Abstract
Portosystemic shunts (PSS), congenital or acquired, occur uncommonly in the feline population. The diagnostic approach is similar to one in dogs suspected of a PSS based on the clinical signs and haematological and biochemical changes. Diagnostic imaging, however, is key for the confirmation of a PSS. Although abdominal ultrasound is the first-choice diagnostic imaging modality, the results are not always unequivocal. Transsplenic portal scintigraphy (TSPS) using 99mTc-pertechnetate is a well-established technique in canine medicine, providing relatively fast and easy confirmation of the presence or absence of a PSS. As the prevalence of PSS is much lower in the feline population, this technique has not been widely used in cats. This retrospective study of 12 cases gives an overview of the potential of TSPS in the diagnostic work-up of PSS in cats (2005–2012).
Congenital or acquired portosystemic shunts (hereafter referred to both types as PSSs) are not frequently diagnosed in cats.1–4 Some cat breeds are reportedly more prone to PSSs (eg, Himalayan or Persian cats) although the majority are domestic shorthairs.5–8 Male cats are reported to be at slightly increased risk.9–11 The majority of cats are <1 year old at the time of diagnosis, but PSSs should not be excluded from the differential diagnosis in older cats.10,12,13 A striking feature may be copper-coloured irises.6,7,10
Initial clinical signs related to the circulatory bypass of the liver and subsequent persistence of nutrients and toxic metabolites in the systemic circulation are often an early indication for a PSS. These signs can be relatively mild (eg, retarded growth compared with littermates) or severe neurological/hepatoencephalopathic signs (eg, seizures, blindness, dullness, ptyalism).5,6,9,10,12,13 A small proportion of cats with PSSs have gastrointestinal problems (eg, vomiting, anorexia) or urinary problems (eg, urolithiasis). They are, however, outnumbered by cats with mainly neurological signs.5,6,10
The next step in the diagnostic procedure comprises a blood examination. Specific attention needs to be given to ammonia and the pre- and postprandial bile acid levels, as they increase when hepatic functionality is impaired3,14–18 Urinalysis can reveal ammonium (bi)urate cristalluria, possibly accompanied by secondary urinary tract infection.6,12,19
Confirmation of the presence of a PSS requires diagnostic imaging. Conventional radiography is of little help for the detection of the shunt itself.5,6,12 Portography reveals more information, but is invasive, time-consuming and requires prolonged anesthesia.13,14,17,20 Ultrasound (US) remains the first imaging modality in the initial work-up to the patient, but experience is a key factor for visualisation of an abnormal vessel.7,11,14,17,20–23 Where US examination is inconclusive, scintigraphy can be an option.17,24 It is a fast and minimally invasive aid in diagnosing PSSs, with a high sensitivity and specificity, although anatomical detail is rather poor.24–27 Per-rectal portal scintigraphy (PRPS) using pertechnetate (99mTcO4-) is a widely used method for PSS imaging in dogs and cats.25,27–32 After deposition of pertechnetate in the rectum or the distal descending colon, the tract of the reabsorbed activity indicates the presence or absence of a PSS. Instigated by the inherent disadvantages of this method (relatively high radiation burden and susceptibility to artefacts),25,26,30 an adaptation to this technique was made where the tracer is injected under US guidance directly into the splenic parenchyma [transsplenic portal scintigraphy (TSPS)]. The advantages of TSPS lie in the smaller amount of radioactivity necessary, a shorter scanning procedure than with PRPS, and the obtained images possibly render more information on shunt conformation. 26 The TSPS technique is well-established in dogs, where the prevalence of PSSs is much higher than in the feline population. 1
This retrospective study (2005–2012) describes the application and feasibility of the TSPS method in cats.
Case description
Patients
All animals (n = 12) were privately owned cats presented at the Small Animal Clinic of the Faculty of Veterinary Medicine, Ghent University between 2005 and 2012, and suspected of having a PSS based on the history, the clinical examination, the clinical signs, results of a blood analysis and/or abdominal US findings. Most cats were referred to the Veterinary Nuclear Medicine Division (Faculty of Veterinary Medicine, Ghent University) to confirm presence or absence of a PSS after an inconclusive US examination combined with other diagnostic results that indicated the presence of a PSS. Two cats had a postsurgical TSPS examination to evaluate the result of surgical PSS attenuation; one of them also had a presurgical TSPS scan.
