Abstract
The objective of this study was to assess the prevalence of renal cysts and other renal abnormalities in purebred Maine Coon cats, and to characterise these through genetic typing. Voluntary pre-breeding screening programmes for polycystic kidney disease (PKD) are offered for this breed throughout Switzerland, Germany and other northern European countries. We performed a retrospective evaluation of Maine Coon screening for renal disease at one institution over an 8-year period. Renal ultrasonography was performed in 187 healthy Maine Coon cats. Renal changes were observed in 27 of these cats. Renal cysts were found in seven cats, and were mostly single and unilateral (6/7, 85.7%), small (mean 3.6 mm) and located at the corticomedullary junction (4/6, 66.7%). Sonographical changes indicating chronic kidney disease (CKD) were observed in 10/187 (5.3%) cats and changes of unknown significance were documented in 11/187 (5.9%) cats. All six cats genetically tested for PKD1 were negative for the mutation, and gene sequencing of these cats did not demonstrate any common genetic sequences. Cystic renal disease occurs with a low prevalence in Maine Coons and is unrelated to the PKD observed in Persians and related breeds. Ultrasonographical findings compatible with CKD are not uncommon in juvenile Maine Coons.
Introduction
Polycystic kidney disease (PKD) represents a heterogenous set of disorders that have been found in humans to result from single gene defects transmitted as either autosomal dominant or autosomal recessive traits. 1 In the domestic cat, the autosomal dominant form of the disease (AD-PKD) has been described in Persian and related breeds, with a worldwide prevalence estimated at 37–49% less than a decade ago,2–6 to 25.9% in a more recent study. 7 The disease, characterised by the growth of cysts within renal parenchyma, is progressive and may be responsible for irreversible renal failure in affected cats. Hepatic and pancreatic cyst formation has been associated with this disease complex. 8 Eradication of AD-PKD is possible through active selection of PKD-free progenitors. Ultrasonography has proven to be a cost-effective modality for large-scale phenotypical evaluation of potentially affected individuals, with a sensitivity for cyst detection of 91% and a specificity of 100% at 36 weeks of age. 9 For this reason, some authors recommend screening cats older than 10 months.3,10 Following the identification of the genetic mutation responsible for AD-PKD, 6 highly specific and sensitive commercial assays have been made available for disease screening in Persians and their crosses.11,12
In Switzerland, Maine Coon breeders commonly have their cats’ kidneys scanned for cyst detection on a voluntary basis. Breeder awareness appears high, with many websites either announcing PKD-free animals or discussing PKD being a concern in this breed. Maine Coons are popular in Switzerland, being the most commonly treated purebred cats at the Vetsuisse Faculty of the University of Bern. They are 3.5 times more numerous than the second most common breed, the Persian. In 2009 and 2010 Maine Coons represented 9.2% and 11% of our 1550 yearly feline admissions, respectively.
The Vetsuisse Faculty, consisting of the veterinary hospitals of the Universities of Bern and Zürich, has an established protocol for official sonographical PKD evaluation in this breed. Web searches, as well as ultrasound and anecdotal reports from the Netherlands, Austria and Germany, suggest these screenings are not a local phenomenon. Radiology diplomates report having observed a higher incidence of renal cysts in Maine Coons in the past (U Geissbühler and J Lang, personal communications). It was initially assumed that cystic renal disease had been introduced into the breed as a result of inadvertent crossbreeding with Persians. Breeders have reported the disease skipping generations, which would be unexpected with AD-PKD. Documentation of one litter with two affected offspring, where neither parents were affected, and the observation of male and female cases would be more compatible with a monogenic autosomal recessive mode of inheritance. The behaviour of cystic renal disease in Maine Coons thus appears different from AD-PKD recognised in Persians and related breeds. We hypothesised that a different mutation or origin would be responsible for the disease. There are, to our knowledge, no descriptions of cystic renal disease in Maine Coon cats in the scientific literature.
The purposes of this study were:
to document the frequency and ultrasonographical appearance of renal abnormalities in purebred Maine Coons participating in a pre-breeding screening programme;
to establish whether the same genetic mutation is responsible for cystic renal disease in Maine Coons and Persians.
