Abstract
The clinical presentation and response to treatment of cats infected with Tritrichomonas foetus have not been sufficiently described in a large number of pet cats. The aim of this study was to collect and analyze clinical data from pet cats diagnosed with intestinal T foetus infection. Clinical information was collected for 104 cats that tested polymerase chain reaction-positive for T foetus. The most common clinical sign was diarrhea (98%) with a median duration of 135 days (range 1–2880 days). Forty-nine of 83 (59%) cats had diarrhea since adoption. Other clinical signs included anorexia (22%), depression (24%), weight loss or failure to gain weight (20%), vomiting (19%), abdominal pain (9%) and increased appetite (3%). A total of 45 cats had completed treatment with ronidazole, 29 of which (64%) showed a good clinical response to treatment. Sixteen (36%) cats had either partial or no improvement, or a relapse shortly after discontinuation of treatment.
Over the past decade, the protozoan parasite Tritrichomonas foetus has emerged as a new and important cause of feline diarrhea with virtually worldwide distribution.1–5 Feline T foetus isolates were initially believed to be identical to the bovine isolates, but recent genetic studies have shown that T foetus from domestic cats and cattle are genetically distinct.6–8 Furthermore, a recent study suggested that feline intestinal trichomoniasis might be caused by a new Tritrichomonas species (Tritrichomonas blagburni nova species). 9 Further studies are needed to elucidate the classification, genetics, and properties of the feline T foetus isolates and their similarities to Tritrichomonas isolates from other species.9,10
The pathogenesis of T foetus infection in the cat has been investigated in experimental studies and the pathogenicity of the organism in cats is unequivocal.1,4,11,12 However, it has been shown that many cats, especially those of older age, may be asymptomatically infected with T foetus.13–17 Natural T foetus-associated intestinal disease has been described mainly in cats from multi-cat environments, such as catteries,14,16 shelters18,19 or cats presented at cat shows.5,17,20 The signalment, history, clinical presentation and response to treatment in a large number of pet cats naturally infected with T foetus have not been sufficiently described.11,13,15,21 Therefore, the aim of the present study was to collect and analyze clinical data from pet cats that were diagnosed with intestinal T foetus infection.
Materials and methods
Case enrolment
The database of the Gastrointestinal Laboratory at Texas A&M University (College Station, TX, USA) was searched for the period January 2006 to December 2009 for cats that had tested polymerase chain reaction (PCR)-positive for T foetus in fecal samples. The veterinarians who had submitted the fecal samples of the cats that were positive for T foetus were contacted and asked to fill out a questionnaire for each cat. Questions covered date of birth, sex and sexual status, body weight and body condition score (BCS), medical history, clinical signs, diagnostics performed, concurrent conditions and treatment. Emphasis was given on the onset, severity and duration of gastrointestinal clinical signs that were potentially associated with intestinal T foetus infection. For the purposes of the present study, diarrhea was defined as one or more episodes of the passage of feces that contained more water than normal.
Diagnosis of intestinal T foetus infection
The diagnosis of T foetus infection was based on detection of the microorganism’s DNA in fecal samples, using a single-tube nested PCR, as described previously. 13 Fecal samples had been submitted to the Gastrointestinal Laboratory for evaluation of potential T foetus infection. DNA for PCR analysis was extracted from fecal samples within 3 days of receipt (during which time they were kept frozen at −4oC) using a commercially available DNA extraction kit (ZR Fecal DNA kit; Zymo Research), which has been validated for the extraction of DNA for the detection of T foetus DNA in fecal samples from cats. 22 Fecal samples from all cats were extracted in duplicate fashion, and negative controls (sterile water) were extracted with each batch to ensure that contamination of extraction reagents was not present. The quality of DNA and presence of endogenous PCR inhibitors in each DNA sample were assessed by PCR amplification of a 876 base pair sequence of the bacterial 16S ribosomal RNA gene using universal bacterial primers (517F [5’ GTGCCAGCAGCCGCGGTAA 3’] and 1391R [5’ GACGGGCGGTGAGTGCA 3’]), as described previously. 23 The single-tube nested PCR was carried out using two sets of specific primers targeting the T foetus internal transcribed spacer region (ITS1 and ITS2) and the 5.8S rRNA gene. 23 Positive T foetus DNA purified from a positive culture specific for this parasite (In PouchTF; Biomed Diagnostics) 24 and negative (sterile water) controls were run with each batch to identify failure of the assay (positive control), or reagent contamination (negative control) respectively. PCR amplicons were verified by electrophoresis on 2% agarose gels (E-Gels 2%; Invitrogen) and visualized under ultraviolet light.
