Abstract
A 1-year-old sexually intact male Korat cat was referred for ophthalmological consultation due to anisocoria. Mydriasis with external ophthalmoplegia and absence of pupillary light responses in the right eye and nasofacial hypalgesia were seen. Cavernous sinus syndrome (CSS) was suspected. Bilateral deformities of the jaw and phalangeal bones, severe spinal pain and abnormal conformation of the lumbar spine were also present. Radiographic examination revealed several mineralised masses in the appendicular and axial skeleton, indicative of multiple cartilaginous exostoses. For further investigation of the CSS-related neurological deficits, the cat underwent computed tomography (CT) examination of the skull. CT images revealed a non-vascularised, calcified, amorphous mass originating from the right lateral skull base and superimposing on the sella turcica. Based on the severity of diffuse lesions and owing to the clinical signs of extreme pain, the cat was euthanased. A diffuse skeletal and intracranial osteochondromatosis was diagnosed histologically.
Case Report
A 1-year-old sexually intact male Korat cat weighing 4 kg was referred for ophthalmological consultation due to anisocoria that had started 1 week prior to presentation. The cat was born and raised in Italy, and was up-to-date with vaccinations for feline calicivirus, rhinotracheitis and panleukopenia. The cat lived strictly indoors and did not share the environment with other pets. A few days before presentation, the owner had observed a swelling in the right masseteric region. There was no history of previous illness or trauma.
The cat’s mental status was responsive on presentation. General physical examination was difficult owing to the cat’s aggressive behaviour elicited on palpation of the cervical, thoracic and lumbar spine, which was suggestive of intense pain. Firm immobile masses at the level of the lumbar spine were palpated. A bilateral, almost symmetrical, swelling of the mandibular bone was appreciable on palpation. The first toe of the right forepaw was thicker than expected, with angular deviation of the nail. Ophthalmological and neurological examinations showed an evident anisocoria with right eye mydriasis and external ophthalmoplegia. Right palpebral areflexia and ipsilateral nasofacial hypalgesia were also seen. Direct and consensual pupillary light responses were absent in the right eye, but they were normal in the left eye. The rest of the ophthalmological and neurological examinations were normal. The cranial nerve deficits were consistent with cavernous sinus syndrome (CSS). Differentials were inflammatory and neoplastic diseases.
A complete cell blood count revealed a slight lymphopenia (1660/μl; reference interval 2000–7200 lymphocytes/μl). Serum biochemical abnormalities included increased alkaline phosphatase (990 U/l; reference interval <100 U/l) and creatine kinase (1488 U/l; reference interval 18–230 U/l) concentrations. Serological testing for feline leukaemia virus (FeLV) was positive.
