Abstract
Fifty-six healthy Ragdoll cats underwent an ultrasonographical examination of the urinary tract to evaluate if gender, age, bodyweight and presence of a medullary rim sign had a significant influence on renal length and corticomedullary ratio (CM). Individual variation percentage was much more pronounced for renal length in comparison with CM ratio. Mean renal length measured 3.83 ± 0.45 cm (range 2.98–5.09 cm), mean cortical thickness 0.73 ± 0.15 cm (range 0.36–1.18 cm), mean medullary thickness 0.87 ± 0.19 cm (range 0.46–1.39 cm) and mean CM ratio 0.88 ± 0.29 (range 0.29–1.78). Renal length showed a significant positive correlation with bodyweight (P <0.0001), age (P = 0.0073) and male gender (P <0.0001). Therefore, these parameters have to be kept in mind when evaluating renal length on ultrasound. The CM ratio was solely influenced by the presence of a medullary rim sign (P <0.0001). Further research, however, is needed to investigate the usefulness of the CM ratio for the detection of kidney disease by ultrasonography.
Introduction
Kidney diseases are one of the most common health problems suffered by cats, particularly as they become older. 1 The Ragdoll is a popular cat breed worldwide, but, according to several Ragdoll breeder organisations, such as the Scandinavian Ragdoll Club and the Ragdoll Club Benelux, Ragdoll cats may develop chronic kidney disease (CKD) due to polycystic kidney disease (PKD) or chronic interstitial nephritis (CIN).2,3 In several European countries Ragdoll cats are therefore screened for PKD and CIN.2 –4 Abdominal ultrasonography is one of the tests included in this screening programme, and it is considered to be the reference modality for imaging the feline kidney because relevant anatomic information concerning the size, shape and internal architecture can be obtained.5 –8
Renal length can be easily measured by ultrasonography with a high degree of reproducibility.9,10 The normal size of the feline kidney has been reported.9,11 –13 Acute kidney injury (AKI) often results in enlarged kidneys with a smooth contour, whereas CKD may result in small, irregularly outlined kidneys.7,14 –16 Several studies in humans, dogs and cats have revealed correlations between renal length and different variables, like age, gender and bodyweight.13,17 –22
In humans, cortical thickness instead of renal length is used to differentiate between CKD and AKI as cortical thinning is often present in patients with CKD and can precede changes in renal length.22 –24 In addition, Beland et al 24 concluded that cortical thickness might be related more closely to the estimated glomerular filtration ratio than renal length in patients with CKD. It is not known if the same is true for veterinary patients. Similar to renal length, cortical thickness is influenced by different variables like age, gender, race and bodyweight/body mass of the human patient.21 –23,25 Only a few studies describe the cortical and medullary thickness of normal feline kidneys.9,13
The major study aim was to evaluate if gender, age and bodyweight had a significant influence on renal length and corticomedullary (CM) ratio within a single cat breed. A secondary aim was to describe normal kidney morphology and dimensions in healthy Ragdoll cats, which will help veterinarians to screen Ragdoll cats for kidney disease.
Material and methods
In this prospective study, 75 Ragdoll cats presented at our institution for PKD and CIN screening were used. Most of these cats also participated in a prospective study, which evaluated the presence of kidney disorders in this particular breed. 26 The cats were considered healthy on the basis of history, physical examination, routine urinalysis [urine specific gravity (USG), urinary protein to creatinine ratio, urinary sediment and urinary dipstick], blood examination (complete blood count and serum biochemistry) and absence of ultrasonographical abnormalities (irregular kidney outline, presence of cavitary lesions, solid masses or nodules, segmental/triangular cortical lesions, nephroliths). Serum creatinine concentration was measured with a colorimetric assay (Spotchem; Menarini) and the reference interval (80–164 µmol/l) was determined by the machine manufacturer through the evaluation of more than 100 healthy cats of different ages and weights. USG was determined with a refractometer and was determined to be normal if it was higher than 1.035. The presence of hyperechoic cortices and/or medullary rim signs, a thin linear hyperechogenic line in the outer medulla parallel to the corticomedullary junction, was considered as a normal finding if no other abnormalities were observed.