Statistical analysis
All statistical analyses were conducted using a commercially available statistical software program (Prism 5; GraphPad). Continuous data were tested for normality using the Kolmogorov–Smirnov test. Descriptive statistics were performed for all variables. Variables with a Gaussian distribution were reported as mean ± standard deviation (SD), while variables with a non-Gaussian distribution were reported as median and range. The frequencies of clinical signs were reported as percentages. Ninety-five percent confidence intervals (95% CI) were reported for all variables.
Results
Study population
Questionnaires for 104 cats living in the USA and Canada were completed and returned to the Gastrointestinal Laboratory. The number of cats per household was reported for 92 cases; the median number of cats per household was two (range 1–50). Ninety-one (90%)/101 for which information was available were exclusively indoor, while 10/101 cats (10%) were both indoor and outdoor.
Signalment
Fifty-four of 104 cats (52%) were purebred. A total of 24 breeds were represented, the most common being domestic shorthair (DSH) (33/104; 32%), Bengal (13/104; 13%), Persian (6/104; 6%), Abyssinian (6/104; 6%), Ragdoll (5/104; 5%), Maine Coon (4/104; 4%), domestic longhair (4/104; 4%), domestic mediumhair (4/104; 4%) and Siamese (4/104; 4%).
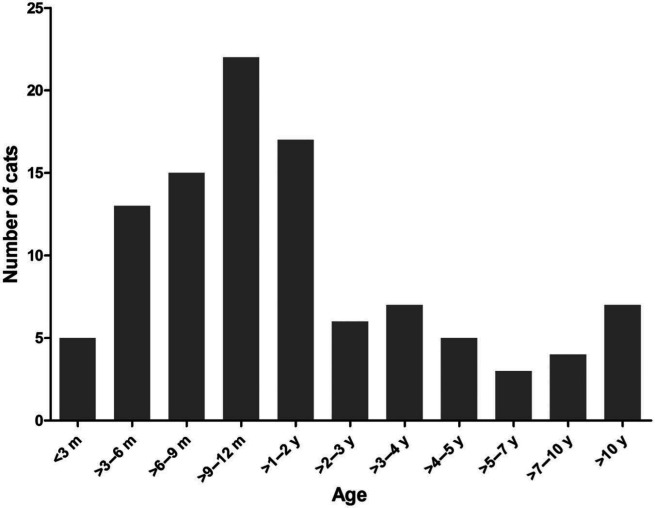
Forty-six (44%) cats were female and 58 (56%) were male. The median age of the cats was 1 year, with a range of 1 month to 16.3 years. Fifty-eight (56%) cats were 12 months of age or older at the time of T foetus diagnosis (Figure 1). The mean BCS was 2.9/5.
Figure 1.
Age distribution of cats diagnosed with intestinal Tritrichomonas foetus infection. m = months; y = years
Clinical signs
The most common clinical sign was diarrhea, which was reported in 101/103 cats (98%). The median duration of the diarrhea was 135 days, with a range of 1 day to 7.9 years. Forty-nine of 83 cats (59%) were reported to have had diarrhea since adoption. The fecal consistency was reported in 86 cases; 36 (42%) cats had soft stool, 29 (34%) had watery stool, and 21 (24%) cats were reported to have both watery diarrhea and soft stool. Haematochezia was reported in 45/99 cats (46%), tenesmus in 42/98 cats (43%) and mucus in 59/100 cats (59%). The frequency of episodes of diarrhea ranged from 1 to 8 times per day. The fecal characteristics, the clinical signs related to the diarrhea and their frequencies are shown in Table 1.
Table 1.
Fecal characteristics, clinical signs related to the diarrhea and their frequencies in cats with diarrhea and intestinal Tritrichomonas foetus infection
| Fecal characteristics and clinical signs related to the diarrhea | Percentage of cats |
|---|---|
| Semi-formed feces | 41 |
| Watery feces | 33 |
| Semi-formed/watery feces | 24 |
| Mucus | 59 |
| Hematochezia | 46 |
| Tenesmus | 43 |
| Melena | 7 |
Other clinical signs reported included depression (24/102 cats; 24%), decreased appetite or anorexia (22/103 cats; 22%), weight loss (20/101 cats; 20%), vomiting (20/103 cats; 19%), abdominal pain (9/103 cats; 9%) and increased appetite (3/103 cats; 3%).