Scans
The cats were fasted for a minimum of 12 h. Propofol (Propovet, 10 mg/ml; Abbott Laboratories) was used (2–4 mg/kg, bolus injection to effect) for anaesthesia. They were placed in right lateral recumbency above the gamma camera (Toshiba GCA401A) equipped with a low-energy, high-resolution collimator. The spleen was ultrasonographically localised (MyLab30Vet, Esaote Pie Medical, 7.5 MHz microconvex probe), and a 22-G needle was carefully inserted into the splenic parenchyma as far caudally and ventrally in the spleen as possible to avoid deposition of the radioactivity too close to the liver, thus possibly impeding correct scan processing and interpretation. A bolus of 99mTcO4- in a shielded syringe (mean ± SD 86.2 ± 34.78 MBq), diluted in 0.2–0.3 ml of 0.9% NaCl solution was then injected under constant ultrasonographical guidance. A few seconds prior to tracer injection a dynamic acquisition was started. Immediately upon injection the needle and syringe were removed from the spleen in a manner such that they did not pass over the cranial abdomen or thorax to avoid interference with the scan. The sequences were obtained at a rate of 4 frames per second over 60 s (240 frames in total). Images were stored in a 128 × 128 × 16 matrix size.26,33
The images were first reviewed in a dynamic film mode for visual analysis. Appearance of the bolus of activity in the liver before any activity arrives in the thorax indicates the absence of a PSS. Conversely, arrival of the bolus of activity in the heart first means the liver is bypassed, which is diagnostic for a PSS, whether congenital or acquired. The different frames were then summated to obtain a single composite image. On this image, regions of interest (ROIs) were manually placed: one over the heart and one over the liver. A time–activity curve (TAC) was calculated, portraying the appearance, progression and intensity of the activity in the different ROIs over time (Figures 1 and 2). A correction for background activity in the TAC calculation was made by an additional ROI over ‘background tissue’, ie, a ROI in the dorsal cervical area. The shunt fraction (SF) was calculated using the following equation: 26,29,33
Figure 1.
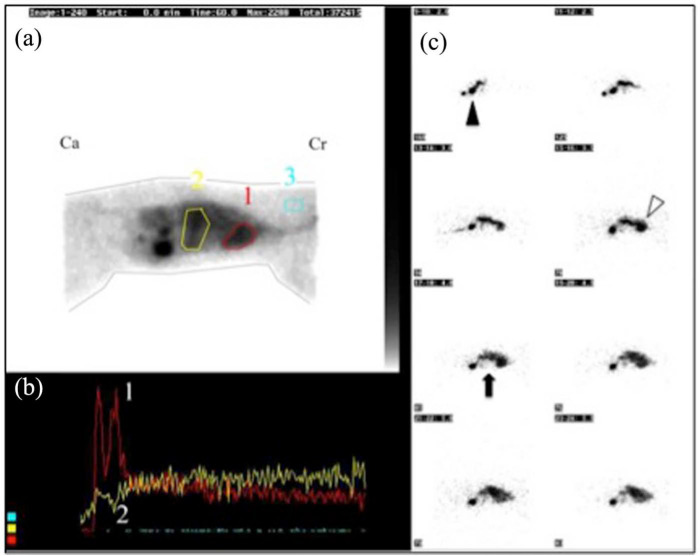
Positive transsplenic portal scintigraphy. (a) Summed image of all individual frames (head is towards the right, Cr = cranial, Ca = caudal). A region of interest (ROI) is drawn over the heart (ROI 1), over the liver (ROI 2) and over cervical soft tissue for background activity correction (ROI 3). (b) The time (x-axis) versus radioactivity (y-axis) curve (time–activity curve) depicts the progression of radioactivity in the separate ROIs. The radioactivity arrived in the heart (curve 1) before arriving in the liver (curve 2). (c) Eight individual frames (2.0–5.5 s after injection) demonstrate the trajectory of the radioactivity from the splenic injection site (black arrowhead) towards the heart first (white arrowhead). The hepatic area (black arrow) remains relatively void of radioactivity
Figure 2.