Materials and methods
Clinical evaluation
Medical records of purebred Maine Coon cats presented at the Small Animal Hospital of the Vetsuisse Faculty, University of Bern, for official ultrasonographical PKD screening between January 2005 and January 2012 were retrospectively evaluated. At our institution the recommended age at time of screening is 10 months, but younger patients were occasionally scanned and included in the study. Patients were required to be microchipped, and their identity was systematically controlled comparing the scanned microchip number with data registered on the original version of the pedigree; a photocopy of the latter was kept in the patient file. Pre-breeding screening in Maine Coons at our institution normally begins with an echocardiographic evaluation; Cats diagnosed with hypertrophic cardiomyopathy (HCM) are not recommended for breeding and are usually not examined any further.
Abdominal ultrasonography was performed by an ECVDI Diplomate accredited for this evaluation. Patients were manually restrained in lateral recumbency, hairs were parted over each kidney, and skin soaked with a liberal amount of alcohol and coupling gel to achieve adequate contact. The kidneys were evaluated with 5–13MHz (Aloka Prosound α-10; Hitachi) or 7.5–13MHz (Aloka Prosound 5500; Hitachi) linear probes. Anechoic circular lesions within renal parenchyma with associated distal acoustic enhancement, with a very thin or absent lesion wall were interpreted as cysts. Changes in the renal architecture and echogenicity were documented.
Follow-ups were conducted at time of manuscript preparation either by telephone or email correspondence with the owner or breeder of the cat.
DNA isolation
Ethylenediamine tetra-acetic acid (EDTA) blood samples of subjects affected with renal cysts, their relatives of known PKD status and blood from cyst-free Maine Coons were collected prospectively, with written owner consent. The study was approved by the ‘Cantonal Committee for Animal Experiments’ (Canton of Bern; permit 32/11). Genomic DNA was isolated from the EDTA blood samples using the Nucleon Bacc2 kit (GE Healthcare).
Genotyping of the PKD1:c.10063C>A variant 6
Primers TTCTTCCTGGTCAACGACTG and CAGGTAGACGGGATAGACGA were used to amplify a 569-base pair product encompassing exon 29 of the feline PKD1 gene. After purification with shrimp alkaline phosphatase and exonuclease the PCR products were directly sequenced with the BigDye v3.1 kit on an ABI 3730 capillary sequencer (Life Technologies).
Genome-wide association study and homozygosity mapping
Genomic DNA from six cats with renal cysts and seven PKD-negative Maine Coon controls was genotyped on the illumina feline 70k single nucleotide polymorphism (SNP) array. The control cats underwent the same ultrasonographical screening, and were deemed cyst-free at a median age of 43.8 months (range 8–66 months). Plink software 13 was used to analyse the data. Animals and markers with a call rate of less than 90% were removed. Furthermore, non-informative markers (minor allele frequencies <0.01) and markers strongly deviating from Hardy–Weinberg equilibrium (P <0.00001) were also removed. With the remaining markers an allelic association study was performed. Correction for multiple testing was done using 100,000 permutations and only Pgenome-values (the p value after 100’000 permutations) of less than 5% were considered significant. Plink was also used to determine shared runs of homozygosity between the cats using the options –homozyg and –homzyg-group.
Results
Clinical findings
One hundred and eighty-seven Maine Coons, all deemed to be in good health by their owners, underwent ultrasonography of the kidneys during the study period. Of these, 10 had one repeat examination, and one had a total of three examinations. Median age at time of first ultrasound scan was 14 months (range 8–122 months). Of these, four cats were younger than 10 months. There were 55 males and 132 females.
Over the 7 years covered by this study, renal ultrasonography revealed abnormalities in 27 cats. These abnormalities consisted of renal cysts in seven cats, chronic kidney disease (CKD) in 10 cats (one of which was also included in the group with renal cysts) and 11 cats with changes of unknown significance.