Other intestinal parasites
Fecal flotation had been performed in 85 cases and three of those cats (4%) were found to be positive for coccidia. Testing for Giardia species had been performed in 67 cats using different test methods (eg, SNAP Giardia, zinc flotation); 15 (22%) of those cats were positive for Giardia species.
Treatment
A total of 79/102 cats (78%) were treated with ronidazole. Of these, 30 cats were either still early on the treatment cycle with ronidazole at the time of the study and therefore the effect of treatment could not be appreciated or were lost to follow-up. In four cats, the treatment was discontinued owing to neurological signs (n = 3) and/or anorexia (n = 3). Of the remaining 45 cats, 29 (64%) had a good clinical response to treatment, defined as complete or close to complete resolution of clinical signs. Sixteen cats (36%) were reported to have either partial or no improvement, or a relapse shortly after discontinuation of treatment. Ten of those cats (22% of 45 cats) had been treated with the currently recommended dosages of ronidazole (30 mg/kg, q24h, for 2 weeks; n = 4) or higher (n = 6). Five cats had been treated with doses lower than those currently recommended, while one cat had been treated for only 1 week.
Discussion
To our knowledge, this is the largest study investigating the clinical picture and response to treatment of pet cats naturally infected with T foetus. While a plethora of other studies have described the clinical picture of cats with intestinal T foetus infection, the vast majority of them5,14,16–20 have enrolled cats living in multi-cat environments, such as catteries and shelters, which may not reflect the average pet cat population living at homes alone or in small numbers.
Diarrhea was by far the most common clinical sign in the present study, which was present in almost all cats. Because diarrhea has been described as a major clinical sign associated with clinical trichomonosis in cats,4,11,15,16,19–21 the submitting veterinarians might have been biased in testing mainly for T foetus cats that had diarrhea. However, in agreement with our findings, studies that have investigated the prevalence of T foetus infection in cats both with and without diarrhea, have reported that diarrhea typically represents the most common clinical sign. 16 More importantly, a study that investigated pet cats in the USA reported that all 17 cats that were positive for T foetus (100%) presented with chronic diarrhea. 21 The duration of the diarrhea in the present study ranged from 1 day to several years. Previous studies have shown that T foetus-induced diarrhea typically resolves within 2 years in most cats. 11 However, as suggested by the present study, diarrhea might persist for much longer in some cats.
Interestingly, 59% of 83 cats were reported to have diarrhea since adoption. This might suggest that the majority of pet cats acquire the infection in catteries, shelters, pet shops, etc, where cats live in densely populated environments. Although epidemiological differences may occur in this disease depending upon the geographical region, these environments, especially catteries, have been shown repeatedly to represent important risk factors for T foetus infection in cats.1,5 The present study suggests that cats might be living in single-cat environments for years and still display clinical signs of an infection that they acquired during early stages of their life while they were still living in multi-cat environments. Therefore, T foetus infection should not be considered unlikely in cats with chronic diarrhea that live in single-cat environments.
About 20% of cats each had complete or partial anorexia, depression, vomiting and/or weight loss. These clinical signs have not been commonly associated with T foetus infection in previous studies, 16 although there are some exceptions, especially in young cats and kittens.15,19 These systemic clinical signs might be the result of concurrent intestinal infections or other diseases and not T foetus infection per se. Although enteric co-infections have frequently been documented in T foetus-infected cats, their association with clinical signs has not been well established.5,21 In recent studies, co-infection with Giardia species or coccidia was not associated with the prevalence or severity of diarrhea in T foetus-positive cats, and clinical signs of T foetus infection were not exacerbated by co-infection with other intestinal parasites.16,17 Regardless, the findings of the present study might suggest that in cats without the expected typical clinical sings (ie, chronic or intermittent large bowel diarrhea without systemic signs), T foetus infection should not necessarily be ruled out without testing because it may contribute to the clinical signs caused by other diseases.