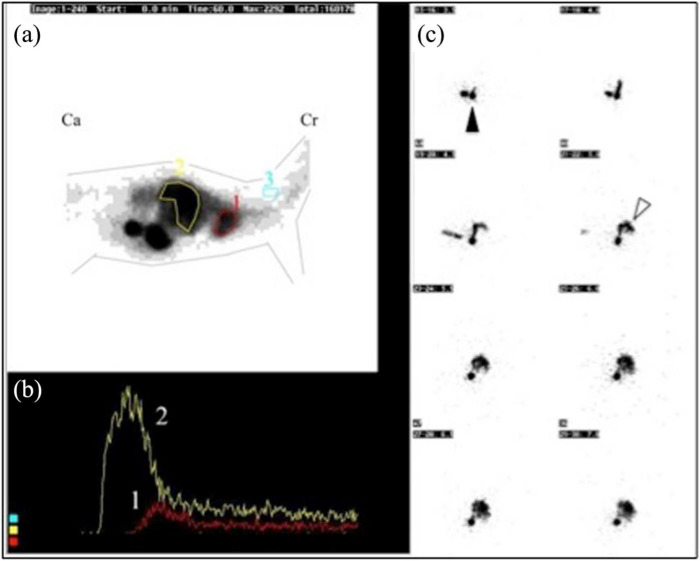
Negative transsplenic portal scintigraphy. (a) Summed image of all individual frames (head is towards the right, Cr = cranial, Ca = caudal). A region of interest (ROI) in drawn over the heart (ROI1), over the liver (ROI2) and over cervical soft tissue for background activity correction (ROI3). (b) The time (x-axis) versus radioactivity (y-axis) curve (time–activity curve) depicts the progression of radioactivity in the separate ROIs. The radioactivity arrived in the liver (curve2) before arriving in the heart (curve1). (c) Eight images of 2 frames per image (3.5–7 s after injection) demonstrate the trajectory of the radioactivity from the splenic injection site (black arrowhead) towards the hepatic area (white arrowhead) first. The heart only appears later and is not yet seen on these frames
The heart and liver counts were registered for the first 7 s of the scan with T = 0 s being the first peak of activity either in the liver (normal vasculature) or in the heart (abnormal vasculature corresponding with PSS). Eight seconds is the approximate time interval for the blood to travel from the liver to the heart in normal animals. 26 An increased SF (>4.5%) is consistent with the presence of a PSS. 33
Results
Fifteen TSPS scans was performed in total. Table 1 lists the signalment, clinical signs and most relevant diagnostic test results.
Table 1.
Data of cats presented for transsplenic portosystemic shunt examination. Breed, age, sex, most relevant clinical signs, and results for ammonia and bile acids are listed (when postprandial, bile acids are marked with *)
| Cat | Breed | Age | Sex | Clinical signs | Bile acid | Ammonia | Result US | Result TSPS | Remarks |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Birman | 4 y | Mc | Constipation, minor weight loss | 105 µmol/l* (Ref <10 µmol/l) |
N/A | – | + | |
| 2 | Domestic SH |
6 mo | F | Dullness, retarded growth, epileptic episodes, ptyalism | 69 µmol/l and 212* µmol/l (Ref <10 μmol/l) |
N/A | – | + | |
| 3 | Domestic SH |
6.5 mo | Fn | Slow recovery from anaesthesia (OVHX), epileptic episodes, ptyalism | 180 µmol/l and 188* µmol/l (Ref <8 μmol/l) |
N/A | – | + | US was indicative, but not diagnostic for IHPSS |
| 4a | British LH | 15 mo | Mc | Dullness, retarded growth, ptyalism, abnormal behaviour | 105 μmol/l (Ref <10 µmol/l) |
N/A | – | Intra-abdominal injection |
|
| 4b | British LH | 15 mo | Mc | Dullness, retarded growth, ptyalism, abnormal behaviour | 105 µmol/l (Ref <10 µmol/l) | N/A | – | + | Second TSPS repeated immediately after first TSPS |
| 5 | Maine Coon | 5 mo | F | Slow recovery from anaesthesia (hip luxation), retarded growth, epileptic episodes, ptyalism, abnormal behaviour | N/A | 449 µmol/l (Ref <95 µmol/l) |
– | + | |
| 6a | Maine Coon | 7 mo | M | Ptyalism, abnormal behaviour, diarrhoea, disturbed balance | 81 µmol/l (Ref <10 µmol/l) |
255 µg/dl (Ref <100 µg/dl) |
– | + | Presurgical TSPS |
| 6b | Maine Coon | 1 y | M | Recurrent signs: abnormal behaviour, disturbed balance, headshaking | 7 µmol/l (Ref <10 µmol/l) |
65 µmol/l (Ref <90 µmol/l) |
Tortuous vessels, but unclear if shunt | – | Postsurgical TSPS (6 mo after surgery) |
| 7 | British SH | 10 mo | F | Ptyalism, abnormal behaviour, (temporary) blindness, diarrhoea, increased vocalisation, tremor |
Before surgery: 115 µmol/l* After surgery: 23 µmol/l* (Ref <10 µmol/l) |
Before surgery: 1417 µg/dl After surgery: 143 µg/dl (Ref <100 µg/dl) |
Before surgery: + After surgery: N/A |
Before surgery: N/A After surgery: + |
Only postsurgical TSPS (4 mo after surgery) |
| 8 | Birman | 9 mo | M | Periodical ptyalism, apathy, circling | 100 µmol/l (Ref <10 µmol/l) |
155 µg/dl (Ref <75 µg/dl) |
N/A | Equivocal | Suspicion of hepatic portovenous hypoplasia |
| 9 | Domestic SH | 11 mo | Mc | Copper-coloured irises | 89 µmol/l (Ref <8 µmol/l) Repeated after 1 week: 4 µmol/l (Ref <8 µmol/l) |
N/A | – | – | Laboratory error in bile acid determination |
| 10a | British SH | 3.5 y | Fn | Low body weight | 113 µmol/l (Ref <10 µmol/l) Repeated after 1 week: 3 µmol/l (Ref <10 µmol/l) |
N/A | – | Intra-abdominal injection |
Laboratory error in bile acid determination |
| 10b | British SH | 3.5 y | Fn | Low body weight | 113 µmol/l (Ref <10 µmol/l) Repeated after 1 week: 3 µmol/l (Ref <10 µmol/l) |
N/A | N/A | – | Second TSPS repeated 24 h after first TSPS |
| 11 | Domestic SH | 2 y | Mc | Dullness, retarded growth, impaired balance | N/A |
