Abstract
Aim:
The overarching purpose of the AAFP Anesthesia Guidelines (hereafter referred to as the ‘Guidelines’) is to make anesthesia and sedation safer for the feline patient.
Scope and accessibility:
It is noteworthy that these are the first exclusively feline anesthesia guidelines authored by an expert panel, making them particularly useful as an extensively referenced, practical resource for veterinary practice teams. Because much of the key content is presented in tabular or visual format, the Guidelines have a high level of accessibility and convenience that invites regular usage. While the recommendations in the Guidelines focus primarily on client-owned cats, the content is also applicable to community-sourced animals with an unknown medical history.
Keywords: Anesthesia, sedation, anesthetic equipment, comorbidities, monitoring, airway management
Introduction
Data and empirical experience have shown that cats undergoing anesthesia continue to have an increased mortality rate compared with dogs.1,2 These Guidelines address specific causes of this disparity and ways of avoiding perioperative complications associated with monitoring, airway management, fluid therapy and recovery. Additionally, the Guidelines discuss other important aspects of feline anesthesia, including perianesthetic anxiety and stress, perianesthetic monitoring by physical and electronic means, the role of underlying diseases such as hypertrophic cardiomyopathy (HCM), the correct use of anesthesia equipment, and total injectable anesthesia.
Although pain mitigation is integral to anesthesia, anesthesia techniques should not be confused with pain management, which is the subject of previously published clinical guidelines. 3 Using both sets of Guidelines together will allow the practitioner to provide comprehensive perianesthetic care.
Anesthesia equipment: safety considerations for feline patients
Anesthetic equipment is considered ‘life-critical’ because the wellbeing of patients can be adversely affected if equipment is functioning suboptimally or is used incorrectly. Table 1 lists the essential and recommended anesthesia equipment for cats. Equipment must be well maintained and tested at scheduled times (Table 2).
Table 1.
Anesthesia equipment
|
Essential equipment
✜ IV catheters – 20 and 22 G ✜ Face masks ✜ Endotracheal tubes with a Murphy eye; variety of sizes (2.0–5.5 mm), cuffed or uncuffed ✜ Laryngoscope and appropriate blade and size: ✜ eg, Miller, Cranwall or Seward, size 0–2 ✜ Anesthesia machine with out-of-circuit vaporizer ✜ Non-rebreathing anesthesia circuit with manometer ✜ Safety pop-off relief valve ✜ Esophageal stethoscope ✜ Thermometer ✜ Blood pressure monitor ✜ Pulse oximeter with waveform display |
|
Recommended additional equipment
✜ High-pressure breathing circuit alarm ✜ End-tidal carbon dioxide (ETCO2) monitor with waveform display ✜ Fluid pump ✜ Electrocardiograph |
Table 2.
Routine maintenance for anesthesia equipment safety
| Equipment | Routine maintenance |
|---|---|
| Breathing circuits | Leak test prior to each anesthetic
Cleaning * : wash in warm water between patients; hang to air dry |
| Endotracheal tubes (ETTs) | Leak test ETT cuff prior to each use
Cleaning * : Thoroughly clean inside ETT using bottle brush and warm water Discard if patient has respiratory disease |
|
CO2
absorbents |
Replace granules (granules become dry due to CO2 absorption from patients and exposure to air)
†
:
Single canisters – after every 8 h of use A system for recording hours and date(s) of use (eg, a tag attached to the canister) should be in place |
| Anesthesia machine | Clean as needed; replace O-rings and tubing yearly |
| Vaporizer | Output test: according to the manufacturer’s recommendations
Full service: clean inside, perform high-pressure leak test, replace parts, and recalibrate every 3 years (requires outside maintenance service) |