A dorsoventral radiograph of the skull, an anteroposterior radiograph of the forepaws and a lateral view of the spine were performed to evaluate the mandibular, appendicular and spinal masses palpated during physical examination and spinal pain.
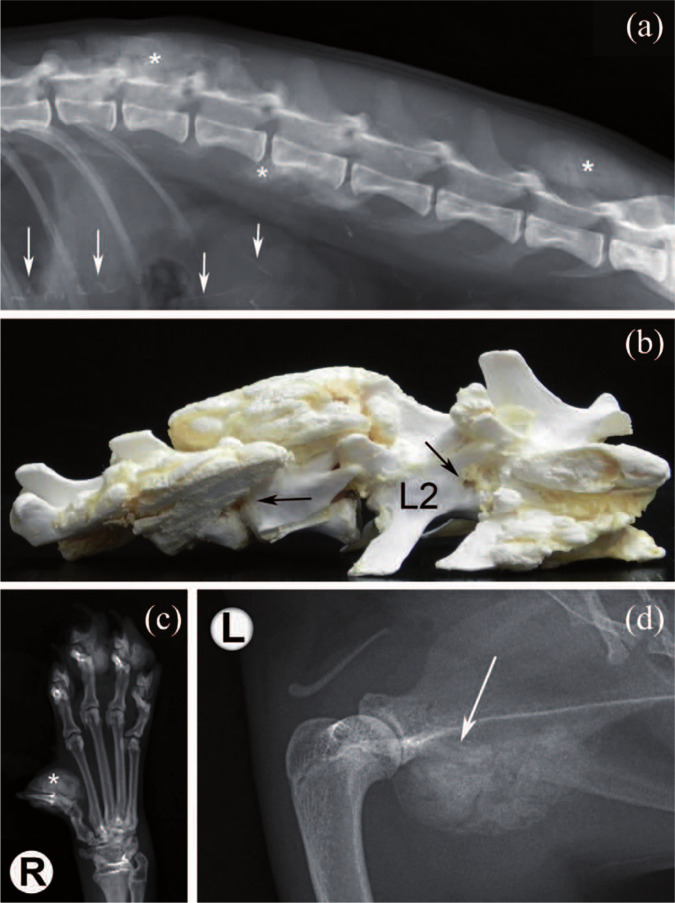
Several mineralised masses with well-defined, smoothly or irregularly contoured borders were observed laterally to the right and left masseteric region. More precisely, as a result of what was palpated during the physical examination they were supposed to be localised laterally to the right and left mandibular ramus, as well as latero-caudally to the right tympanic bulla (Figure 1). Moreover, variably sized, amorphous, calcified masses were present in the soft tissues along the middle and distal phalanges of different toes of both forepaws (Figure 2c). A lateral radiograph of the spine revealed amorphous calcified masses superimposed on vertebral arches and spinous processes of the T13–L3 and L6–L7 vertebral segments, and transverse process of L3. Linear mineral opacities superimposed with the cranial abdomen in the same lateral radiographic view of the thoraco-lumbar spine were visible (Figure 2a). Moreover, the lateral view of the thoracic spine revealed additional calcified amorphous masses arising from the scapulae and superimposing with the scapulo-humeral joints (Figure 2d).
Figure 1.
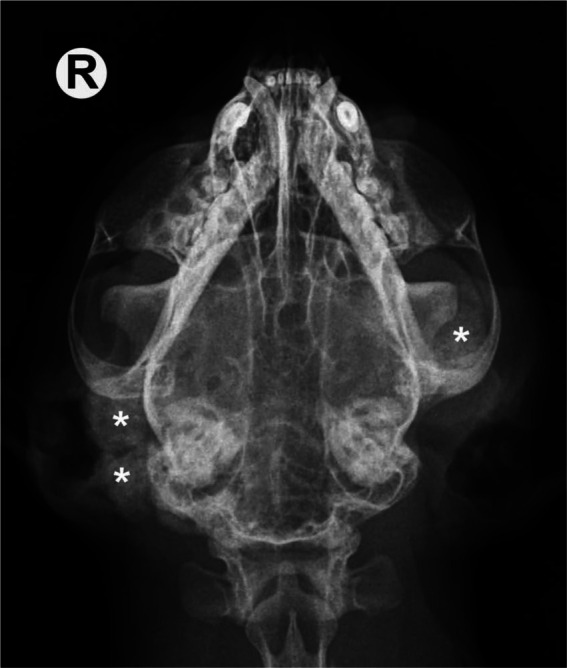
Dorsoventral radiograph of the skull. Several mineralised masses laterally to the right and left mandibular ramus, as well as laterally and caudally to the right tympanic bulla (asterisks), are evident. R = right
Figure 2.
(a) Lateral view of the T11–S1 spine. Amorphous calcified masses superimposed on vertebral arches and spinous processes of the T13–L3 and L6–L7 spinal segments, and transverse process of L3 are visible (asterisks). Linear mineral opacities within the cranial abdomen are visible (arrows). (b) Gross evaluation of the T12–L3 spinal segment; multifocal calcified masses stenosing lateral foramina (arrows), and involving L1–L2 spinous processes and articular facets. (c) Dorsoventral radiograph of the right forepaw. The amorphous, calcified mass along the middle and distal phalanges of the first toe is well appreciable (asterisk). (d) Mediolateral view of the left scapulo-humeral joint. Amorphous calcified mass arising from the scapula and superimposing on the joint is clearly visible (arrow). R = right; L = left
Based upon the radiographical findings, differential diagnosis included: (i) osteochondromatosis (multiple cartilaginous exostoses), (ii) multifocal tumoural calcinosis and (iii) multifocal neoplasia (eg, osteochondroma, chondrosarcoma).