Initial value: 160 µg/dl (Ref <100 µg/dl) Repeated after 1 mo: 41 µg/dl (Ref <100 µg/dl) |
– | – | Laboratory error in ammonia determination |
| 12 | Domestic SH | 6 y | Mc | Dullness, ptyalism, haematuria, tremor, weakness, pollakiuria, dysuria | 138 µmol/l, (Ref <10 µmol/l) |
323 µg/dl (Ref <100 µg/dl) |
+ | Intra-abdominal injection |
TSPS not repeated |
US = ultrasound; TSPS = transplenic portal scintigraphy; SH = shorthair; LH = longhair; y = years; mo = months; Mc = male castrated; F = female; Fn = female neutered; M = male; OVHX = ovariohysterectomy surgery; N/A = not applicable; + = positive PSS diagnosis on ultrasound (US) or scintigraphy; – = negative PSS diagnosis on US or scintigraphy; IHPSS = intrahepatic portosystemic shunt
Cat 1–7: positive TSPS
In six cats (Table 1, cats 1–6), TSPS clearly indicated the presence of a PSS; the seventh cat (Table 1, cat 7) was only referred for TSPS after surgical ligation of an extrahepatic portocaval shunt, previously diagnosed by abdominal US. At the time of the diagnosis all cats had abnormal behaviour (apathy/dullness), two were blind and two had seizure episodes. Ptyalism was present in six cats; in three cats copper-coloured irises were noted, and of the three cats it was known that they were smaller than their littermates. Two cats were reported to have diarrhoea. Bile acids were elevated in all six cats in which this was determined. In three cats venous ammonia levels were measured, and they were found to be elevated in these three cats.
Abdominal US was undertaken in all cats. In 5/6 cats for which TSPS was needed for diagnosis there was a suspicion of PSS on US based on secondary signs, such as microhepatia, renomegaly and the presence of urinary stones/sediment, although the shunting vessel itself could not be identified. The SF ranged from 67.3% to 96.2%. The cat (Table 1, cat 3) with the SF of 67.3% was suspected of having an intrahepatic shunt, based on the pattern of the TAC (very short interval between appearance of activity in the liver and activity in the heart) and the equivocal US results. In one cat (Table 1, cat 4a + b) TSPS was performed twice, as the first scan was non-diagnostic. Although the US image confirmed needle placement in the splenic parenchyma, perforation of the spleen at the moment of injection cannot be excluded. The scan was then repeated immediately to avoid a second anaesthesia and distribution of the injected activity throughout the entire cat, possibly hampering interpretation of the repeated scan. The second time a higher amount of activity (first scan: 44.4 MBq, second scan: 120,25 MBq) was injected, and this TSPS was positive. Only one of the six cats (cat 6) in which TSPS was needed for diagnosis went to surgery. Two were euthanased owing to their poor clinical condition and worsening neurological signs; three were controlled by medical and nutritional treatment only.
Cat 7 was initially diagnosed with a PSS by US, but was referred for TSPS when the bile acids and venous ammonia level remained high 4 months after corrective portosystemic shunt surgery. The cat underwent a celiotomy for ovariohysterectomy, during which multiple tortuous shunting vessels were noticed at cranial pole of the left kidney, although the initial shunting vessel was fully occluded, as demonstrated by intraoperative mesenteric portography. These collateral vessels indicate acquired PSSs and can explain the high postoperative SF (92.2%), and the persistent abnormal ammonia and bile acids levels despite the improved clinical condition. Figure 1 depicts a positive diagnosis for PSS by means of TSPS.
Cat 8: equivocal TSPS
Cat 8 was a 9-month-old Birman and had episodes of ptyalism, apathy and abnormal behavioural (circling), with increased pre- and postprandial bile acids and ammonia (Table 1, cat 8). The SF in this cat was 17.7%, and the TAC showed that the liver and heart simultaneously received the tracer, although the liver clearly received the majority of the radioactivity. A macroscopic PSS was deemed unlikely based on trajectory of the intrasplenic injected activity as seen on the TSPS scan result and the lack of US signs of a macroscopic PSS. Hepatic portovenous hypoplasia was suspected in this patient. A macroscopic shunt was not found on surgical abdominal exploration and liver biopsies were histopathologically examined, confirming hepatic portovenous hypoplasia.