Disinfectants such as Virkon (DuPont), chlorhexidine and accelerated hydrogen peroxide are often used; the manufacturer’s instructions for dilution, contact time and rinsing must be strictly adhered to
The need to change CO2 absorbents is highly variable and depends on several factors including, but not limited to, the size of the canister, oxygen flow rates and humidity. If end-tidal CO2 is being monitored, exhaustion of the absorbent can be tracked (one cause of a rise in inspired CO2 levels is exhausted CO2 absorbents)
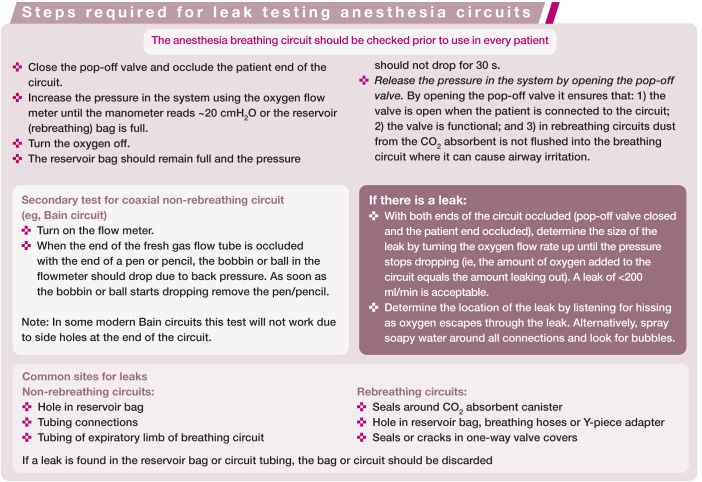
Anesthesia machines and breathing circuits are designed to deliver oxygen and inhalant anesthetic agents and prevent rebreathing of carbon dioxide (Co2). Leak testing the circuit before use in each patient ensures oxygen will flow to the patient and that there is minimal leakage of waste anesthetic gases. The steps required for leak testing are described in the box on page 604.
Two essential safety features are: 1) an in-circuit manometer, and 2) a safety pop-off relief valve (Figure 1).
Figure 1.

Bain non-rebreathing circuit (NRC) adapter with in-circuit manometer (white arrow) and safety pop-off valve (black arrow). Courtesy of Heidi Shafford
A manometer allows safe delivery of manual and mechanical breaths, and checking of the seal between the endotracheal tube (ETT) cuff and trachea. Safety pop-off relief valves prevent excessively high airway pressures and potential barotrauma. These devices can be installed on most anesthesia machines for use with non-rebreathing or rebreathing circuits. The risk of high airway pressures is significant in cats because of their small lung capacity (~400 ml in an average sized cat); the high oxygen flow rates used with non-rebreathing circuits (NRCs) can lead to damage of the cat’s airway in less than 30 s. Another option is to insert a high pressure alarm between the common gas outlet and the circuit; this does not allow escape of gas but emits a loud noise if the pressure in the circuit rises (Figure 2).
Figure 2.

Example of a battery-powered high pressure alarm with an adapter (white arrow) that is inserted into the expiratory limb of a Bain NRC. Courtesy of Heidi Shafford

Anesthesia circuits
NRCs are widely used in feline anesthesia because they offer less resistance than a rebreathing circuit, which is an important consideration in small patients. Rebreathing of Co2 is prevented by high oxygen flow rates; ⩾200 ml/kg/min is usually recommended but rebreathing can be monitored with a capnograph, allowing the flow rate to be set so that inspired Co2 is <5 mmHg. Another benefit of NRCs is their low equipment dead space. The Panel recommends using NRCs in all cats, but modern circle systems with lightweight plastic rebreathing valves and minimal dead excessive in small cats. investment in pediatric circuits and low dead space adapters is recommended.
The federal government of the United States has not established official standards for environmental control of waste anesthetic gases. The occupational Safety and Health Administration (oSHA) provides advisory guidelines and some US states have specific regulations. This Panel strongly recommends scavenging of waste anesthetic gases – especially with active systems, which are more effective than passive scavenging systems (eg, activated charcoal canisters). For more information consult the American College of Veterinary Anesthesia and Analgesia position statement on the control of waste anesthetic gases in the workplace: www.acvaa.org/docs/Position_Statements .
Preanesthetic assessment
Patient history
Obtaining a complete patient history from an owner can be challenging. For example, history regarding food and water intake, and urinary and fecal output may be unknown in cats that spend time outdoors and also in multi-cat households. Cats often show no obvious space can also be used safely in cats >3 kg (6.6 lb). The oxygen flush valve should never be used when a cat is connected to an NRC.