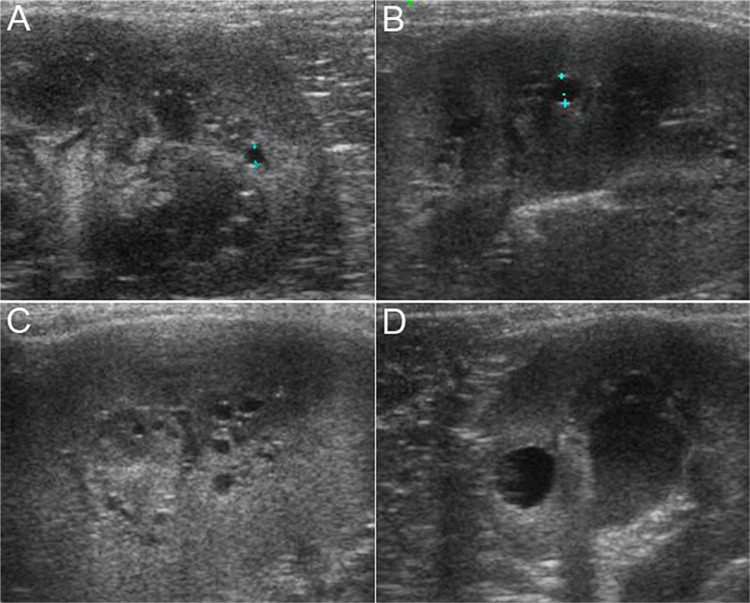
In the seven cats found to have renal cysts, median age was 14 months (range 12–20 months) (Table 1). Of these cats, one showed ultrasonographical signs of CKD. This cat died of renal disease at 4 years of age, while the other 4/5 cats with renal cysts for which follow-up was available were reported alive and well at ages ranging from 4 to 5.5 years. Cysts were located at the corticomedullary junction in 4/6 cats (67%); 2/6 (33%) were within the cortex. Cyst location could not be clearly established in the cat with signs of CKD either from stored images or radiological report. Cysts were most commonly single and unilateral (6 /7, 85.7%) and small (average size for single cysts 3.5 mm). Patient 2 in Table 1 had a repeat ultrasound 1 year later, in which the cyst had more than doubled in diameter. In patient 3, numerous very small cortical cysts were clustered bilaterally along the corticomedullary junction (Figure 1B and C).
Table 1.
Maine Coon cats with renal cysts – individual data
| Patient | Age (months) | Gender | Kidney affected | Cyst number | Cyst size (mm) | Ultrasonographical findings | Outcome |
|---|---|---|---|---|---|---|---|
| 1* | 12 | M | L | 1 | 3.3 | Bilateral nephrolithasis. Bilaterally reduced corticomedullary distinction. Mildly irregular left renal contours | Died of renal disease at 4 years |
| 2 | 12 | F | L | 1 | 2.2 | Cortical cyst. Control ultrasound 1 year later demonstrated moderate growth, cyst irregular (3.5 × 5 mm) | Lost to follow-up |
| 3* | 12 | M | Both | Countless | Very small, largest 3 | Cysts clustered at the corticomedullary junction. UspG 1.041; BUN 10.8 mmol/l (6.6–12.5 mmol/l); creatinine 105 μmol/l (53–141 μmol/l) | Alive and well at 5 years |
| 4* | 18 | M | L | 1 | 3 | Cyst at corticomedullary junction | Lost to follow-up |
| 5* | 17 | F | R | 1 | 5.9 | Cortical cyst | Alive and well at 4 years |
| 6* | 20 | F | R | 1 | 1.8 | Medullary rim sign. Cyst at corticomedullary junction. UspG 1.053, pH 5.8, chemical and sediment analysis normal, with 3+ fat droplets |
Alive and well at 4.5 years |
| 7* | 14 | M | L | 1 | 5.3 | Cyst at corticomedullary junction | Alive and well at 5.5 years |
M = male; F = female; R = right; L = left; UspG = urine specific gravity; BUN = blood urea nitrogen
Cats which underwent PKD1 exon 29 allele mutation testing
Figure 1.
Ultrasonographical images of the kidneys of Maine Coons with renal cysts. (A) Patient 6, sagittal view of the caudal pole of the right kidney. Between the calipers, a 1.8 mm cyst at the corticomedullary junction. Fine hyperechoic dots are aligned along the deeper portions of the medulla (rim sign). Renal parenchyma was interpreted as otherwise normal. Sagittal (B) and parasagittal (C) views of the left kidney of patient 3. Between the calipers is a 3 mm cyst. Countless minuscule cysts are visualised along the corticomedullary junction. (D) Patient 5, transverse view of the right kidney. The largest cyst observed in this series (5.9 mm in diameter) is found within the cortex
Two cyst-positive cats were siblings, another was half-sibling to these; all three were sired by a male negative for cysts at 16 and 65 months. Both mothers of these three cats were negative for cysts at 14 and 48 months, and 36 and 72 months, respectively. One cyst-positive female (Figure 1D) had recently been imported from the Netherlands.
