: disease, diagnosis, and treatment. Top Companion Anim Med 2010; 25: 224–230. [DOI] [PubMed] [Google Scholar]
- 6. Ader P. Heartworm (Dirofilaria immitis) in the brain of a cat - review and case report. Cal Vet 1979; 33: 23–25. [Google Scholar]
- 7. Cusick PK, Todd KS, Jr, Blake JA, et al. Dirofilaria immitis in the brain and heart of a cat from Massachusetts. J Am Anim Hosp Assoc 1976; 12: 490–491. [Google Scholar]
- 8. Mandelker L, Brutus RL. Feline and canine dirofilarial encephalitis. J Am Vet Med Assoc 1971; 17: 57–59. [PubMed] [Google Scholar]
- 9. American Heartworm Society. Current feline guidelines for the diagnosis, prevention, and management of heartworm (Dirofilaria immitis) infection in cats. http://www.heartwormsociety.org/veterinary-resources/feline-guidelines.html (revised January 2012).
- 10. Cooley AJ, Clemmons RM, Gross TL. Heartworm disease manifested by encephalomyelitis and myositis in a dog. J Am Vet Med Assoc 1987; 190: 431–432. [PubMed] [Google Scholar]
- 11. Mansour AE, McCall JW, McTier TL, et al. Epidemiology of feline dirofilariasis. Infections induced by simulated natural exposure to Aedes aegypti experimentally infected with heartworms. In: Proceedings of the Heartworm Symposium 1995, Auburn, AL, USA, 31 March - 2 April, pp 87–95. Batavia, IL: American Heartworm Society. [Google Scholar]
- 12. Blass CE, Holmes RA, Neer TM. Recurring tetraparesis attributable to a heartworm in the epidural space of a dog. J Am Vet Med Assoc 1989; 194: 787–788. [PubMed] [Google Scholar]
- 13. Healey TS. Recurrent cervical pain and ataxia in a Chihuahua. Vet Med 2003; 98: 656–662. [Google Scholar]
- 14. Luttgen PJ. Posterior paralysis caused by epidural dirofilariasis in a dog. J Am Anim Hosp Assoc 1981; 17: 57–59. [Google Scholar]
- 15. Shires PK, Turnwald GH, Qualls CW, et al. Epidural dirofilariasis causing paraparesis in a dog. J Am Vet Med Assoc 1982; 180: 1340–1343. [PubMed] [Google Scholar]
- 16. Lichtenfels L, Pilitt PA, Kotani L, et al. Morphogenesis of development stages of Dirofilaria immitis (Nematoda) in the dog. Proc Helm Soc Wash 1985; 5: 98–113. [Google Scholar]
- 17. Sonin MD. Filariata of animals and man and disease caused by them. Part III. Filariidae, Onchocercinae. New Delhi: Oxonian Press, 1985, p 476. [Google Scholar]
- 18. Gioia G, Lecová L, Genchi M, et al. Highly sensitive multiplex PCR for simultaneous detection and discrimination of Dirofilaria immitis and Dirofilaria repens in canine peripheral blood. Vet Parasitol 2010; 172: 160–163. [DOI] [PubMed] [Google Scholar]
- 19. Faries FC, Jr, Mainster ME, Martin PW. Incidental findings of Dirofilaria immitis in domestic cats. Vet Med Small Anim Clin 1974; 69: 599–600. [PubMed] [Google Scholar]
- 20. Lindquist WD, Winters KD. Cerebral feline dirofilariasis [case report]. Feline Pract 1981; 11: 37–39. [Google Scholar]
- 21. Fukushima K, Hutsell D, Patton S, et al. Aberrant dirofilariasis in a cat. J Am Vet Med Assoc 1984; 184: 199–201. [PubMed] [Google Scholar]
- 22. Miura H, Kanamoto T, Morita T, et al. A case of feline dirofilariasis with intracerebral aberration. J Vet Med Assoc 2001; 54: 701–705. [Google Scholar]
- 23. Donahoe JMR, Holzinger EA. Dirofilaria immitis in the brains of a dog and a cat. J Am Vet Med Assoc 1974; 164: 518–519. [PubMed] [Google Scholar]
- 24. McCall JW, Dzimianski MT, McTier TL, et al. Biology of experimental heartworm infections in cats. In: Proceedings of the Heartworm Symposium 1992, Austin, TX, USA, pp 71–79. [Google Scholar]
- 25. Theis JH. Public health aspects of dirofilariasis in the United States. Vet Parasitol 2005; 133: 157–180. [DOI] [PubMed] [Google Scholar]
- 26. Litster AL, Atwell RB. Feline heartworm disease: a clinical review. J Feline Med Surg 2008; 10: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]