Abstract
To compare glomerular filtration rate (GFR) estimated by a single blood sample method, the non-ionic contrast medium iodixanol (40 mg I/kg) and the standard GFR tracer inulin (50 mg/kg) were co-administered as a bolus intravenous injection to 12 cats, followed by blood collection 60 and 90 mins later. Serum iodixanol and inulin concentrations were measured separately by high-performance liquid chromatography and colourimetric assay. A correlation (r = 0.90, P <0.01) was noted between GFR values estimated by the single-blood-sample method using iodixanol and inulin, indicating that this procedure can apply to feline GFR estimates, even if different GFR tracers are used. In a feline kidney transplantation study, the GFR was monitored subsequently by this simplified iodixanol method throughout a 750-day observation period with no adverse reactions. The results demonstrate that the simplified method, including the volume of distribution, can be used as an alternative or expedient tool in a clinically relevant situation.
Short Communication
Estimation of glomerular filtration rate (GFR) is the gold standard for precise assessment of feline kidney function. 1 Although the traditional method involves monitoring urinary clearance, there are methodological difficulties with its application in practice. For example, use of the standard GFR tracer inulin requires accurately timed repeated blood and urine samplings. We reported recently that the GFR estimated by a single blood sample using the isotonic, non-ionic, dimeric x-ray contrast medium iodixanol 2 or inulin 3 based on Jacobsson’s equation 4 was closely correlated with that determined by the conventional multi-sample strategy. Jacobsson’s formula included the sampling time, volume of distribution (Vd), dosage level and serum concentration as variables. To determine whether Jacobsson’s equations (single blood sample method) with iodixanol and inulin are utilised in the same way for cats, it is necessary to confirm that GFR values are identical, even with use of different tracers. Thus, iodixanol and inulin were co-administered intravenously to healthy cats and cats with spontaneous chronic kidney disease (CKD) or polyuria, and the GFRs obtained from the respective single blood sample methods were compared. Next, the single blood sample method using iodixanol was used to monitor sequential changes in the GFR of a kidney transplant recipient cat with a donor cat up to day 750 postoperatively. Iodixanol contains twice the iodine content per molecule than the non-ionic, monomeric x-ray contrast medium iohexol, which is frequently used in feline practice.1,5 Iodixanol is assumed to show the pharmacodynamic activity identical to that of iohexol at half the whole-body exposure dose. 6 Meanwhile, iohexol has been reported to be a cause for concern regarding deteriorated renal function. 5
Five clinically healthy, purpose-bred male mix cats (aged 3–7 years weighing 3–6 kg), four cats (aged 6–10 years and weighing 2–4 kg) with CKD and three cats (aged 5–9 years and weighing 4–7 kg) with polyuria were used. Healthy cats were owned by the Veterinary Teaching Hospital at Iwate University (IUVTH, Morioka, Japan) and regarded as ‘healthy’ from the results of clinical observations, haematology, serum chemistry and/or urinalysis. Cats with CKD and polyuria were used after obtaining the owners’ consents for their participation in this study. The former was defined from blood urea nitrogen (BUN) (>30 mg/dl) and creatinine (>1.6 mg/dl) concentrations in sera in conjunction with proteinuria. The latter was judged based on clinical signs, although their causes were unknown.