All cats underwent an ultrasonographical examination without sedation. The cats were placed in dorsal recumbency and were manually restrained. The hair was not clipped because most of the cats were show cats. The hair was parted and water, alcohol and acoustic coupling gel were used to provide an appropriate acoustic contact between the transducer and the skin. Multifrequency (6–10 MHz) microconvex or multifrequency (7.5–12 MHz) linear transducers (Logiq 7; GE Medical Systems) were used for scanning.
For each kidney, sagittal, dorsal and transverse scans were obtained as described by Widmer et al. 8 Delineation (smooth or irregular), echogenicity (hypo-, iso- or hyperechogenic compared with the liver/spleen), renal parenchyma, renal length, and cortical and medullary thickness were evaluated. The length (in cm) was determined on the sagittal sonogram (Figure 1), which is characterised by the appearance of two bright parallel bars formed by cross-sectioned pelvic diverticula. The cortical and medullary thickness (in cm) were defined as (i) the distances from the renal margin to the corticomedullary junction (the interface between the hyperechoic renal cortex and hypoechoic renal medulla) and (ii) from the corticomedullary junction to the renal pelvis, respectively. The edge of the renal pelvis was defined as the interface between the hypoechoic medulla and the hyperechoic renal pelvis fat. The measurements were taken from a dorsal sonogram in the middle of the kidney and were orientated perpendicular to the long axis (Figure 2). When a medullary rim sign was present, it was included in the measurements of the medulla (Figure 3).
Figure 1.
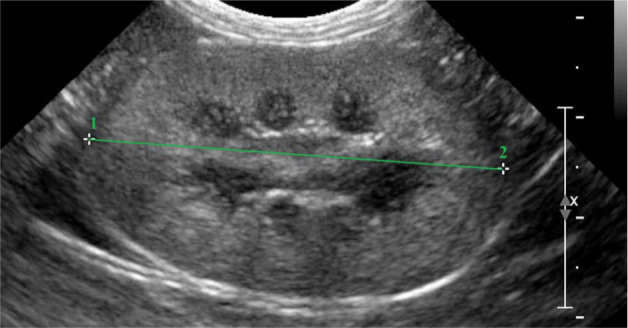
Sagittal sonogram of a normal feline kidney. The stippled line from 1 to 2 shows the measurement of the renal length
Figure 2.
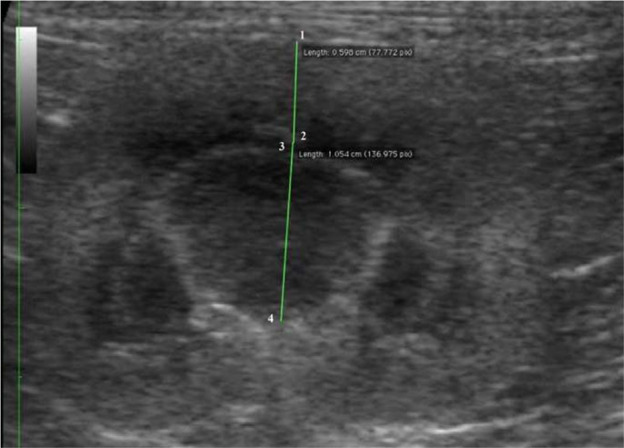
Dorsal sonogram of a normal feline kidney. The green lines indicate the cortical thickness (1–2) and the medullary thickness (3–4)
Figure 3.