Rebreathing circuits (eg, circle systems) depend on functional one-way valves to ensure gas flow in one direction, and Co2 absorbents to prevent rebreathing of Co2. Benefits include lower oxygen flow rates (compared with NRCs), less use of inhalant agents and less waste anesthetic gas. Pediatric rather than adult breathing hoses are recommended as they have lower equipment dead space.
Tables 3 and 4 provide a quick reference guide to the two types of anesthesia breathing circuits, suggested oxygen flow rates and reservoir (rebreathing) bag size based on circuit type and the cat’s body weight.
Table 3.
Comparison of non-rebreathing and rebreathing anesthesia circuits
| Functional consideration | Non-rebreathing circuit (NRC) | Rebreathing circuit (circle system) |
|---|---|---|
| Rebreathing potential | No rebreathing of exhaled gases | Rebreathing exhaled oxygen and inhalant |
| Mechanism for preventing rebreathing of CO2 | High oxygen flow rates move exhaled CO2 along the expiratory limb away from the patient | Functional CO2 absorbent and one-way valves |
| Conservation of body heat | High flow rates of oxygen may lead to increased loss of body heat | Lower flow rates of oxygen and rebreathing of exhaled gases may help to conserve body heat |
| Resistance to breathing | Minimal | Resistance is caused by CO2 absorbent and one-way valves |
| Equipment dead space | Minimal | Minimized by using a pediatric Y circuit |
| Time taken for a change in anesthetic depth | Short, due to small circuit volume and higher oxygen flow rates | Longer, due to larger circuit volume and lower oxygen flow rates |
Optimal circuit based on patient size:
✜ Very small cat (<3 kg; 6.6 lb): NRC
✜ Small to medium cat (3–6 kg; 6.6–13.2 lb): NRC or rebreathing circuit
✜ Large cat (>6 kg; 13.2 lb): NRC or rebreathing circuit
Table 4.
Minimum oxygen flow rates and reservoir (rebreathing) bag sizes for cats
| Non-rebreathing circuit (NRC)
Oxygen flow rate ⩾200 ml/kg/min |
Patient body weight | ||||
|---|---|---|---|---|---|
| kg | lb | Rebreathing bag size | Minimum oxygen flow rate | ||
| <1 | <2 | 0.5 l | 0.5 l/min | ||
| 1 | 2 | 0.5 l | 0.5 l/min | ||
| 2 | 4 | 0.5 l | 0.5 l/min | ||
| 3 | 7 | 0.5 l | 0.6 l/min | ||
| >3 kg | >7 lb | May use rebreathing circuit | |||
| 4 | 9 | 0.5 l | 0.8 l/min | ||
| 5 | 11 | 0.5 l | 1.0 l/min | ||
| 6 | 13 | 1 l | 1.2 l/min | ||
| 7 | 15 | 1 l | 1.5 l/min | ||
| 8 | 18 | 1 l | 1.6 l/min | ||
| 9 | 20 | 1 l | 1.8 l/min | ||
| 10 | 22 | 1 l | 2.0 l/min | ||
| Rebreathing circuit (circle system) | Patient body weight | ||||
| kg | lb | Rebreathing bag size | Oxygen flow rate – first 15 mins * | Minimum oxygen flow rate – maintenance † | |
| 0–3 | 0–7 | Use non-rebreathing circuit | |||
| 3–7 | 7–15 | 1 l | 2–3 l/min | 0.5 l/min | |
| 7–10 | 15–22 | 1 l | 2–3 l/min | 0.5 l/min | |
Higher oxygen flow rates in the first 15 mins accelerate the rate of change in inhalant concentration in the rebreathing anesthetic circuit
Monitor inspired CO2 to assess rebreathing of CO2 and the need for higher or lower oxygen flow rates. With an active scavenging system, higher oxygen flow rates may be needed (this will not harm patients)
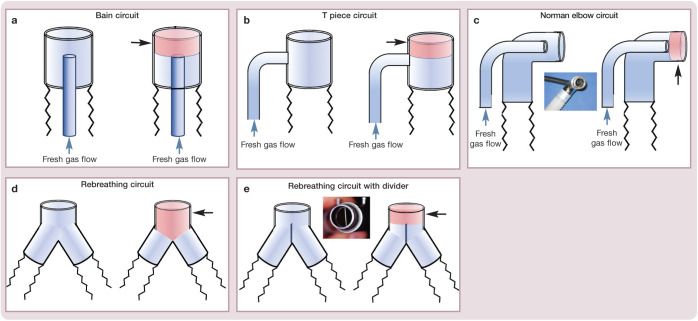
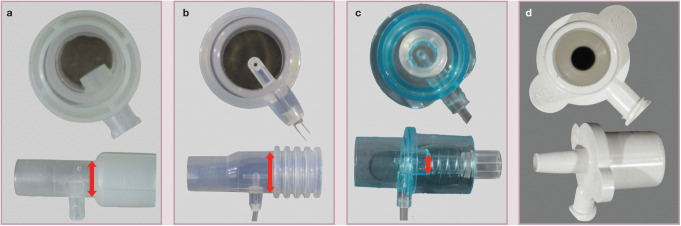
It is important to minimize equipment dead space. Common sources include the patient end of breathing circuits (Figure 3), side-stream capnograph adapters (Figure 4), inline capnographs and elbow adapters. At the same time, it is important not to increase airway resistance, so the diameter of the capnograph adapter should always exceed the internal diameter of the ETT (Figure 4c).