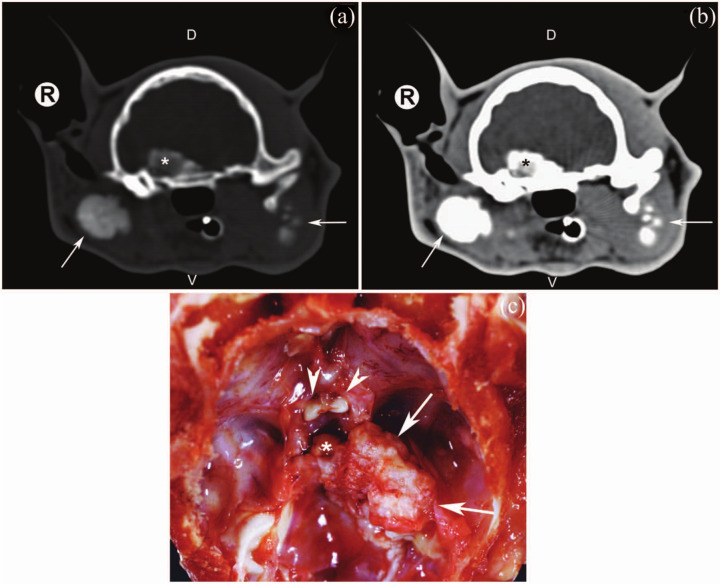
The cat underwent computed tomography (CT) examination (Tomoscan LX; Philips Medical Systems) of the skull to further investigate the CSS-related neurological deficits. The CT examination (Figure 3a, b) revealed a (13.6 × 13 × 10.3 mm) non-vascularised, amorphous mass, the density of which was suggestive of a mineral nature (HU: +230 independent of contrast medium injection) originating from the right lateral skull base and superimposing on the sella turcica. Furthermore, CT examination confirmed the presence of the other three calcified masses previously described as located laterally to the bilateral mandibular ramus (left mass: 15.2 × 10.3 × 18.5 mm; right mass: 16.6 × 9.3 × 16.8 mm) and the right timpanic bulla (16.2 × 9 × 19.5 mm). Involvement of the basisphenoid, mandibular and bulla bones could not be ruled out.
Figure 3.
Pre-contrast bone window (WW = 2000 HU; WL = 350 HU) (a) and post-contrast soft tissue window (WW = 360 HU; WL = 40 HU) (b) transverse computed tomographic images of the skull of the same cat as in (a). A non-vascularised, amorphous, mineralised mass, measuring 13.6 × 13 × 10.3 mm, originating from the right lateral skull base and superimposing on the sella was revealed (asterisk). Images were obtained with a third-generation CT and a 1.5 mm slice thickness. Arrows indicate mineralised masses lateral to the right tympanic bulla and left mandibular ramus. (c) Dorsal view of the basisphenoid bone after brain removal of the same cat as in (a); mineralised mass (arrows), optic nerves (arrow heads) and hypophisis (asterisk). R = right; V = ventral; D = dorsal; WW = window width; WL = window level
Differential diagnosis for such a type of intracranial lesion includes: (i) cartilaginous exostoses, (ii) calcified granuloma and (iii) neoplasia (osteochondroma, chondrosarcoma, calcified meningioma). On the basis of the clinical, radiographical and CT features, the differential diagnosis could be limited to two pathological conditions, namely osteochondromatosis or, less likely, multifocal tumoural calcinosis. In fact, the co-existence of two different intracranial and skeletal pathologies, even if not impossible, is to be considered highly unlikely in a 1-year-old patient.
Based on the severity of the multiple injuries and owing to the extreme pain clinical signs, the cat was euthanased upon the owner’s request and necropsy followed immediately.
Necropsy confirmed the presence of multifocal calcified masses affecting the appendicular and axial skeleton. Osteocartilaginous exostoses bilaterally deformed the ventro-caudal outline of the mandibular ramus. On the right side, the tympanic bulla with associated external auditory meatus, and both the mastoid and jugular processes were involved. After opening the skull and removing the brain, a calcified mass in the right middle cranial fossa involving the basisphenoid bone was observed (Figure 3c).
In the spine, multifocal calcified masses were consistent with the radiographical findings. The most extensive masses involved the spinous processes of T13–L2 (3.8 × 1.5 cm) and L6–L7 (3 × 1 cm) vertebrae (Figure 2b). In the first case the mass also involved the corresponding transverse processes on one side, reaching the transverse process of T12 on the opposite side. The newly formed bone tissue seemed to develop as a distinct bony cup in some points, while in others it clearly infiltrated the underlying bone tissue. The linear mineral opacities observed in the lateral radiograph of the thoracolumbar spine were not clearly identified and therefore escaped further histological examination. On the contrary, necropsy confirmed the presence of irregular calcified masses involving scapular-homeral joints and soft tissue of fore limb phalanges.