The exact association between feline T foetus infection, the development of clinical signs associated with T foetus infection, and age has not been fully elucidated. Although the majority of studies have suggested that T foetus primarily infects and causes disease in kittens and young cats,11,15,16,21,25,26 it is increasingly recognized that older cats are also often infected with T foetus.13,14,17,19 In one study at a rescue colony in Italy the majority of infected cats (67% of 24 cats infected) was found to be older than 1 year of age. 19 In another study that investigated healthy cats presented at cat shows in Norway, the mean age of T foetus-infected cats was 20.1 months, and 55% of these cats were >1 year of age. 17 Similar to the findings of the two above-mentioned studies, most cats (nearly 56%) in our study were 1 year of age or older and about 7% of cats were more than 10 years of age. The reason for the discrepancy among studies regarding the age of affected cats is not known, but differences in study populations (which may be related to differences between cats living in catteries and cats kept as pets) and previous exposure to T foetus may have played a role. Regardless of the reason, these data suggest that T foetus should not be considered an unlikely diagnosis in older cats with a compatible history and compatible clinical signs.
Reports regarding predisposition of different cat breeds to T foetus infection have also been puzzling. Different studies have identified different breeds as being at high risk for T foetus infection, and results from one study are not typically verified by subsequent studies.15,16,21,26,27 In general, a genetic predisposition of pure-bred cats to T foetus infection has previously been suggested by some, but the observed over-representation of pure-bred cats might be a result of high-risk husbandry conditions of many catteries rather than true genetic predisposition.5,11,15 In the present study, although several breeds were over-represented, DSH cats were by far the most commonly affected. This finding is in accordance with the findings of another study at a rescue colony in Italy. 19 However, the results of our study might be simply because DSH cats are very commonly kept as pets, while previous studies have mainly evaluated cats from catteries and cat shows where cats are exclusively pure-bred. Inclusion of a reference feline population could have provided more accurate interpretations of breed predispositions in the present study, but, unfortunately, one was not available. The susceptibility of certain feline breeds or pure-bred cats, in general, to T foetus infection requires further study. However, any cat, regardless of breed, could be infected with T foetus.
In accordance with previous studies,5,15,16,20,21 a relatively large percentage of cats (more than 20%) tested were found to have concurrent Giardia species infection. A smaller portion of cats (4%) was found to have concurrent infection with coccidia. These percentages should be interpreted with caution because different methods were used by the submitting veterinarians to test for each parasite. Ideally, the submitted fecal samples should have been tested using the same methods, but this was not possible owing to the retrospective nature of the study. Although T foetus and Giardia species co-infections seem to occur frequently, a recent study that investigated naturally infected cats did not find any association between co-infection with other enteric parasites (eg, Giardia species or coccidia) and T foetus. 16 In the same study, 16 and in agreement with another study, 15 T foetus-associated diarrhea was not exacerbated by infection with Giardia species or coccidia, and infection with T foetus alone was sufficient to cause clinical signs. Therefore, the clinical picture of the cats described in the present study was unlikely to have been affected by co-infection with other enteric parasites.
Sixty-four percent of cats treated with ronidazole were judged to have a good response to treatment, while about 36% of cats had an inadequate response to or a relapse shortly after treatment. This is in disagreement with the results of a smaller study in Australia, in which ronidazole was effective in almost 100% of treated cats. 15 About 60% of the cats that had an inadequate response to ronidazole or a relapse shortly after treatment was only treated for one 2-week cycle with ronidazole at a commonly recommended therapeutic regiment (30 mg/kg, q24h PO), which might suggest that this therapeutic regimen might not be effective in all cats. This is supported by the findings of another study, 19 in which some of the cats treated with ronidazole continued to have occasional loose stools. In a study by Gookin et al, 28 it was recommended that cats that have a relapse should be treated with higher dosages of ronidazole (30–50 mg/kg PO q12h for 14 days). 28 Also, lower ronidazole dosages (10 mg/kg q24h) were associated with relapses in this study. 28 Alternatively, the relapses in this group of cats might have been associated with worsening or inadequate control of concurrent diseases (including co-infection with other enteric pathogens), resistance to ronidazole 29 and/or poor owner compliance. Another possible explanation for poor response to ronidazole treatment in the present study is problems with compounding of the drug or the use of inappropriate ronidazole preparations. Owing to the retrospective nature of the study, information on compounding and specific preparations was not available. About 40% of the cats that had an inadequate response to or a relapse shortly after treatment had actually been treated with ronidazole dosages that are considered inadequate. This means that treatment failure is often due to the prescription of inadequate dosages of ronidazole. Ideally, the response to treatment should be judged in the light of a follow-up PCR examination of fecal samples. However, this is often not done owing to financial constraints, as was the case in the present study, in which the vast majority of the submitting veterinarians had not submitted a follow-up sample. The effectiveness of ronidazole in the treatment of feline intestinal T foetus infection requires further investigation in prospective and controlled clinical trials.