Figure 3.
Dead space (in red; black arrows) associated with the patient end of various types of anesthetic breathing circuit: (a) Bain circuit; (b) T piece circuit; (c) Norman elbow circuit; (d) rebreathing circuit; and (e) rebreathing circuit with divider. Courtesy of Peter Pascoe
Figure 4.
Dead space related to various capnograph adapters. (a) Side-stream adapter for use with a removable sampling line; arrow shows diameter width. (b) An ‘all-in-one’ side-stream adapter; arrow shows diameter width. (c) A pediatric adapter; note the small diameter of the lumen, as shown by the arrow. The diameter of the capnograph adapter should always exceed the internal diameter of the ETT. (d) Low dead space ETT adapters are available for attachment to side-stream sampling lines. Courtesy of Gregg Griffenhagen
Excessive equipment dead space leads to rebreathing of Co2 and decreases the effective tidal volume available for gas exchange. Dead space should be ⩽2–3 ml/kg, which is ⩽20% of tidal volume. A single elbow adapter may add up to 8 ml of dead space, which can be clinical signs early in a disease process because the signs are subtle and go unnoticed or are assumed to be ‘normal’ (eg, decreased mobility with age). Many owners are also unaware that their ‘apparently healthy’ cat is over- or underweight. 4 Therefore, a thorough physical examination and appropriate pre-anesthetic laboratory testing is important in all cats presented for general anesthesia. The history gathering process can be enhanced by the development of a standardized questionnaire or discussion points document, such as the one presented in the AAFP-AAHA Feline Life Stage Guidelines. 5

Patient physical examination
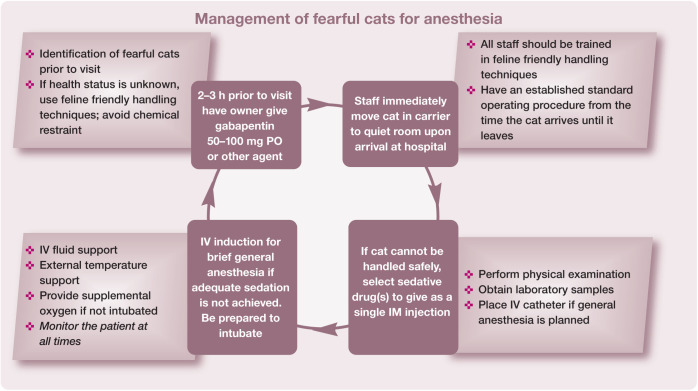
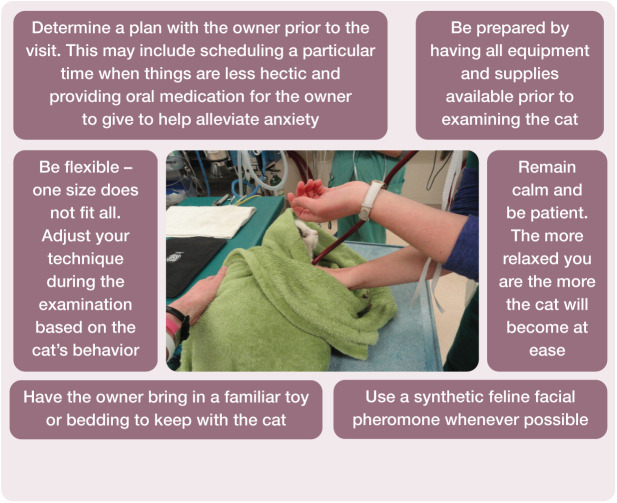
All cats should have a preanesthetic physical examination that evaluates their signalment, physiologic parameters and all body systems. These results should be compared with those from previous visits, if available, to identify any potential trends. if a cat is anxious or stressed during this time, several physiologic variables, including heart rate, blood pressure and respiratory rate, can be elevated, and it may not be possible to complete the examination. 6 Practice teams are often faced with cats that are fearful and difficult to handle. Many of these challenges can be overcome with team training as detailed in the AAFP and ISFM Feline-Friendly Nursing Care Guidelines and Feline-Friendly Handling Guidelines.7,8 Pre-hospital administration of gabapentin is highly recommended for many cats to reduce the fear response, decrease stress and facilitate physical examination.9,10 In this setting gabapentin is used as an anxiolytic to decrease negative emotions including fear, which can lead to unwanted behaviors including aggression. 11 Figures 5 and 6 summarize key components for working with these cats.
Figure 5.
Multi-step protocol for the management of fearful cats for anesthesia
Figure 6.
Key recommendations for minimizing anxiety and facilitating handling of feline patients presented for general anesthesia. Photograph courtesy of Sheilah Robertson
If the procedure is elective and the cat has become distressed, a viable option is to reschedule with a plan to address fear before the cat returns to the hospital. Without a complete physical examination, the potential for missing or misdiagnosing comorbidities is significantly increased, thereby increasing anesthetic risk.