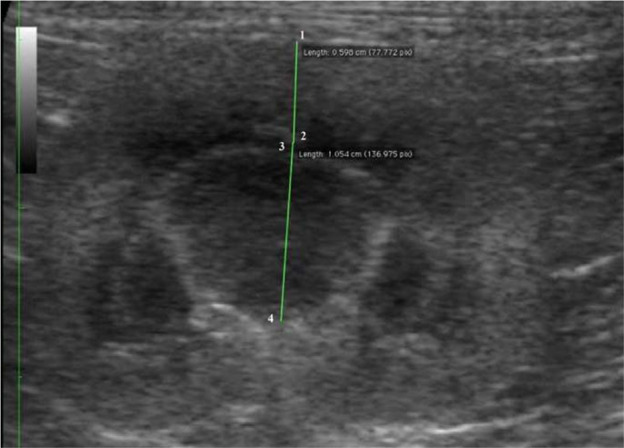
Dorsal sonogram of a normal feline kidney with a medullary rim sign. The green lines indicate the cortical thickness (1–2) and medullary thickness (3–4). The rim sign is included in the measurement of the medulla
The CM ratio was calculated as the ratio of the cortical and medullary thickness. Statistical analysis was performed with SAS (SAS Institute). To assess the variability linked with the individual cat (individual variation) and the two (left vs right) kidneys within the cat (kidney side variation) a random effects model with cat nested in breed and kidney nested in cat was fitted, and variance components for each of the two sources of variation were obtained by restricted maximum likelihood. Further analysis was based on a linear mixed model with animal and kidney nested in animal as random effects and different covariates as categorical fixed effects. F-tests, at a global significance level of 5%, were used to evaluate if renal length and CM ratio were significantly influenced by the different categorical (gender, presence/absence of a medullary rim sign) and continuous (bodyweight, age, renal length, CM ratio) covariables. Means are reported with their standard deviation.
Results
The Ragdoll cats showed a mean serum creatinine of 109.27 µmol/l (range 63.6–236 µmol/l) and a mean USG of 1.047 (range 1.016–1.055). From the 75 Ragdoll cats examined, 19 cats were excluded because of a cavitary lesion (n = 2), ultrasonographical signs (irregular kidney outline, increased echogenicity of the cortex and/or medulla and decreased corticomedullary distinction) of chronic kidney disease (n = 8), 21 segmental/triangular cortical lesion (n = 4), presence of a solid mass (n = 1) and irregular outline of one or both kidneys (n = 4). All of these cats also showed elevated creatinine concentrations. Finally, 40 females (38 intact and two neutered) and 16 males (14 intact and two neutered) were included. The mean bodyweight was 4.1 ± 0.91 kg (range from 2.84–6.9 kg). The mean age was 35 ± 21 months (range from 12–91 months). The ultrasonographical findings are summarised in Table 1.
Table 1.
Kidney dimensions as determined by use of ultrasonography in 56 healthy Ragdoll cats
| Kidney dimension | Mean ± SD | Range |
|---|---|---|
| Length (cm) | 3.83 ± 0.45 | 2.98–5.09 |
| Cortical thickness (cm) | 0.73 ± 0.15 | 0.36–1.18 |
| Medullary thickness (cm) | 0.87 ± 0.19 | 0.46–1.39 |
| Corticomedullary ratio | 0.88 ± 0.29 | 0.29–1.78 |
Eight Ragdoll cats, five intact females and three intact males, showed a medullary rim sign at one or both kidneys. Seven cats, five males (four intact) and two intact females, had one or both kidneys where the cortex was hyperechoic compared to the liver (right kidney) or spleen (left kidney). None of these cats showed other sonographical renal abnormalities, and so both the medullary rim sign and the hyperechoic cortices were considered incidental findings.
The highest overall variation was observed for the renal length (0.203 cm2) compared with the CM ratio (0.086 cm2). Most of the variation in renal length (81%) was owing to differences between cats, whereas the largest part of the variation in the CM ratio (62%) was owing to differences between the two kidneys within a cat .
Bodyweight (P <0.0001) and age (P = 0.0073) had a significant effect on renal length: the kidney increases in size with an increase in bodyweight and age. A highly significant, positive correlation (P <0.0001, r = 0.71) was also observed between bodyweight and renal length (Figure 4). There was also a significant influence of gender (P <0.0001), with males (4.29 ± 0.40 cm) showing larger kidneys than females (3.64 ± 0.32 cm). Renal length was not influenced by the presence or absence of a medullary rim sign (P = 0.542) or by the CM ratio (P = 0.328).
Figure 4.