Cats 9–11 and cat with repeated scan after surgery: negative TSPS
Two cats (Table 1, cats 9 and 10) were referred for TSPS based on increased bile acids, found in a complete blood examination as part of a general check-up (cat 9) or as part of a work-up for severe food allergy and failure to gain weight on a newly installed diet (cat 10). In both cases abdominal US could not identify a PSS nor were their clinical signs compatible with a PSS. The TSPS scan of cat 10 was repeated 24 h after the first scan (Table 1, cat 10a + b) owing to accidental intra-abdominal tracer injection causing a non-diagnostic scan. The repeated scan confirmed absence of a PSS. The SF for cat 9 was 0.1% and 3.2% for cat 10. Cat 11 was suspected of having a PSS based on its history (relatively small, slow growth and impaired balance) and a one-off abnormal ammonia value. The increased ammonia was not confirmed in a second blood examination, thus the cat was referred for TSPS. This, however, was negative (SF 0.1%).
The fourth cat with negative TSPS (cat 6) also had a scintigraphical diagnosis of PSS before surgical treatment (SF 93.6%) (Table 1, cat 6a). After surgery, the clinical condition of the cat improved dramatically, and bile acids and ammonia levels normalised. At 6 months after surgery, however, the headshaking, abnormal behaviour and disturbed balance recurred, but to a lesser extent and only shortly after feeding. The repeated TSPS was negative (SF 3.7%), which is in accordance with the normal results of the blood examination. However, this cat showed ultrasonographical signs of an acquired PSS (tortuous vessels), which is compatible with the clinical signs (Table 1, cat 6b). No further diagnostic procedures were pursued. Figure 2 represents a negative TSPS scan.
Cat 12
Although already 6 years old at time of presentation, this cat had chronic urinary problems for over 1 year (haematuria, pollakiuria, dysuria). These signs were accompanied by muscle weakness, lethargy, head tremors and severe ptyalism. Abdominal US revealed urinary sediment and bladder stones. Urinalysis confirmed urate cristalluria, suggestive for ammonium urate urolithiasis. After surgical removal of the stones, clinical signs did not resolve and subsequent blood analysis showed increased ammonia and increased pre- and postprandial bile acids. An abdominal US was repeated but hampered by a large amount of food and faeces in the intestinal tract, and TSPS was performed. The intrasplenic injection was unsuccessful owing to intra-abdominal injection. The TSPS scan was not repeated. The US examination, however, was redone when the cat was properly fasted, clearly visualising an extrahepatic PSS. The aberrant vessel was surgically attenuated (Table 1, cat 12).
Discussion
Portosystemic shunts, both congenital and acquired, are rare in cats, with a much lower prevalence than in dogs.1–4 Signalment and clinical signs may raise the suspicion of a PSS, but are often vague and non-specific, and further work-up is necessary. The most commonly tested blood parameters are venous ammonia and (pre- and postprandial) bile acids. Laboratory assays solely can be inconclusive, however, as two of the cats with a negative TSPS scan were initially referred for scintigraphical examination based on increased bile acids (Table 1, cats 9 and 10); a third cat with a negative TSPS scan was referred after a one-time increased ammonia level (Table 1, cat 11). Although repeat blood measurements can be performed, only visualisation of the PSS grants a definite diagnosis.
Conventional radiography cannot directly visualise the shunting vessel. It can only reveal secondary, indirect signs such as microhepatia, renomegaly, reduced serosal detail and/or uroliths.5,6,12 In contrast, portography provides highly detailed information on the hepatic/abdominal vasculature, but remains invasive and requires general anaesthesia.10,14,17,20 More recently, computed tomography (CT) angiography and magnetic resonance angiography allow shunt detection. These procedures provide detailed anatomical information, but they require prolonged anaesthesia and are relatively expensive as a first screening method, especially if the presence of a shunt is uncertain.24,34,35 Generally, abdominal US is the first imaging technique to be performed in the diagnostic work-up, providing information on the size and shape of the liver, on the liver parenchyma and its vasculature, and secondary changes, such as renomegaly or urolithiasis. It is non-invasive and, in the hands of an experienced operator and with the use of Doppler technique, the abnormal vessel can often be detected.7,11,14,17,20,22,23 However, a negative result on US does not exclude a shunt.