Preanesthetic laboratory tests
The decision to perform preoperative testing should be based on the history and physical examination findings, perioperative risk assessment, clinical judgement and practice policy. Abnormal test results may reveal problems not identified from the physical examination and history, and lead to modification of the anesthetic plan. 12 A minimum database should be developed based on individual patient assessment (Table 5).
Table 5.
| Diagnostic test | Neonatal/pediatric and junior Adult/prime (0 months–2 years) (3–6 years) | Mature (7–10 years) | Senior/geriatric (>10 years) | |
|---|---|---|---|---|
|
Chemistry screen
As a minimum include: BUN, creatinine, ALP, ALT, TP, glucose, albumin, globulin, phosphorus, K+, Na+, Ca2+ |
± | ± | + | + |
|
Complete blood count
Hematocrit, RBCs, WBCs, differential count, cytology, platelets |
± | ± | + | + |
|
Urinalysis
Specific gravity, sediment, glucose, ketones, bilirubin, protein |
± | ± | + | + |
| Retroviral testing | + | ± | ± | ± |
| T4 | ± | ± | + | |
| Blood pressure (NIBP) | ± | ± | + | |
| ECG* | ± | ± | ± | |
| Thoracic radiography * † | ± | ± | + | |
| NT-proBNP * | ± | ± | ± | |
ALP = alkaline phosphatase; ALT = alanine aminotransferase; BUN = blood urea nitrogen; CBC = complete blood count;
ECG = electrocardiography; NIBP = non-invasive blood pressure; NT-proBNP = N-terminal pro-brain natriuretic peptide; RBCs = red blood cells; T4 = thyroxine; TP = total protein; WBCs = white blood cells
Specific tests indicated based on physical examination findings and signalment
In some jurisdictions, regulations do not allow personnel to hold animals for radiography; therefore, the pros and cons of sedation or anesthesia must be weighed against the benefits of obtaining images
Because many cats are stressed at the time of presentation, physical examination and laboratory results may reflect this, making interpretation difficult; for example, is the blood glucose, heart rate and blood pressure elevated due to a disease process or stress? 6 if feline friendly handling techniques have failed, it is in the best interests of the patient to forego physical restraint to obtain laboratory samples and consider chemical restraint. 8 if chemical restraint is used, the practitioner should understand the impact that some drugs may have on the physical examination and laboratory data. one example is dexmedetomidine, which produces a decrease in heart rate, changes in the echocardiogram, and increased blood pressure and blood glucose values.18,19
A retrospective study of preanesthetic blood screening of cats with a mean age of 11.6 years reported that approximately 16% had abnormal results that were concerning for the clinician and that, in 1% of these cases, no abnormalities had been identified from the history or physical examination; as a result anesthetic protocols were changed or the procedure postponed in 9% of the cases. 12
Annual testing for hyperthyroidism is recommended, starting between the ages of 7–10 years, because it affects up to 10% of cats aged 10 years and older.13,14,20,21 Depending upon the patient’s history, signalment and physical examination results, other specific tests such as electrocardiography, thoracic radiography, echocardiography and N-terminal pro-brain natriuretic peptide (NT-proBNP) screening may be indicated. 22 Cardiomyopathy is often a ‘silent’ disease in cats and NT-proBNP screening should be considered in at-risk breeds.15,16,22–24 This test is reported to have a 90% sensitivity and 85% specificity in detecting cats with asymptomatic heart disease. 25 The NT-proBNP test has also been shown to help differentiate cardiac from non-cardiac disease in dyspneic cats and is a useful tool in cats that are not good candidates for other diagnostic testing, such as thoracic radiography and echocardiography, because of their physical status.16,22,26