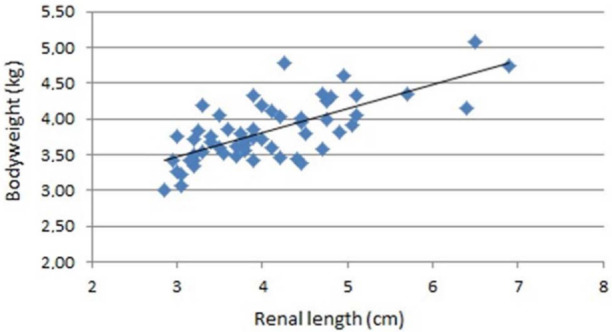
Renal length (cm) and bodyweight (kg) in 56 healthy Ragdoll cats
The presence of a medullary rim sign had a significant effect (P <0.0001) on the CM ratio: CM ratio decreased when a medullary rim sign was present. The CM ratio was not significantly influenced by renal length (P = 0.095), age (P = 0.132), bodyweight (P = 0.068) or gender (P = 0.301) of the cat.
Discussion
As mentioned earlier the aim of our study was to describe normal kidney morphology and dimensions in healthy Ragdoll cats, and to evaluate if gender, age and bodyweight had a significant influence on renal length and CM ratio. By direct anatomical measurement, renal length in cats ranges from 3.8 to 4.4 cm. 9 Previous ultrasonographical studies have reported that the normal size of the feline kidney varies between 3.0 and 4.3 cm, and can even be up to 5.3 cm .9,11 –13 Our result of 3.83 ± 0.45 cm (range 2.98–5.09) fits well within this interval and corresponds with the renal length found by Park et al 13 in healthy Korean domestic shorthair cats.
In the present study a highly significant, positive correlation (P <0.0001, r = 0.71) was observed between bodyweight and renal length (Figure 4), as also reported by Park et al. 13 This finding is comparable with dogs, where a positive relationship exists between renal length/ volume and bodyweight. 17 In dogs, ranges for renal length have been made as a function of their bodyweight. 17 Although differences in bodyweight among cats are minor compared with those in dogs, this may also be pertinent for cats (Table 2). Further studies with larger groups of cats are indicated to determine these ranges. Mantis 27 has suggested that Persian and Chinchillas tend to have smaller renal length than other breeds of cats, but further research is needed to evaluate if clinically relevant differences exist between cat breeds.
Table 2.
Renal length as a function of bodyweight
| Weight range (kg) | n | Renal length (cm) |
||
|---|---|---|---|---|
| Range | Mean | SD | ||
| 2.5–3.5 | 18 | 3.01–4.19 | 3.55 | 0.32 |
| 3.5–4.5 | 24 | 3.39–4.78 | 3.75 | 0.33 |
| 4.5–5.5 | 10 | 3.58–4.60 | 4.12 | 0.30 |
| >5.5 | 4 | 3.91–4.75 | 4.58 | 0.41 |
In the current study, male cats (4.29 ± 0.40 cm) tended to have larger kidneys than females (3.64 ± 0.32 cm). This corresponds to the study of Shiroma et al 19 and to studies performed in human patients.18,21 This difference in renal length between male and female cats may be partially explained by the difference in bodyweight, as in our study male cats had a larger mean bodyweight (4.83 ± 1.14 kg) than females (3.77 ± 0.58 kg); nevertheless, the influence of the gender itself cannot be excluded. Shiroma et al 19 also found that there was a significant influence of the reproductive status on renal length, with intact cats showing larger kidneys than neutered ones. This was also true in our study, although the difference between intact and castrated animals was not significant, probably owing to the fact that our cat population mainly consisted of intact cats. As the difference in renal length between male and female cats was quite obvious, it could be useful to keep the gender of the cats in mind when evaluating the renal length.