Ultrasonographical findings compatible with CKD were recorded in 10 cats (10/187, 5.3%), including one cat with a single renal cyst mentioned above. The three most common findings in this group were reduced corticomedullary distinction (6/10, 60%), irregular kidney contours in addition to other changes compatible with CKD (6/10, 60%), mineral sand or reflectors in the renal pelvis (5/10, 50%). None of these patients underwent blood or urine chemistry testing at the time of the ultrasound. Median age in this group was 13 months, with a range of 12–82 months.
During the study period, 11 cats (11/187, 5.9%) were found to have an irregular renal form without changes in renal echogenicity (Table 2). These irregularities of unknown significance included:
Table 2.
Maine Coon cats with ultrasonographical findings of unknown significance
| Patient | Age (months) | Gender | Kidney affected | Ultrasonographical findings | Outcome |
|---|---|---|---|---|---|
| 8 | 11 | F | Both | Capsular constriction | Euthanased at 4.5 years for renal disease |
| 9 | 13 | F | Both | Capsular constriction | Died of renal disease |
| 10 | 13 | F | R | Capsular constriction | Died at 3 years of renal disease |
| 11 | 12 | F | Both | Mild irregularity of renal capsule | Was alive and well at 2 years, then lost to follow-up |
| 12 | 12 | F | L | Capsular constriction | Euthanased because of renal disease |
| 13 | 12 | F | L | Capsular constriction | Alive and well at 7 years |
| 14 | 15 | M | L | Capsular constriction | Lost to follow-up |
| 15 | 14 | M | L | Flattened cranial pole | Alive and well at 7 years |
| 16 | 14 | F | Both | Mild irregularity of renal capsule. Left kidney somewhat too small (3.2 cm) | Alive and well at 6.5 years |
| 17 | 18 | F | R | Flattened cranial pole | Alive and well at 6.5 years |
| 18 | 12 | F | R | Capsular constriction | Lost to follow-up |
M= male; F= female; L= left; R= right
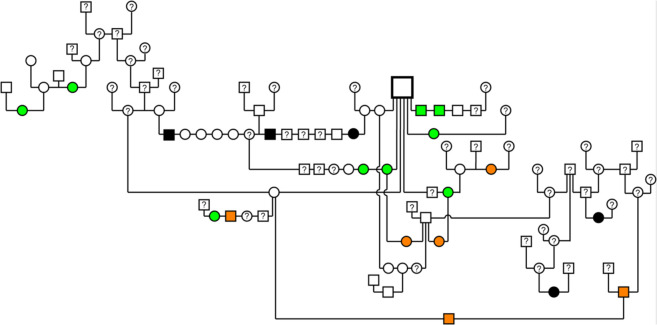
deep constrictions in the capsule similar to lobulation in 7/11 (63.6%), of which six had a common male ancestor (five as father, one as grandfather) (Figure 2); five of these seven (71.4%) cats presented unilateral changes, three on the left and two on the right side;
unilateral flattening of a pole (2/11, 18.2%);
mild bilateral irregularity of the renal capsule (2/11, 18.2%).
Figure 2.
Pedigree of selected Maine Coon cats from this study. Open symbols represent cats that showed regular kidneys in the ultrasound examination. Black filled symbols indicate cats affected with renal cysts. Green filled symbols and orange filled symbols indicate changes of unknown significance and chronic kidney disease, respectively. For animals with a question mark the renal phenotype is unknown. Note that three cats with renal cysts in this pedigree descend from non-affected parents. Clear familial clustering around a popular sire can be noted for renal changes of unknown significance; three cats with such changes also descend from non-affected parents
Limited follow-up information was obtained for nine of these 11 cats (81.8%). While five were alive and well according to their owners, four had had died of or had been euthanased because of renal failure.