Neurotoxicity is the most commonly reported and serious side effect of ronidazole treatment in cats.15,28,30 In the present study, neurological signs were observed in 3/49 cats (6%) treated with ronidazole. Anorexia was another relatively common clinical sign associated with ronidazole treatment in the present series.
Conclusions
In this study, the majority of cats infected with T foetus were DSH cats, and slightly more than 50% of cats were over 1 year of age. Also, the majority of infected cats were not from multi-cat environments. Diarrhea was the predominant clinical sign, and most cats had diarrhea since adoption. Other clinical signs, such as anorexia, depression, vomiting and weight loss, were less commonly present. In most cats, ronidazole treatment at the currently recommended dosage was efficacious in ameliorating clinical signs. However, in some cats clinical signs persisted despite use of ronidazole at the currently recommended dosages.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case series.
The authors do not have any potential conflicts of interest to declare.
Accepted: 26 May 2013
This study was presented in abstract form at the 28th annual forum of the American College of Veterinary Internal Medicine, June 2010, Anaheim, CA, USA
References
- 1. Tolbert MK, Gookin J. Tritrichomonas foetus: A new agent of feline diarrhea. Compend Contin Educ Vet 2009; 31: 374–381. [PubMed] [Google Scholar]
- 2. Xenoulis PG. Tritrichomonas foetus: a new cause of diarrhea in cats in Greece. J Hellenic Vet Med Soc 2011; 62: 132–140. [Google Scholar]
- 3. Levy MG, Gookin JL, Poore M, et al. Tritrichomonas foetus and not Pentatrichomonas hominis is the etiologic agent of feline trichomonal diarrhea. J Parasitol 2003; 89: 99–104. [DOI] [PubMed] [Google Scholar]
- 4. Gookin JL, Levy MG, Law JM, et al. Experimental infection of cats with Tritrichomonas foetus. Am J Vet Res 2001; 62: 1690–1697. [DOI] [PubMed] [Google Scholar]
- 5. Gookin JL, Stebbins ME, Hunt E, et al. Prevalence of and risk factors for feline Tritrichomonas foetus and giardia infection. J Clin Microbiol 2004; 42: 2707–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Slapeta J, Craig S, McDonell D, Emery D. Tritrichomonas foetus from domestic cats and cattle are genetically distinct. Exp Parasitol 2010; 126: 209–213. [DOI] [PubMed] [Google Scholar]
- 7. Sun Z, Stack C, Slapeta J. Sequence differences in the diagnostic region of the cysteine protease 8 gene of Tritrichomonas foetus parasites of cats and cattle. Vet Parasitol 2012; 186: 445–449. [DOI] [PubMed] [Google Scholar]
- 8. Reinmann K, Muller N, Kuhnert P, et al. Tritrichomonas foetus isolates from cats and cattle show minor genetic differences in unrelated loci ITS-2 and EF-1alpha. Vet Parasitol 2012; 185: 138–144. [DOI] [PubMed] [Google Scholar]
- 9. Slapeta J, Muller N, Stack CM, et al. Comparative analysis of Tritrichomonas foetus (Riedmuller, 1928) cat genotype, T foetus (Riedmuller, 1928) cattle genotype and Tritrichomonas suis (Davaine, 1875) at 10 DNA loci. Int J Parasitol 2012; 42: 1143–1149. [DOI] [PubMed] [Google Scholar]
- 10. Frey CF, Muller N. Tritrichomonas — systematics of an enigmatic genus. Mol Cell Probes. 2012; 26: 132–136. [DOI] [PubMed] [Google Scholar]
- 11. Foster DM, Gookin JL, Poore MF, et al. Outcome of cats with diarrhea and Tritrichomonas foetus infection. J Am Vet Med Assoc 2004; 225: 888–892. [DOI] [PubMed] [Google Scholar]
- 12. Yaeger MJ, Gookin JL. Histologic features associated with Tritrichomonas foetus-induced colitis in domestic cats. Vet Pathol 2005; 42: 797–804. [DOI] [PubMed] [Google Scholar]