In our study, a positive, but weak, correlation (r = 0.35) was observed between age and renal length. This was quite surprising as previously only a negative correlation has been described with the kidney decreasing in size with increasing age of the cats.20,28 However, Glodny et al 21 found that in humans a decrease in renal length only starts in the fifth decade of life and that before this age the kidneys can increase in size. 21 This explains our findings as we only included young-to-middle-aged Ragdoll cats, but no elderly cats. However, there was also a weak positive correlation (r = 0.36) between age and bodyweight. Therefore, the effect of age on renal length could have been influenced by bodyweight
On the basis of previous anatomic evaluations the cortical thickness of healthy cats measures 0.2–0.5 cm. 9 This does not correlate with our findings, where we measured cortical thickness as 0.73 ± 0.15 cm. This value lies in between the ultrasonographical results of Park et al, 13 who found a cortical thickness of 0.47 ± 0.08 cm, and Walter et al, 9 who found a cortical thickness of 0.82 ± 0.14 cm. The values in our study for medullary thickness (0.87 ± 0.19 cm) were higher than the results mentioned in previous studies.9,13 However, in both studies, cortical and medullary thickness were determined on a sagittal sonogram as opposed to the dorsal sonogram used in our study. As kidney width (sagittal plane) differs from kidney height (dorsal plane), the scan plane used to perform the measurements has an influence on the measurements of the cortical and medullary thickness. Park et al 13 observed a high degree of correlation between bodyweight and renal cortical thickness, and a low degree of correlation with the medullary thickness. Unfortunately, no statistical significance of this correlation is mentioned in their study. In our study, no significant correlation between CM ratio and bodyweight was found. In humans, Kubodera et al 25 found that both the cortical and medullary thickness were negatively correlated with age, but that there was no correlation between CM ratio and age. This corresponds with our results, as we also could not find a relationship between CM ratio and age. In our study, we only found a negative correlation between the presence of a medullary rim sign and the CM ratio. This finding was expected, as the medullary rim sign is a part of the medulla.
Although the CM ratio showed a wide range, overall variance was lower than for renal length. Renal length is not only a larger numerical measurement but, as showed above, is also influenced by many factors, such as age, gender and bodyweight. Measurement of renal cortical and medullary thickness has not found its way into clinical practice. Its use is considered limited because the corticomedullary junction and renal sinus borders cannot always be clearly defined on ultrasound images and may contribute to the high observer variation in measurement of the cortical thickness.13,29 However, it should be mentioned that few studies have looked into the CM ratio and cortical and medullary thickness in cats. Further research is therefore needed to evaluate the usefulness of the CM ratio for the detection of kidney disease by ultrasonography, similar to human medicine.
Eight cats showed a medullary rim sign, a thin (1–3 mm) linear hyperechogenic line in the outer medulla parallel to the corticomedullary junction. This is caused by microscopic mineral deposits within the lumen of the proximal renal tubules and can occur as an incidental finding or in animals with a wide variety of pathological renal lesions.7,8,15,30,31 None of our cats showed clinical or other sonographical renal abnormalities, so the medullary rim sign was considered as an incidental finding.
A hyperechoic cortex, namely a cortex that is iso- or hyperechoic compared with the liver/spleen, was observed in seven cats. Cortical echogenicity is more variable in cats than in dogs owing to the presence of fat vacuoles in the cortical–tubular epithelium.7,16,28,31,32 A plentiful number of fat vacuoles in the renal cortex is associated with an increased difference in echogenicity between cortex and medulla, so that hyperechoic cortices can occur in healthy cats. 12 The amount of fat content in the renal cortex is not related to body weight, but only to sex hormones and maturity.6,12 Drost et al 33 did not find a significant difference in echogenicity related to the gender of the cats. Owing to the low number of kidneys showing hyperechoic cortices, we could not statistically evaluate this in our study, although hyperechogenic cortices seem to appear more in male (5/16) than female cats (2/40).