Nuclear medicine is a proven method for detecting the presence of a PSS. Per-rectal portal scintigraphy with pertechnetate is a frequently reported method used in veterinary medicine, especially in dogs, but feline patients with PSS have also been diagnosed using PRPS.25–32 This method, however, entails several disadvantages. The amount of radioactivity to be placed in the rectum or distal descending colon is relatively high to obtain a qualitatively good study, as only ±15% of the pertechnetate is taken up from the gastrointestinal tract into the blood stream.28,30 Low absorption results in a low count rate study that makes interpretation more challenging, whereas the radioactivity remaining at the deposition site can create large artefacts.24,26,33 Shielding the caudal abdomen from the gamma camera with lead can improve scan quality, but may also cover the activity trajectory, especially in very small animals.
Similar to portography with intrasplenic injection of contrast agent, 36 the TSPS method was developed to ameliorate scintigraphical investigation of animals suspected of PSS.26,33,37,38 It requires only a quarter of the radioactivity needed for PRPS, reducing radiation burden to the animal and the clinician. 26 The extraction rate of tracer from the splenic parenchyma is much higher compared with absorption through the intestinal mucosa — about 52.5% on average — yielding qualitatively better studies than those obtained with PRPS. 26 From an anaesthetic point of view, there is little difference between both scintigraphical procedures. Anaesthesia is performed as described earlier, using bolus injections of propofol, and, in our institute, similarly for both TSPS and PRPS scans. Although the scan duration is slightly longer for PRPS procedures, this does not seem to necessitate a different anaesthetic approach.24,26,33
As demonstrated in this series of patients, the application of the TSPS method is not limited to the canine population, but felines also suspected of PSS can be successfully examined with TSPS. The literature only reports one cat diagnosed with a PSS using TSPS 38 , whereas the PRPS method seems to be applied more frequently.6,14,27,32 The main challenge of the TSPS technique lies in the technical difficulty of injecting into the relatively small feline spleen. Three initially non-diagnostic studies in this case series were caused by faulty tracer injection. In one cat (cat 4a + b) the investigation was repeated immediately, using a higher activity than with the first injection, resulting in a diagnostic study. The combined activity of these both studies was still lower than the activity that would have been administered for a PRPS study. In a second cat (cat 10a + b) the study was repeated successfully 24 h later; for the third cat (cat 12) the owners declined to repeat the investigation. The activity that was deposited intra-abdominally did not hamper interpretation of the repeated scans nor did it seem to cause any intra-abdominal lesions as assessed by US after termination of the TSPS scan.
A known shortcoming of scintigraphical PSS diagnosis is the lack of morphological detail. The distinction between portocaval and porto-azygos shunts can be made, as the radioactivity will approach the heart from caudally in a portocaval shunt, or from a craniodorsal direction in a porto-azygos shunt.24,26,33 The shunt origin and morphology, however, must be determined with other diagnostic imaging modalities, such as portovenography or CT angiography. False -negative TSPS scans are possible in case the shunting vessel originates caudal from the spleen.24,26,33 This is, however, an uncommon situation. The majority of the portosystemic shunts are reported to originate from the portal vein itself, from the gastrosplenic veins or gastroduodenal veins, all located cranial to the phrenicoabdominal veins.39,40 Visualisation of hepatic portovenous hypoplasia is not possible with TSPS although the shortened transit-time of activity from the liver to the heart is considered indicative, warranting further corroborative examinations.31,33 The SF in these cases may also be abnormal, even though visual assessment of the scan may not indicate a macroscopic PSS. One cat (cat 8) in this study with an equivocal TSPS result (a mildly increased SF and a very short delay of transit in the liver) had histopathologically-confirmed hepatic portovenous hypoplasia.
Although all procedures were performed without any complication, the absence of splenic laceration or bleeding after injection should always be checked ultrasonographically after termination of the TSPS scan.
Conclusions
This series of cats, although small in number, demonstrates that TSPS can be applied successfully in feline patients suspected of a PSS. The most important drawback of this patient series is that only in one cat was surgery performed, thereby confirming the positive TSPS result. None of the cats with a negative TSPS scan underwent further diagnostic work-up as the presence of a PSS was thought to be very unlikely after the repeated blood examinations. The owners of the cat with a negative TSPS scan after previous surgical intervention declined further diagnostic imaging. The main disadvantage of TSPS is the use of radioactive substances and related regulatory issues. The technical challenges of this investigation are similar to those reported in dogs, but are not insurmountable, as proven by these cases, and TSPS can have a place in the diagnostic work-up of PSSs in cats.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case series.
The authors do not have any potential conflicts of interest to declare.