- 13. Xenoulis PG, Saridomichelakis MN, Read SA, et al. Detection of Tritrichomonas foetus in cats in Greece. J Feline Med Surg 2010; 12: 831–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miró G HL, Hernández L, Montoya A, et al. First description of naturally acquired Tritrichomonas foetus infection in a Persian cattery in Spain. Parasitol Res 2011; 109: 1151–1154. [DOI] [PubMed] [Google Scholar]
- 15. Bell ET, Gowan RA, Lingard AE, et al. Naturally occurring Tritrichomonas foetus infections in Australian cats: 38 cases. J Feline Med Surg 2010; 12: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuehner KA, Marks SL, Kass PH, et al. Tritrichomonas foetus infection in purebred cats in Germany: prevalence of clinical signs and the role of co-infection with other enteroparasites. J Feline Med Surg 2011; 13: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tysnes K, Gjerde B, Nødtvedt A, et al. A cross-sectional study of Tritrichomonas foetus infection among healthy cats at shows in Norway. Acta Vet Scand 2011; 20: 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bissett SA, Stone ML, Malik R, et al. Observed occurrence of Tritrichomonas foetus and other enteric parasites in Australian cattery and shelter cats. J Feline Med Surg 2009; 11: 803–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holliday M, Deni D, Gunn-Moore DA. Tritrichomonas foetus infection in cats with diarrhoea in a rescue colony in Italy. J Feline Med Surg 2009; 11: 131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kingsbury DD, Marks SL, Cave NJ, et al. Identification of Tritrichomonas foetus and Giardia spp infection in pedigree show cats in New Zealand. N Z Vet J 2010; 58: 6–10. [DOI] [PubMed] [Google Scholar]
- 21. Stockdale HD, Givens MD, Dykstra CC, et al. Tritrichomonas foetus infections in surveyed pet cats. Vet Parasitol 2009; 160: 13–17. [DOI] [PubMed] [Google Scholar]
- 22. Stauffer SH, Birkenheuer AJ, Levy MG, et al. Evaluation of four DNA extraction methods for the detection of Tritrichomonas foetus in feline stool specimens by polymerase chain reaction. J Vet Diagn Invest 2008; 20: 639–641. [DOI] [PubMed] [Google Scholar]
- 23. Gookin JL, Birkenheuer AJ, Breitschwerdt EB, et al. Single-tube nested PCR for detection of Tritrichomonas foetus in feline feces. J Clin Microbiol 2002; 40: 4126–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gookin JL, Foster DM, Poore MF, et al. Use of a commercially available culture system for diagnosis of Tritrichomonas foetus infection in cats. J Am Vet Med Assoc 2003; 222: 1376–1379. [DOI] [PubMed] [Google Scholar]
- 25. Gookin JL, Breitschwerdt EB, Levy MG, et al. Diarrhea associated with trichomonosis in cats. J Am Vet Med Assoc 1999; 215: 1450–1454. [PubMed] [Google Scholar]
- 26. Gunn-Moore DA, McCann TM, Reed N, et al. Prevalence of Tritrichomonas foetus infection in cats with diarrhoea in the UK. J Feline Med Surg 2007; 9: 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frey CF, Schild M, Hemphill A, et al. Intestinal Tritrichomonas foetus infection in cats in Switzerland detected by in vitro cultivation and PCR. Parasitol Res 2009; 104: 783–788. [DOI] [PubMed] [Google Scholar]
- 28. Gookin JL, Copple CN, Papich MG, et al. Efficacy of ronidazole for treatment of feline Tritrichomonas foetus infection. J Vet Intern Med 2006; 20: 536–543. [DOI] [PubMed] [Google Scholar]
- 29. Gookin JL, Stauffer SH, Dybas D, et al. Documentation of in vivo and in vitro aerobic resistance of feline Tritrichomonas foetus isolates to ronidazole. J Vet Intern Med 2010; 24: 1003–1007. [DOI] [PubMed] [Google Scholar]
- 30. Rosado TW, Specht A, Marks SL. Neurotoxicosis in 4 cats receiving ronidazole. J Vet Intern Med 2007; 21: 328–331. [DOI] [PMC free article] [PubMed] [Google Scholar]