Conclusions
In our study a randomised population of Ragdoll cats was observed. The Ragdoll cats showed a mean renal length of 3.83 ± 0.45 cm (range 2.98–5.09 cm), a mean cortical thickness of 0.73 ± 0.15 cm (range 0.36–1.18), a mean medullary thickness of 0.87 ± 0.19 cm (range 0.46–1.39) and a mean corticomedullary ratio of 0.88 ± 0.29 (range 0.29–1.78). A greater individual variability was found for renal length compared with the CM ratio. The renal length showed a positive correlation with age, bodyweight and male gender. Therefore, it can be useful to keep the age, bodyweight and gender of the investigated cat in mind when evaluating renal length on ultrasound. The CM ratio, however, was solely influenced by the presence of a medullary rim sign (P <0.0001) and not by the renal length, gender, age or bodyweight of the cats. Further research, however, is needed to investigate the usefulness of the CM ratio to detect kidney disease with ultrasonography.
Footnotes
Funding: This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
The authors do not have any potential conflicts of interest to declare.
Accepted: 28 March 2013
References
- 1. Lulich JP, O’Brien TD, Osborne CA, Polzin DJ. Feline renal failure: questions, answers, questions. Comp Contin Educ Pract Vet 1992; 14:127–152. [Google Scholar]
- 2. Scandinavian Ragdoll Club. Health: Scandinavian Ragdoll clubs health programme. http://www.weikums.se/health.htm (2004, accessed 15 September 2011).
- 3. Ragdoll Club Benelux. Gezondheid: CIN. http://www.Ragdollclubbenelux.com/nl_gezondheidcin.html (2003, accessed 15 September 2011).
- 4. Paepe D, Saunders JH, Bavegems V, et al. Screening of Ragdoll cats for kidney disease: a retrospective evaluation. J Small Anim Pract 2012; 53: 572–577. [DOI] [PubMed] [Google Scholar]
- 5. Walter PA, Johnston GR, Feeney DA, O’Brien TD. Renal ultrasonography in healthy cats. Am J Vet Res 1987; 48: 600–607. [PubMed] [Google Scholar]
- 6. Walter PA, Johnston GR, Feeney DA, O’Brien TD. Applications of ultrasonography in the diagnosis of parenchymal kidney disease in cats: 24 cases (1981–1986). J Am Vet Med Assoc 1988; 192: 92–9. [PubMed] [Google Scholar]
- 7. Nyland TG, Mattoon JS, Herrgesell EJ, Wisner ER. Urinary tract. In: Nyland TG, Mattoon JS. (eds). Small animal diagnostic ultrasound. 2nd ed. Philadelphia: WB Saunders, 2002, pp 158–195. [Google Scholar]
- 8. Widmer WR, Biller DS, Adams LG. Ultrasonography of the urinary tract in small animals. J Am Vet Med Assoc 2004; 225: 46–54. [DOI] [PubMed] [Google Scholar]
- 9. Walter PA, Feeney DA, Johnston GR, Fletcher TF. Feline renal ultrasonography: quantitative analyses of imaged anatomy. Am J Vet Res 1987; 48: 596–599. [PubMed] [Google Scholar]
- 10. Barr FJ. Evaluation of ultrasound as a method of assessing renal size in the dog. J Small Anim Pract 1990; 31: 174–179. [Google Scholar]
- 11. Barrett RB, Kneller SK. Feline kidney mensuration. Acta Radiol Suppl 1972; 319: 279–280. [PubMed] [Google Scholar]
- 12. Yeager AE, Anderson WI. Study of association between histologic features and echogenicity of architecturally normal cat kidneys. Am J Vet Res 1989; 50: 860–863. [PubMed] [Google Scholar]
- 13. Park I, Lee H, Kim J, et al. Ultrasonographic evaluation of renal dimension and resistive index in clinically healthy Korean domestic short-hair cats. J Vet Sci 2008; 9: 415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mannion P. Diagnostic ultrasound in small animal practice. 1st ed. Oxford: Blackwell Science, 2006, pp 109–127. [Google Scholar]
- 15. d’Anjou M. Kidneys and ureters. In: Penninck D, d’Anjou M. (eds). Atlas of small animal ultrasonography. 1st ed. Ames: Blackwell Publishing, 2008, pp 339–364. [Google Scholar]