The newly formed bone tissue was sampled from skull, spine, phalanges and scapulae. After complete fixation in 10% neutral-buffered formalin it was decalcified with formic acid decalcifying solution (37% HCl, 99% HCOOH, H2O). Paraffin-embedded sections were stained with haematoxylin and eosin and submitted to routine histological examination.
All lesions consisted of irregular spongy bone showing scattered marrow cavities outlined by osteoblasts and surrounded externally by chondromatous tissue (Figures 4 and 5). The latter showed an infiltrative growth pattern towards adjacent muscle. At these sites, except for the phalanges, the masses were continuous with adjacent bone plate.
Figure 4.
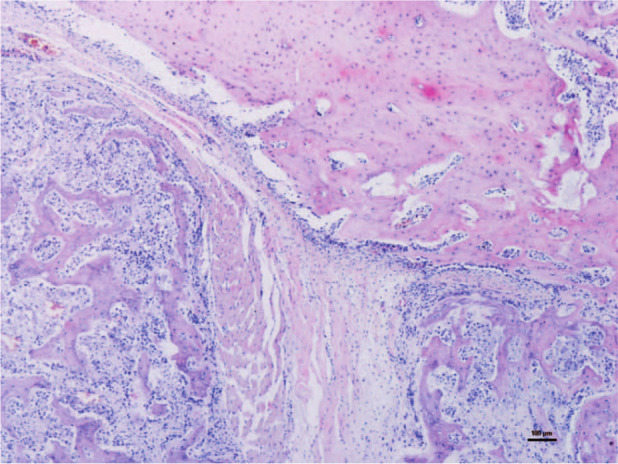
Histopathology of the lesion affecting the right mandibular ramus. A hyaline cartilage cap rich in normal chondrocytes covers the newly formed irregular spongy bone tissue (haematoxylin and eosin)
Figure 5.
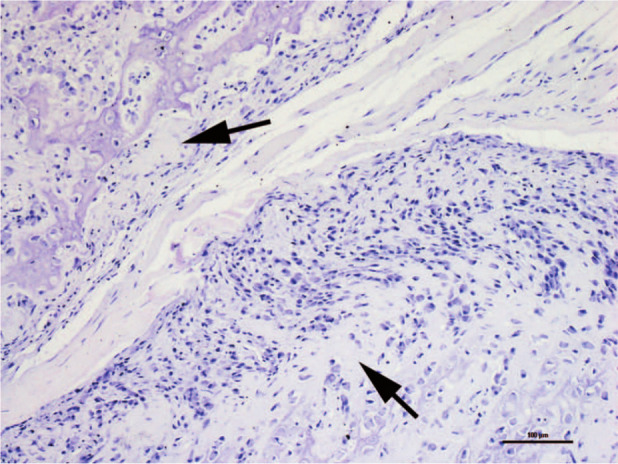
Detail of Figure 4. The mass-like lesion involves the adjacent muscle. Note the progressive replacement of active cartilage (arrows) with bone tissue
The gross and microscopic findings were consistent with diffuse cranial, spinal and appendicular osteochondromatosis.
Discussion
CSS is a clinical disorder described in both dogs and cats, characterised by dysfunction of the cranial nerves that course close to the cavernous sinuses, including the oculomotor nerve (III), trochlear nerve (IV), abducent nerve (VI), and the maxillary and ophthalmic branches of the trigeminal nerve (V).1,2
Reviewing canine and feline CSS literature, all the cases with a histopathological diagnosis were due to either an infection or a tumour, and most patients were euthanased as a result of their disease.2–7
There is discrepancy in the literature regarding the definition of the cavernous sinus syndrome. 8 It has been debated whether the maxillary branch of the trigeminal nerve should be added to the cranial nerves affected by lesions in the cavernous sinus. 2 The cat in the present study showed a clear facial hypoalgesia lateral to the nose, on the same side as the CSS. Moreover, cutaneous stimulation of its muzzle on the right side elicited no facial muscle contraction or whisker movements despite normal facial nerve function, which was suggestive of maxillary branch involvement.