Genetic findings
All cats with renal cysts were homozygous wild-type at the variant causing AD-PKD in Persian cats (PKD1:c.10063C>A). Thus, the ultrasonographical changes seen in the Maine Coons are not caused by the known AD-PKD allele.
A pedigree analysis of the cats with renal changes revealed familial clustering indicating a genetic cause (Figure 2). Male and female cats were equally affected suggesting an autosomal mode of inheritance. Three Maine Coons affected with renal cysts from two litters were offspring of non-affected parents, excluding a simple dominant mode of inheritance.
As the pedigree seemed compatible with a monogenic autosomal recessive mode of inheritance, an attempt was made to map the causative mutation by genome-wide association analysis. A total of six PKD-affected Maine Coons and seven non-affected controls was genotyped on the feline 70 k SNP microarray. However, no significant differences in allele frequencies between cases and controls were seen. The six affected cats also did not show extended regions of shared homozygosity (data not shown).
Discussion
In this study, renal cysts were observed in Maine Coon cats that were tested clear of the ‘Persian’ PKD allele. The renal cysts identified in seven young Maine Coons were mainly unilateral (86%), small (mean 3.5 mm) and, in those where this distinction could be confidently made, located at the corticomedullary junction (67%). In humans, the simple renal cyst is considered an acquired lesion, associated mainly with increasing age. Autopsy studies report a 50% incidence of renal cysts after 50 years of age. 14 Single, large cysts are occasionally encountered in middle-aged and older veterinary patients. 8 Weakening of the tubular basement membrane of the distal convoluted or collecting duct cells has been postulated as a mechanism to single cyst formation, resulting in a diverticulum which expands in time. Simple renal cysts are also occasionally observed in children, 15 with a prevalence of 0.22% irrespective of age or gender, and tend not to increase in size with time. Whether these occur in juvenile cats is unknown. In the case of patient 1, a 12-month-old cat with ultrasonographical signs of chronic renal disease, it is unclear if the cyst formed secondary to the degenerative renal changes, or if it was present before the degeneration began.
Patient 3 is particularly interesting in that countless small cysts were bilaterally distributed along the corticomedullary junction in otherwise sonographically normal kidneys. PKD, either autosomal recessive or dominant, is characterised by renal enlargement and uniform cyst distribution over the entire organ. 16 Numerous cysts at the corticomedullary junction have been described in glomerulocystic kidney disease in a 4-month-old kitten in end-stage renal disease. 17 Ultrasonographically, bilateral renomegaly, hyperechoic cortices and mild pyelectasia were observed in this kitten, but the cysts themselves were not identified. No ultrasonographical equipment specifications are provided in the above case report; however, cysts were macroscopically in the 1 mm range, similar in size to those observed in patient 3 with a high-frequency transducer.
In humans, location of the cysts at the cortico-medullary border is a feature of a restricted number of conditions. 18 Recently, a nephropathy has been described in a 4-year-old Boxer, which resembled human nephronophtisis (NPHP) and medullary cystic kidney disease complex, two genetic conditions associated with the development of cysts at the corticomedullary junction. 19 NPHP is an autosomal recessive condition associated with extra-renal features in 10–20% of cases and end-stage renal disease before 25 years of age, whereas medullary cystic kidney disease is an autosomal dominant condition leading to end-stage renal disease in the third to fourth decades of life. 18 Tubular atrophy, interstitial fibrosis and changes in the tubular basement membranes are the main characteristics of both these conditions, and in the case of NPHP the appearance of cysts is said to occur in the later phases of the disease. 20 Even though renal function was not specifically assessed in 5/7 of the cyst-positive cats in our series, the absence of clinical symptoms and lack of ultrasonographical parenchymal changes in this group make the above-described conditions less likely.
‘Maine Coon PKD’ thus appears to represent a form of juvenile nephropathy other than AD-PKD, and its exact nature needs to be settled through histological evaluation of affected kidney tissue. It is unclear at this time if these seven cases exemplify different presentations of the same pathology or different pathologies altogether. Owing to the retrospective nature of the study and the minimal follow-up data, there remains a possibility that cyst-free Maine Coons went on to develop cysts at a later age. Future prospective studies following cats with increasing age are needed.