- 16. Larson MM. The kidneys and ureters. In: O’Brien R, Barr F. (eds). BSAVA manual of canine and feline abdominal imaging. 1st ed. Gloucester: British Small Animal Veterinary Association, 2009, pp 185–204. [Google Scholar]
- 17. Barr FJ, Holt PE, Gibbs C. Ultrasonographic measurement of normal renal parameters. J Small Anim Pract 1990; 31: 180–184. [Google Scholar]
- 18. Emamian SA, Nielsen MB, Pedersen JF, Ytte L. Kidney Dimensions at sonography: correlation with age, sex, and habitus in 665 adult volunteers. Am J Roentgenol 1993; 160: 83–86. [DOI] [PubMed] [Google Scholar]
- 19. Shiroma JT, Gabriel JK, Carter RL, et al. Effect of reproductive status on feline renal size. Vet Radiol Ultrasound 1999; 40: 242–245. [DOI] [PubMed] [Google Scholar]
- 20. Seyrek-Intas D, Kramer M. Renal imaging in cats. Vet Focus 2008; 18: 23–30. [Google Scholar]
- 21. Glodny B, Unterholzner V, Taferner B, et al. Normal kidney size and its influencing factors – a 64-slice MDCT study of 1.040 asymptomatic patients. BMC Urology 2009; 9: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ozmen CA, Akin D, Bilek SU, et al. Ultrasound as a diagnostic tool to differentiate acute from chronic renal failure. Clin Nephrol 2010; 74: 46–52. [PubMed] [Google Scholar]
- 23. Adibi A, Emami Naini A, Salehi H, Matinpour M. Renal cortical thickness in adults with normal renal function measured by ultrasonography. Iran J Radiol 2008; 5: 163–166. [Google Scholar]
- 24. Beland MD, Walle NL, Machan JT, Cronan JJ. Renal cortical thickness measured at ultrasound: is it better than renal length as an indicator of renal function in chronic kidney disease? Am J Roentgenol 2010; 195: 146–149. [DOI] [PubMed] [Google Scholar]
- 25. Kubodera T, Asakawa M, Ito H. Morphometric studies on the cortex and medulla of frontally sectioned kidneys in the Japanese adult. Kaibogaku Zasshi 1993; 68: 504–512. [PubMed] [Google Scholar]
- 26. Paepe D, Bavegems V, Combes A, et al. Prospective evaluation of Ragdoll and control cats for kidney disease by routine laboratory parameters and ultrasonography. J Vet Intern Med 2012; 26: 1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mantis P. Ultrasonography of the urinary and genital system of the dog and cat. Iran J Vet Surg .2008; Suppl.: 63–71. [Google Scholar]
- 28. Burk RI, Feeney DA. Small animal radiology and ultrasonography: a diagnostic atlas and text. 3rd ed. St. Louis: WB Saunders, 2003, pp 262–265, 360–388. [Google Scholar]
- 29. Emamian SA, Nielsen MB, Pedersen JF. Intraobserver and interobserver variations in sonographic measurements of kidney size in adult volunteers: A comparison of linear measurements and volumetric estimates. Acta Radiol 1995; 36: 399–401. [PubMed] [Google Scholar]
- 30. Biller DS, Bradley GA, Partington BP. Renal medullary rim sign: ultrasonographic evidence of renal disease. Vet Radiol Ultrasound 1992; 33: 286–290. [Google Scholar]
- 31. Dennis R, McConnell F. Diagnostic imaging of the urinary tract. In: Elliott J, Grauer GF. (eds). BSAVA manual of canine and feline nephrology and urology. 2nd ed. Gloucester: British Small Animal Veterinary Association, 2007, pp 126–141. [Google Scholar]
- 32. Dennis R, Kirberger RM, Barr F, Wrigley RH. Handbook of small animal radiology and ultrasound: techniques and differential diagnoses. 2nd ed. Edinburgh: Elsevier, 2010, pp 305–308. [Google Scholar]
- 33. Drost WT, Henry GA, Meinkoth JH, et al. Quantification of hepatic and renal cortical echogenicity in clinically normal cats. Am J Vet Res 2000; 61: 1016–1020. [DOI] [PubMed] [Google Scholar]