Fasting
The goal of preoperative fasting is to reduce the volume of stomach contents, and prevent gastroesophageal reflux (GER), regurgitation and aspiration. Withholding food for 6–12 h prior to anesthesia or instructions for ‘nothing after midnight’ have traditionally been recommended but are not evidence based. Studies on the duration of fasting on GER are conflicting in dogs.27,28 Feeding a small meal of canned food 3 h before surgery reduced the incidence of GER in one study but not another, and shorter fasting times were associated with less acidic reflux. other factors, including preanesthetic and anesthetic drugs, procedure, age and position during surgery, also influence GER. Long fasting times do not necessarily ensure that a cat’s stomach will be empty. Stress, meal size and lack of dietary moisture can slow gastric emptying. Therefore, the anesthetist must always be prepared for perioperative vomiting. Although there are no data in cats, shorter fasting times (3–4 h) with provision of a small wet food meal 3–4 h before anesthesia may be adopted at the clinician’s discretion. Water should be available until the time of premedication.
Patient temperament
The increased release of catecholamines in fearful or stressed cats leads to tachycardia, systemic hypertension and/or tachypnea, all of which can increase the risks associated with anesthesia. 17 For these reasons the Panel emphasizes the importance of respectful handling and the pre-visit use of gabapentin or other chemical restraint when needed.
Surgical procedure and risk
The authors of the confidential enquiry into perioperative small animal fatalities (CEP-SAF) study reported that the intended procedure (minor vs major) and procedural urgency were risk factors for anesthetic-related death. 2 The low mortality rate in young healthy cats undergoing elective neutering procedures supports these findings. 29
Individualized patient plan
in humans the preanesthetic evaluation includes as a minimum a review of the patient’s medical record, a patient interview, a preanesthetic physical examination, preoperative tests when appropriate, and other consultations if needed (American Society of Anesthesiologists Task Force on Preanesthesia Evaluation). 30 Abnormal test results in apparently healthy animals should be considered carefully along with the history and physical examination findings as they may or may not be clinically significant.12–14,31
American Society of Anesthesiologists’ (ASA) physical status classification
ASA assessment is a means of categorizing levels of preanesthetic health, not the patient’s risk of anesthetic complications. Differentiating healthy from sick patients is important for creating anesthetic plans that minimize risk, and assigning an ASA status is one way to achieve this (Table 6). The potential for occult disease in cats means it can be difficult to accurately assign them to an ASA category. Because ASA status is subjective, this Panel does not consider it essential for every patient, although it may be beneficial in anesthetic planning.
Table 6.