Accepted: 8 April 2013
References
- 1. Levy JK, Bunch SE, Komtebedde J. Feline portosystemic vascular shunts. In: Bonagura JD, Kirk RW. (eds). Kirk’s current veterinary therapy XII, small animal practice. Philadelphia: WB Saunders, 1995, pp 743–749. [Google Scholar]
- 2. Center SA. Hepatic vascular diseases. In: Guilford WG, Center SA, Strombeck DR, et al. (eds). Strombeck’s small animal gastroenterology. 3rd ed. Philadelphia: WB Saunders, 1996, pp 802–846. [Google Scholar]
- 3. Ruland K, Fischer A, Reese S, et al. Portosystemic shunts in cats – evaluation of six cases and a review of the literature. Berl Munch Tierartzl Wochenschr 2009; 122: 211–218. [PubMed] [Google Scholar]
- 4. Langdon P, Cohn LA, Kreeger JM, et al. Acquired portosystemic shunting in two cats. J Am Anim Hosp Assoc 2002; 38: 21–27. [DOI] [PubMed] [Google Scholar]
- 5. Tillson DM, Winkler JT. Diagnosis and treatment of portosystemic shunts in the cat. Vet Clin North Am Small Anim Pract 2002; 32: 881–899. [DOI] [PubMed] [Google Scholar]
- 6. Tivers M, Lipscomb V. Congenital portosystemic shunts in cats: Investigation, diagnosis and stabilization. J Feline Med Surg 2011; 13: 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lamb CR, Forster-van Hijfte MA, White RN, et al. Ultrasonographic diagnosis of congenital portosystemic shunt in 14 cats. J Small Anim Pract 1996; 37: 205–209. [DOI] [PubMed] [Google Scholar]
- 8. Schunk CM. Feline portosystemic shunts. Semin Vet Med Surg 1997; 12: 45–50. [PubMed] [Google Scholar]
- 9. Blaxter AC, Holt PE, Pearson GR, et al. Congenital portosystemic shunts in the cat: a report of nine cases. J Small Anim Pract 1988; 29: 631–645. [Google Scholar]
- 10. Havig M, Tobias KM. Outcome of ameroid constrictor occlusion of single congenital extrahepatic portosystemic shunts in cat: 12 cases (1993–2000). J Am Vet Med Assoc 2002; 220: 337–341. [DOI] [PubMed] [Google Scholar]
- 11. Holt DE, Schelling CG, Saunders HM, et al. Correlation of ultrasonographic findings with surgical, portographic, and necropsy findings in dogs and cats with portosystemic shunts: 63 cases (1987–1993). J Am Vet Med Assoc 1995; 207: 1190–1193. [PubMed] [Google Scholar]
- 12. Berger B, Whiting PG, Breznock EM, et al. Congenital feline portosystemic shunts. J Am Vet Med Assoc 1986; 188: 517–521. [PubMed] [Google Scholar]
- 13. Scavelli TD, Hornbuckle WE, Roth L, et al. Portosystemic shunts in cats: seven cases (1976–1984). J Am Vet Med Assoc 1986; 189: 317–325. [PubMed] [Google Scholar]
- 14. Martin RA. Congenital portosystemic shunts in the dog and cat. Vet Clin North Am Small Anim Pract 1993; 23: 609–623. [DOI] [PubMed] [Google Scholar]
- 15. Center SA, Erb HN, Joseph SA. Measurement of serum bile acids concentrations for diagnosis of hepatobiliary disease in cats. J Am Vet Med Assoc 1995; 207: 1048–1054. [PubMed] [Google Scholar]
- 16. Winkler JT, Bohling MW, Tillson DM, et al. Portosystemic shunt: diagnosis, prognosis, and treatment of 64 cases (1993–2001). J Am Anim Hosp Assoc 2003; 39: 169–185. [DOI] [PubMed] [Google Scholar]
- 17. Broome CJ, Walsh VP, Braddock JA. Congenital portosystemic shunts in dogs and cats. N Z Vet J 2004; 52: 154–162. [DOI] [PubMed] [Google Scholar]
- 18. Ruland K, Fischer A, Hartmann K. Sensitivity and specificity of fasting ammonia and serum bile acids in the diagnosis of portosystemic shunts in dogs and cats. Vet Clin Pathol 2010; 39: 57–64. [DOI] [PubMed] [Google Scholar]
- 19. Rothuizen J, Van den Ingh TSGAM, Voorhout MG, et al. Congenital porto-systemic shunts in sixteen dogs and three cats. J Small Anim Pract 1982; 23: 67–81. [Google Scholar]
- 20. Lamb CR, Daniel GB. Diagnostic imaging of dogs with suspected portosystemic shunting. Compendium 2002; 24: 626–635. [Google Scholar]
- 21. Lamb CR. Ultrasonographic diagnosis of congenital portosystemic shunts in dogs: results of a prospective study. Vet Radiol Ultrasound 1996; 37: 281–288. [Google Scholar]