In the kidney transplantation study, a male domestic shorthair cat (8 years of age and weighing 4.8 kg) with end-stage chronic kidney failure was transplanted with an allograft kidney at IUVTH according to the procedure described previously. 7 At the time of initial presentation the cat showed severe azotaemia [BUN 170 mg/dl; creatinine 10.7 mg/dl; albumin 3.4 g/dl; calcium (Ca) 2.75 mmol/l; inorganic phosphate (IP) 3.10 mmol/l] and was classified as International Renal Interest Society CKD stage 4. 8 After kidney transplant, the recipient was managed with a triple-drug immunosuppressive regimen consisting of cyclosporine (ciclosporin fine granule; Mylan), clarithromycin (clarithromycin capsule; Chouseido) and prednisolone (prednisolone tablets; Shionogi) to prevent acute allograft rejection.7,9 A healthy male domestic shorthair cat (aged 3 years and weighing 4.5 kg) was used as a donor graft source. This cat was confirmed to be healthy based on clinical observations, blood and serum examinations, and urinalysis before nephrectomy. In both cats, buprenorphine (0.2 mg/head) was administered intravenously to control pain after surgery. All procedures were performed in accordance with the Guidelines for Animal Experimentation issued by the Japanese Association for Laboratory Animal Science 10 and approved by the Animal Experimental Ethics Committee of Iwate University (A201139).
Iodixanol (Visipaque 320; 320 mg I/ml, 290 mOsm/kg H2O) was purchased from Daiichi-Sankyo ( and inulin (Inulead; 100 mg/ml, chicory) was provided by Fuji Yakuhin. Iodixanol and inulin were co-administered at 40 mg I/kg2 and 50 mg/kg, 3 respectively, into the cephalic vein of cats using a 24 G indwelling catheter (Nipro), and blood (0.5 ml) was collected from the contralateral cephalic vein before (pre-dose) and 60 and 90 mins after administration using a 24 G needle. The blood samples were centrifuged and sera were stored at −80°C until assayed.
For both recipient and donor cats, GFR was measured prior to operation (days −20, −10, and/or −3), and on days 2, 18, 43, 113, 231, 289, 384, 448, 659, 705 and/or 750 simultaneously with measurement of BUN, creatinine, albumin, Ca and IP levels in sera. In sequential measurements of GFR, the serum samples were collected to check the pre-dose iodixanol residue before administration. The day of the operation was regarded as day 1 for the present investigation. Serum iodixanol concentration was measured with reverse-phase high-performance liquid chromatography according to a procedure reported previously 11 with minor modifications. 12 Serum inulin was measured colourimetrically by a commercially available kit (Dia-color-inulin; Toyobo). It was confirmed beforehand that there was no drug interference to either serum iodixanol or inulin level in the in vitro additive studies.
The GFR was estimated using Jacobsson’s formula:2–4
where the t, Vd, Dose and Ct represent the sampling time (90 mins for iodixanol or 60 mins for inulin), estimated volume of distribution, dosage level (40 mg I/kg for iodixanol or 50 mg/kg for inulin) of the tracer injected and serum concentration (μg I/ml for iodixanol at 90 mins 2 or μg/ml for inulin at 60 mins 3 ), respectively. The estimated Vd value in each cat was determined using the equation Vd = 647.6e–0.023C for iodixanol 2 or Vd = 485.7e–0.015C for inulin. 3 The GFR is represented as ml/min/kg.
Using serum samples at pre-dose, BUN, creatinine, albumin, Ca and IP concentrations in sera were measured with an autoanalyser (Toshiba). Neither iodixanol nor inulin interfered with the determination of these values.
Quantitative data are expressed as means ± standard deviation of each group. Comparison of GFR values between the two methods was performed according to the standard recommendations for comparing analytical techniques based on the Bland and Altman bias presentation13,14 using Prism 5 (GraphPad Software).
Previously, we reported an agreement between GFR values estimated by the single blood sample and multi-sample methods with iodixanol 2 or inulin. 3 In the present work, when iodixanol and inulin were co-administered intravenously to 12 cats, 11 points obtained on the basis of Bland and Altman bias presentation were within the agreement plots (Figure 1), and the correlation between both methods was excellent (r = 0.90, P <0.01). The mean basal GFR value determined from the Bland and Altman plot was 3.38 ml/min/kg (range 2.50–4.20 ml/min/kg, n = 5) for healthy cats. The previously reported reference GFR values for healthy cats were 3.36 ± 0.56 ml/min/kg (n = 15) and 2.95 ± 0.49 ml/min/kg (n = 6) by the single blood sample method using iodixanol 2 and inulin, 3 respectively, although the conditions differed from those used here. The GFR values obtained nearly resembled previously reported GFR data.15,16 No adverse reactions were observed in any of the cats during or after co-administration of iodixanol and inulin, as determined by physiological examination and serum biochemical analysis. The findings suggest that the single blood sample method based on Jacobsson’s formula, including the Vd, can apply to the feline GFR estimates, irrespective of the GFR tracers used.