ASA physical status classification with examples
| Class | Preoperative health status |
|---|---|
| PS 1 | Normal patient with no organic disease; eg elective neutering procedures |
| PS 2 | Patient with mild systemic disease; eg skin
tumor or fracture without shock |
| PS 3 | Patient with severe systemic disease limiting activity but not incapacitating; eg well controlled diabetes mellitus |
| PS 4 | Patient with incapacitating systemic disease that is a constant threat to life; eg perforatedsmall intestine and severe hypovolemia |
| PS 5 | Moribund patient not expected to live 24 h without surgery; eg severe trauma and shock |
PS = physical status
One feline study that evaluated age and ASA physical status as risk factors for perianesthetic morbidity and mortality found that ASA status rather than age was a better predictor of perianesthetic complications. Cats with an ASA status of 3 or higher had a significantly increased risk of complications. 32 Another study found that age (>12 years) was a risk factor independent of ASA status. 2
Life stages
During preanesthetic assessment and planning there are specific factors to consider depending upon the life stage of the cat (Figure 7). 5 Feline friendly handling and risk of hypothermia are applicable to all cats.
Figure 7.
Specific considerations for each feline life stage.2,14,33–38 ASA = American Society of Anesthesiologists. Photographs courtesy of Sheilah Robertson (neonatal/pediatric/kitten) and Susan Gogolski (junior, adult/prime, mature and senior/geriatric)
Comorbidities
The purpose of this comorbidity section is to raise awareness in the practice team of the increased likelihood of the presence of concurrent illness, and the intent of these Guidelines is to highlight the salient factors associated with each comorbidity. For more in-depth information regarding each comorbidity, readers are directed to the listed references.
An extremely important perioperative consideration is that many anesthesia candidates will have comorbidities; some may be subclinical (eg, HCM) or undiagnosed at the time of anesthesia and some may or may not be well controlled.
Hypertrophic cardiomyopathy33,39,40
The use of alpha(α)2-adrenergic agonists in cats with HCM is controversial.
One reference (using medetomidine) suggests a beneficial reduction in outflow obstruction in cats with left ventricular out-flow tract obstruction, which is a subset of HCM. 41 one concern is that the increase in afterload associated with α2-adrenergic agonist administration or stress will decrease cardiac output in cats with HCM. Another concern is that dexmedetomidine alone or combined with other drugs causes changes in atrial and ventricular size and function, and may influence interpretation of echocardiographic variables and thoracic radiographs.19,42,43
The benefit of reducing patient stress, preventing tachycardia, and reducing inhalant anesthetic requirements may out-weigh the potential risks associated with low doses of α2-adrenergic agonist drugs. Echocardiographic measurements have been reported in healthy cats before and after intramuscular alfaxalone (2 mg/kg) combined with butorphanol (0.2 mg/kg), which provided good short-lasting sedation. 44 Although the differences recorded for most echocardiographic measurements were not clinically significant after sedation, 44 this has not been confirmed in cats with HCM.
Hyperthyroidism21,33,39,45
Since hyperthyroidism affects multiple systems, it is desirable to obtain a euthyroid state prior to an elective anesthetic procedure by starting antithyroid medication 2–3 weeks prior to the procedure and rechecking total thyroxine levels at the end of this period. This allows assessment of renal function based on the euthyroid state. if a thyrotoxic cat requires emergency anesthesia for an unrelated problem and has significant tachycardia (>200 bpm), a beta blocker such as atenolol, at 1 mg/kg Po q12–24h, can be started prior to anesthesia. However, if during anesthesia the heart rate increases above 220 bpm with an adequate depth of anesthesia, start a constant rate infusion (CRI) of esmolol with a loading dose of 0.1–0.5 mg/kg iV over 1 min, followed by a CRI of 100–200 µg/kg/min.36,45,46
Renal disease33,39
Preanesthetic fluid therapy may be of value to restore normovolemia and hydration.
International Renal interest Society (IRIS) staging ( www.iris-kidney.com ) should be used to guide therapy. intravenous administration of a balanced electrolyte solution for ⩾4 h at 3–5 ml/kg/h, or based on a daily maintenance dosage calculated as 80 x body weight (kg)0.75 per 24 h, 47 has been suggested by some clinicians prior to anesthesia in cats with IRIS stage ⩾3 kidney disease.