- 22. D’Anjou M-A, Penninck D, Cornejo L, et al. Ultrasonographic diagnosis of portosystemic shunting in dogs and cats. Vet Radiol Ultrasound 2004; 45: 424–437. [DOI] [PubMed] [Google Scholar]
- 23. Szatmari V, van Sluijs FJ, Rothuizen J, et al. Ultrasonographic assessment of hemodynamic changes in the portal vein during surgical attenuation of congenital extrahepatic portosystemic shunts in dogs. J Am Vet Med Assoc 2004; 224: 395–402. [DOI] [PubMed] [Google Scholar]
- 24. Sura PA, Tobias KM, Morandi F, et al. Comparison of 99mTcO4- trans-splenic portal scintigraphy with per-rectal portal scintigraphy for diagnosis of portosystemic shunts in dogs. Vet Surg 2007; 36: 654–660. [DOI] [PubMed] [Google Scholar]
- 25. Daniel GB, bright R, Ollis P, et al. Per rectal portal scintigraphy using 99mTechnetium pertechnetate to diagnose portosystemic shunts in dogs and cats. J Vet Intern Med 1991; 5: 23–27. [DOI] [PubMed] [Google Scholar]
- 26. Cole RC, Morandi F, Avenell J, et al. Trans-splenic portal scintigraphy in normal dogs. Vet Radiol Ultrasound 2005; 46: 146–152. [DOI] [PubMed] [Google Scholar]
- 27. Forster-van Hijfte MA, McEvoy FJ, White RN, et al. Per rectal portal scintigraphy in the diagnosis and management of feline congenital portosystemic shunts. J Small Anim Pract 1996; 37: 7–11. [DOI] [PubMed] [Google Scholar]
- 28. Caride VJ. Rectal absorption of 99mTc-pertechnetate in the dog. J Nuc Med 1973; 14: 600–603. [PubMed] [Google Scholar]
- 29. Daniel GB, Bright R, Monnet E, et al. Comparison of per-rectal portal scintigraphy using 99mtechnetium pertechnetate to mesenteric injection of radioactive microspheres for quantification of portosystemic shunts in an experimental dog model. Vet Radiol 1990; 31: 175–181. [Google Scholar]
- 30. Koblik PD, Komtebedde J, Yen CK, et al. Use of transcolonic 99mtechnetium–pertechnetate as a screening test for portosystemic shunts in dogs. J Am Vet Med Assoc 1990; 196: 925–930. [PubMed] [Google Scholar]
- 31. Koblik PD, Hornof WJ. Transcolonic sodium pertechnetate Tc99m scintigraphy for diagnosis of macrovascular portosystemic shunts in dogs, cats and potbellied pigs: 176 cases (1988–1992). J Am Vet Med Assoc 1995; 207: 729–733. [PubMed] [Google Scholar]
- 32. McEvoy FJ, Forster-van Hijfte MA, White RN. Detection of portal blood flow using per-rectal 99mTc-pertechnetate scintigraphy in normal cats. Vet Radiol Ultrasound 1998; 39: 234–237. [DOI] [PubMed] [Google Scholar]
- 33. Morandi F, Cole RC, Tobias KM, et al. Use of 99mTcO4- trans-splenic portal scintigraphy for diagnosis of portosystemic shunts in 28 dogs. Vet Radiol Ultrasound 2005; 46: 153–161. [DOI] [PubMed] [Google Scholar]
- 34. Frank P, Mahaffey M, Egger C, et al. Helical computed tomographic portography in ten normal dogs and ten dogs with a portosystemic shunt. Vet Radiol Ultrasound 2003; 44: 392–400. [DOI] [PubMed] [Google Scholar]
- 35. Zwingenberger A. CT diagnosis of portosystemic shunts. Vet Clin North Am Small Anim Pract 2009; 39: 783–792. [DOI] [PubMed] [Google Scholar]
- 36. Echandi RL, Morandi F, Daniel WT, et al. Comparison of transsplenic multidetector CT portography to multidetector CT-angiography in normal dogs. Vet Radiol Ultrasound 2007; 48: 38–44. [DOI] [PubMed] [Google Scholar]
- 37. Morandi F, Cole RC, Echandi RL, et al. Transsplenic portal scintigraphy using 99mTc-mebrofenin in normal dogs. Vet Radiol Ultrasound 2007; 48: 286–291. [DOI] [PubMed] [Google Scholar]
- 38. Morandi F, Sura PA, Sharp D, et al. Characterization of multiple acquired portosystemic shunts using transsplenic portal scintigraphy. Vet Radiol Ultrasound 2010; 51: 466–471. [DOI] [PubMed] [Google Scholar]
- 39. Van den Ingh TS, Rothuizen J, Meyer HP. Circulatory disorders of the liver in dogs and cats. Vet Q 1995; 17: 70–76. [DOI] [PubMed] [Google Scholar]
- 40. Lamb CR. Ultrasonography of portosystemic shunts in dogs and cats. Vet Clin North Am Small Anim Pract 1998; 28: 725–751. [DOI] [PubMed] [Google Scholar]