Cyst detectability is highly dependent on instrumentation. The lateral resolution of the linear probe on the ultrasound machine used since 2007 at our institution, specifically its ability to discern as separate two closely spaced objects, is approximately 0.15 mm. Clearly, the ability to detect finer abnormalities would have been reduced prior to this date, as image quality and spatial resolution have since improved.
The clinical significance of the malformed, but otherwise ultrasonographically normal, kidneys in 11 cats is currently unknown. At least four of these 11 cats died of complications related to renal insufficiency, all at less than 6 years of age. Post-mortem evaluation of renal tissue was, unfortunately, not performed in any of these cats. It is unlikely that the lobulations and malformations represent a form of infarction, as the classic ‘linear or wedged-shaped, well defined lesions in the cortex, perpendicular to the cortex’ 21 were neither reported nor seen on the images saved. The incidence of ultrasonographical manifestations of CKD in this Maine Coon population appears lower than in a similar study performed in slightly older Ragdoll cats. 10 In our study group, CKD was observed in 5.4% of cats compared with 10% in Ragdolls, and mineral sand or reflectors in the renal pelvis were noticed in half of the affected cats. The chemical composition of these mineral aggregates was not determined. Urinalyses and renal function measurements were not routinely performed in cats presenting sonographical changes, which limits the informative value of these findings.
Most of the animals evaluated in our pre-breeding screening programmes originate from within a 75 km radius of our institution. This may have biased our findings by including only breeders motivated to screen their cats. Common ancestors were found on inspection of the pedigrees of the majority of cats affected by renal cysts and irregular kidney form. The familial clustering suggests a genetic cause for these abnormalities. Pedigree analysis of cats affected with renal cysts ruled out a fully penetrant monogenic autosomal dominant transmission mode of the condition as seen in AD-PKD. However, as no shared segment of homozygosity could be identified in the six cyst-positive cats tested, it appears renal cysts in Maine Coons likely are not a simple monogenic autosomal recessive trait either. Given the small number of cats in the molecular genetic analysis one has to caution that a single phenotyping error could have led to the same results. Thus, at this time it is not clear whether all observed renal cysts were really caused by the same genetic defect. Finally, it also has to be cautioned that the feline genome is not fully covered by the currently available 70 k SNP array. There is a slight possibility that renal cysts in Maine Coons are inherited as a monogenic autosomal recessive trait, but the resulting shared segment of homozygosity might have been missed if it had been located in a genome region with insufficient marker coverage.
It is unclear if the screening of 2.4 times more females than males influenced the results of the study; however, all three types of findings reported herein were detected in both sexes with no apparent predilection. Similarly, the practical exclusion of HCM-positives, which, in fact, did not go on to renal screening, may have skewed the true incidence of renal changes should a genetic link exist between the two conditions.
Conclusions
Renal abnormalities appear common in juvenile Maine Coons (27/187, 14.4%). Documented abnormalities included chronic renal disease (10/187, 5.3%) and changes of unknown significance (11/187, 5.9%).
To our knowledge, this is the first report of renal cyst formation in Maine Coon cats. Cysts had a low incidence (7/187, 3.7%) and were mostly single and unilateral (6/7, 85.7%). In cats where this distinction could be confidently made, cysts were mostly located at the corticomedullary junction (4/6, 66.7%). This specific location may indicate a different subset of diseases. All tested cats with renal cysts were negative for the ‘Persian’ PKD mutation. PKD may be a misnomer for this condition in Maine Coons, and further research is needed to determine the nature of these changes.
Acknowledgments
We are particularly grateful to the participating Maine Coon breeders and owners for their patience, collaboration and transparency.
Footnotes
Funding: This work was supported by the Morris Foundation, which additionally donated the SNP chips.
The authors do not have any potential conflicts of interest to declare.