In the present cat, masticatory muscle atrophy was neither seen nor expected, as the mandibular branch of trigeminal nerve does not run close to the cavernous sinus. However, mastication muscle atrophy has been either seen or expected in previous case reports on CSS.3,8
The final diagnosis was osteochondromatosis, which is a multiple osteochondroma.9,10 Osteochondroma is defined as a cartilage-capped bony projection arising on the external surface of the bone. Osteochondromatosis in cats may show solitary or multicentric patterns affecting the axial and appendicular skeleton; moreover, extraskeletal or synovial locations have been reported.9–12 Differently from what occurs in humans and dogs, the lesions in cats show continuous growth throughout life and may undergo malignant neoplastic transformation.9,10 The pathogenesis of feline osteochondroma is presumed to be related to FeLV infection of periosteal fibroblasts.9,12 As reported above, FeLV enzyme-linked immunoassay titre was positive in the presented cat.
Osteochondromatosis tends to cause lameness, pain and paresis resulting from compression of the neurological structures.9,12 The spinal pain and neurological deficits described in our case were consistent with a compressive neuropathy involving the spinal nerves at the level of the intervertebral foramina, and the cranial nerves III, IV, V and VI at the level of the right cavernous sinus.
No effective treatment exists for osteochondromatosis, even in a localised condition: a complete surgical excision with wide margins is possible, but difficult to obtain, and local recurrences are likely, mostly due to the connection between cartilaginous caps and adjacent tissues, and to viral aetiology.9–11
Conclusions
The location of the intracranial osteochondroma shown in the present case has never before been described. CT scan enabled visualisation of the intracranial mass, and revealed the relationship between pathological features and clinical signs. Moreover, osteochondroma should be added to infectious and neoplastic diseases as differentials of CSS in cats.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case report.
The authors have no potential conflicts of interest to declare.
Accepted: 8 April 2013
References
- 1. Evans HE, Kitchell RL. Cranial nerves and the cutaneous innervation of the head. In: Evans HE. (ed). Miller’s anatomy of the dog. 3rd ed. Philadelphia: WB Saunders, 1988, pp 953–987. [Google Scholar]
- 2. Theisen SK, Podell M, Schneider T, et al. A retrospective study of cavernous sinus syndrome in 4 dogs and 8 cats. J Vet Intern Med 1996; 10: 65–71. [DOI] [PubMed] [Google Scholar]
- 3. Hernández-Guerra AM, Del Mar López-Murcia M, Planells A, et al. Computed tomographic diagnosis of unilateral cavernous sinus syndrome caused by a chondrosarcoma in a dog: a case report. Vet J 2007; 174: 206–208. [DOI] [PubMed] [Google Scholar]
- 4. Rossmeisl JH, Jr, Higgins MA, Inzana KD, et al. Bilateral cavernous sinus syndrome in dogs: 6 cases (1999–2004). J Am Vet Med Assoc 2005; 226: 1105–1111. [DOI] [PubMed] [Google Scholar]
- 5. Murphy CJ, Koblik P, Bellhorn RW, et al. Squamous cell carcinoma causing blindness and ophthalmoplegia in a cat. J Am Vet Med Assoc 1989; 195: 965–968. [PubMed] [Google Scholar]
- 6. Valentine BA, Summers BA, de Lahunta A, et al. Suprasellar germ cell tumors in the dog: a report of five cases and review of the literature. Acta Neuropathol (Berl) 1988; 76: 94–100. [DOI] [PubMed] [Google Scholar]
- 7. Lewis GT, Blanchard GL, Trapp AL, et al. Ophthalmoplegia caused by thyroid adenocarcinoma invasion of the cavernous sinuses in the dog. J Am Anim Hosp Assoc 1984; 20: 805–812. [Google Scholar]
- 8. Fransson B, Kippenes H, Silver GE, et al. Magnetic resonance diagnosis: cavernous sinus syndrome in a dog. Vet Radiol Ultrasound 2000; 41: 536–538. [DOI] [PubMed] [Google Scholar]
- 9. Rosa C, Kirberger RM. Extraskeletal osteochondroma on a cat’s elbow. J S Afr Vet Assoc 2012; 83: 1–4. [DOI] [PubMed] [Google Scholar]
- 10. Ranade SA, Pacchiana PD. What is your diagnosis? J Am Vet Med Assoc 2011; 238: 1243–1244. [DOI] [PubMed] [Google Scholar]
- 11. Pool RR, Carrig CB. Multiple cartilaginous exostoses in a cat. Vet Pathol 1972; 9: 350–359. [DOI] [PubMed] [Google Scholar]
- 12. Levitin B, Aroch I, Aizenberg I, et al. Linear osteochondromatosis in a cat. Vet Radiol Ultrasound 2003; 44: 660–664. [DOI] [PubMed] [Google Scholar]