Figure 1.
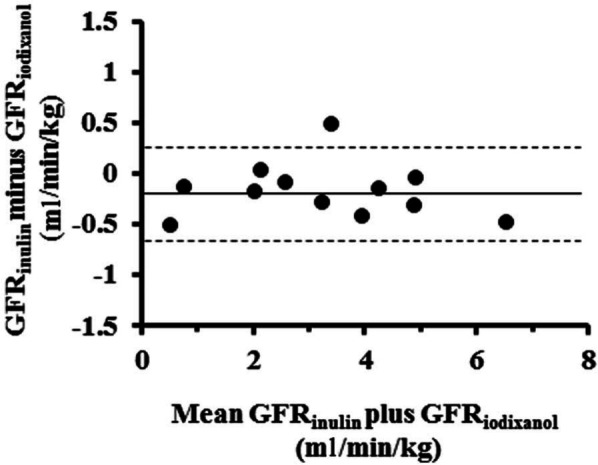
Bland and Altman plots of glomerular filtration rate (GFR) values estimated by the single blood sample method using iodixanol (GFRiodixanol) and inulin (GFRinulin). Iodixanol (40 mg I/kg) and inulin (50 mg/kg) were co-administered as a bolus intravenous injection to 12 cats, and blood samples were collected 90 and 60 mins later. The solid line shows the mean bias (−0.20), and the dotted lines represent the upper and lower 95% limits of agreement (from 0.29 to −0.69)
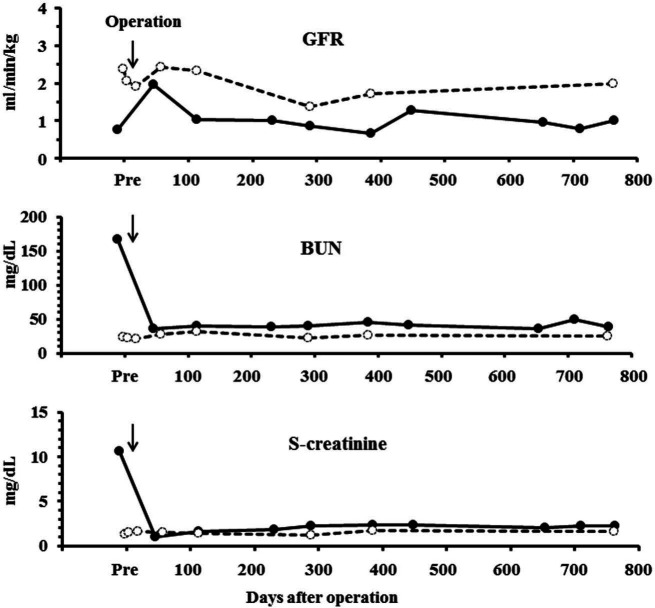
In the kidney transplantation (Figure 2), the extremely low GFR in the recipient was drastically improved after the operation, in conjunction with ameliorations of BUN and creatinine concentrations in sera. Subsequently, the GFR values remained stable within the marginal basal levels over the 750-day observation period. For the donor, although the GFR decreased temporarily to approximately half of the pre-operative value and then returned to basal levels; other kidney parameters were observed within our laboratory background ranges (BUN 10–30 mg/dl; creatinine 0.5–1.2 mg/dl). No serum iodixanol residue was found at pre-dose in sequential GFR measurements. However, further studies are necessary to collect much more background data, including healthy cats and cats with various types of nephropathy.