Accepted: 8 May 2013
References
- 1. Lifton R, Somlo S, Giebisch G. Genetic diseases of the kidney. Oxford: Elsevier, 2009, p 393. [Google Scholar]
- 2. Barthez PY, Rivier P, Begon D. Prevalence of polycystic kidney disease in Persian and Persian related cats in France. J Feline Med Surg 2003; 5: 345–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barrs VR, Gunew M, Foster SF, et al. Prevalence of autosomal dominant polycystic kidney disease in Persian cats and related-breeds in Sydney and Brisbane. Aust Vet J 2001; 79: 257–259. [DOI] [PubMed] [Google Scholar]
- 4. Beck C, Lavelle RB. Feline polycystic kidney disease in Persian and other cats: a prospective study using ultrasonography. Aust Vet J 2001; 79: 181–184. [DOI] [PubMed] [Google Scholar]
- 5. Cannon MJ, Barr FJ, Rudorf H, et al. Prevalence of polycystic kidney disease in Persian cats in the United Kingdom. Vet Rec 2001; 149: 409–411. [DOI] [PubMed] [Google Scholar]
- 6. Lyons L, Biller D, Erdman CA, et al. Feline polycystic kidney disease mutation identified in PKD1. J Am Soc Nephrol 2004; 15: 2548–2555. [DOI] [PubMed] [Google Scholar]
- 7. Lee YJ, Chen HY, Hsu WL, et al. Diagnosis of feline polycystic kidney disease by a combination of ultrasonographic examination and PKD1 gene analysis. Vet Rec 2010; 167: 614–617. [DOI] [PubMed] [Google Scholar]
- 8. Rademacher N. Liver. In: Barr F, Gaschen L. (eds). BSAVA manual of canine and feline ultrasonography. Gloucester: British Small Animal Veterinary Association, 2011, p 92. [Google Scholar]
- 9. Biller DS, Di Bartola SP, Eaton KA, et al. Inheritance of polycystic kidney disease in Persian cats. J Hered 1996; 87: 1–5. [DOI] [PubMed] [Google Scholar]
- 10. Paepe D, Saunders JH, Bavegems V, et al. Screening of ragdoll cats for kidney disease: a retrospective evaluation. J Small Anim Pract 2012; 53: 572–577. [DOI] [PubMed] [Google Scholar]
- 11. Helps CR, Tasker S, Barr FJ, et al. Detection of the single nucleotide polymorphism causing feline autosomal-dominant polycystic kidney disease in Persians from the UK using a novel real-time PCR assay. Mol Cell Probes 2007; 21: 31–34. [DOI] [PubMed] [Google Scholar]
- 12. Helps CR, Tasker S, Harley R. Correlation of the feline PKD1 genetic mutation with cases of PKD diagnosed by pathological examination. Ex Mol Pathol 2007; 83: 264–268. [DOI] [PubMed] [Google Scholar]
- 13. Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Skolarikos A, Laguna PM, de la, Rosette J. Conservative and radiological management of simple renal cysts: a comprehensive review. BJU Int 2012; 110: 170–178. [DOI] [PubMed] [Google Scholar]
- 15. McHugh K, Stringer DA, Hebert D. Simple renal cysts in children: diagnosis and follow-up with US. Radiology 1992; 178: 383–385. [DOI] [PubMed] [Google Scholar]
- 16. O’Leary CA, Ghoddusi M, Huxtable CR. Renal pathology of polycystic kidney disease and concurrent hereditary nephritis in Bull Terriers. Aust Vet J 2002; 80: 353–361. [DOI] [PubMed] [Google Scholar]
- 17. Harkin KR, Biller DS, Balentine HL. Glomerulocystic kidney disease in a kitten. J Am Vet Med Assoc 2003; 223: 1780–1782. [DOI] [PubMed] [Google Scholar]
- 18. Loftus H, Ong ACM. Cystic kidney diseases: many ways to form a cyst. Pediatr Nephrol 2013; 28: 33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Basile A, Onetti-Muda A, Giannakakis K, et al. Juvenile nephropathy in a Boxer dog resembling the human nephronophtisis-medullary cystic kidney disease complex. J Vet Med Sci 2011; 73: 1669–1675. [DOI] [PubMed] [Google Scholar]
- 20. Saunier S, Salomon R, Antignac C. Nephronophtisis. Curr Opin Genet Dev 2005; 15: 324–331. [DOI] [PubMed] [Google Scholar]
- 21. d’Anjou MA. Kidneys and ureters. In: Penninck D, d’Anjou MA. (eds). Atlas of small animal ultrasonography. Ames, IA: Blackwell Publishing, 2008, pp 339–364. [Google Scholar]