Figure 2.
Sequential changes in glomerular filtration rate (GFR) and blood urea nitrogen (BUN), and creatinine concentrations in the recipient (closed circles and black line) and donor (open circles and dotted line). Kidney transplantation was performed on day 1. GFR was estimated pre-operatively and on days 2, 18, 43, 113, 231, 289, 384, 448, 659, 705 and/or 750 postoperatively by the single blood sample method using iodixanol
Conclusions
The results demonstrate that this simplified method using iodixanol can be used as an alternative or expedient tool in a clinically relevant situation.
Acknowledgments
The authors would like to thank Dr Tetsuro Yamashita for his helpful support and suggestions regarding iodixanol measurements.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sections.
The authors do not have any potential conflicts of interest to declare.
Accepted: 8 May 2013
References
- 1. Von Hendy-Willson VE, Pressler BM. An overview of glomerular filtration rate testing in dogs and cats. Vet J 2011; 188: 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Katayama R, Saito J, Katayama M, et al. Simplified procedure for the estimation of glomerular filtration rate following intravenous administration of iodixanol in cats. Am J Vet Res 2012; 73: 1344–1349. [DOI] [PubMed] [Google Scholar]
- 3. Katayama M, Saito J, Katayama R, et al. A single-blood-sample method using inulin for estimating feline glomerular filtration rate. J Vet Intern Med 2013; 27: 17–21. [DOI] [PubMed] [Google Scholar]
- 4. Jacobsson L. A method for the calculation of renal clearance based on a single plasma sample. Clin Physiol 1983; 3: 297–305. [DOI] [PubMed] [Google Scholar]
- 5. Miyamoto K. Clinical application of plasma clearance of iohexol on feline patients. J Feline Med Surg 2001; 3: 143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kishimoto M, Yamada K, Tsuneda R, et al. Effect of contrast media formulation on computed tomography angiographic contrast enhancement. Vet Radiol Ultrasound 2008; 49: 233–237. [DOI] [PubMed] [Google Scholar]
- 7. Katayama M, Nishijima N, Okamura Y, et al. Interaction of clarithromycin with cyclosporine in cats: pharmacokinetic study and case report. J Feline Med Surg 2012; 14: 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Polzin DJ, Osborne CA, Ross SJ. Chronic kidney disease. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine, 6th ed. St Louis, MO: WB Saunders, 2005, pp 1756–1785. [Google Scholar]
- 9. Katayama M, Katayama R, Kamishina H. Effects of multiple oral dosing of itraconazole on the pharmacokinetics of cyclosporine in cats. J Feline Med Surg 2010; 12: 512–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Japanese Association for Laboratory Animal Science. Guidelines for animal experimentation. Exp Anim 1987; 3: 285–288. [Google Scholar]
- 11. Jacobsen PB, Blindheim L, Skotland T. Bioanalytical methods for iodixanol and their application to studies on metabolism and protein binding. Acta Radiol 1995; 36 (Suppl 399): 61–66. [DOI] [PubMed] [Google Scholar]
- 12. Katayama R, Yamaguchi N, Yamashita T, et al. Calculation of glomerular filtration rate in conscious rats by the use of a bolus injection of iodixanol and a single blood sample. J Pharmacol Toxicol Methods 2010; 61: 59–64. [DOI] [PubMed] [Google Scholar]
- 13. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–310. [PubMed] [Google Scholar]
- 14. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res 1999; 8: 135–160. [DOI] [PubMed] [Google Scholar]
- 15. Haller M, Rohner K, Müller W, et al. Single-injection inulin clearance for routine measurement of glomerular filtration rate in cats. J Feline Med Surg 2003; 5: 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ross LA, Finco DR. Relationship of selected clinical renal function tests to glomerular filtration rate and renal blood flow in cats. Am J Vet Res 1981; 42: 1704–1710. [PubMed] [Google Scholar]