Proactive monitoring and management of blood pressure is essential in the perianesthetic period. intravenous fluid therapy should be maintained in recovery until the patient can eat and drink. For cats receiving enalapril, discontinuation 24–48 h prior to anesthesia may reduce the risk of intraoperative hypotension. 48
Diabetes mellitus 49
As with other comorbidities, ideally diabetic patients should be well regulated prior to any anesthetic procedure. Whether or not insulin is required during the perioperative period is based on blood glucose values prior to the procedure. The following recommendations have been made:
✜ If blood glucose is <8 mmol/l (<145 mg/dl) no insulin is required;
✜ At 8–15 mmol/l (145–270 mg/dl) half the cat’s regular dose of insulin is given;
✜ At >15 mmol/l (>270 mg/dl) the full dose of insulin is administered. 49
These patients should be scheduled to be the first anesthetic case of the day so that they can be discharged the same day in order to return to their normal eating behaviors and insulin regimen without disruption. Pre- and intraoperative monitoring (every 30 mins) of blood glucose is recommended. Verifying glucose levels in recovery may be necessary, particularly if insulin has been given during anesthesia and if the patient is not eating. If blood glucose falls below 3 mmol/l (54 mg/dl), 0.25–0.5 g/kg of dextrose is given as an IV bolus; and if severe hyperglycemia occurs (>30 mmol/l, 540 mg/dl), the cat’s normal insulin dose is given. At blood glucose levels between these extremes balanced electrolyte solutions with or without dextrose (2.5–5%) can be used based on results of monitoring. 49
Asthma or lower airway disease39,50
Complications related to sedation and/or general anesthesia can be significantly increased in cats with lower airway disease. Although there are no specific anesthetic protocols in veterinary medicine for asthma, as there are in people, there are some recommendations:
✜ Maintain the patient on its asthma management medications up to and including the morning of anesthesia.
✜ Use preanesthetic sedation (eg, gabapentin, butorphanol, acepromazine) to reduce stress.
✜ If tolerated, preoxygenate with 100% oxygen for 3–5 mins by face mask or flow-by before anesthetic induction.
✜ Place an IV catheter.
✜ Always be prepared to intubate.
✜ Do not attempt to intubate until the cat is at a suitable depth of anesthesia; or, alternatively, consider using a supraglottic airway device (SGAD, see later) if the procedure permits its use.
✜ Oxygen saturation of hemoglobin in arterial blood (SpO2) and end-tidal CO2 (ETCO2) should be monitored and oxygen supplementation continued in recovery, along with close observation of the patient.
These patients can decompensate rapidly so the team needs to approach these cases proactively and have all potentially needed equipment and supplies available before commencing sedation. Terbutaline (0.01 mg/kg SC) reduces complications during bronchoscopy and bronchoalveolar lavage, and should be considered when anesthetizing asthmatic cats. 50 onset of action after SC administration is 15–30 mins, so plan accordingly.
Obesity51–53
Since obesity may affect drug pharmacokinetic parameters such as volume of distribution, bioavailability and clearance, anesthetic drug dosages should be based on the cat’s ideal body weight. Mortality studies suggest a relationship between body weight and outcome; cats weighing more than 6 kg were three times more likely to die during the perioperative period as cats weighing 2–6 kg. 2 Pre-oxygenation using either a face mask or flow-by can assist in lengthening the time to desaturation, particularly in obese brachycephalic breeds. These cats need to have their oxygenation status closely monitored during the recovery period, and may require oxygen supplementation postoperatively due to hypoventilation.
Degenerative joint disease 54
Degenerative joint disease (DJD) may be present in cats of all ages, but the majority are 12 years or older. in addition, clinical signs are typically subtle. Therefore, exercise caution with patient positioning during anesthesia. one study found that 68.8% of cats selected for DJD studies also had concurrent chronic kidney disease. 54
Urinary obstruction33,39,55,56
Although one of the goals of therapy in obstructed cats is to restore urine flow, simply restoring urine flow and not correcting dehydration and electrolyte abnormalities appears to be associated with poor outcomes. in addition, if there are electrocardiographic changes related to hyperkalemia or the blood potassium concentration is >7 mEq/l (mmol/l), institute immediate therapy to protect the heart and to lower the serum potassium concentration prior to sedation or anesthesia.
One or more of the following treatments may be required:
✜ Calcium gluconate 10% 0.5–1.5 ml/kg IV to stabilize cardiac conduction; given over 5–10 mins while monitoring the electro-cardiogram.
✜ IV administration of a balanced electrolyte solution for rehydration, dilution of extra-cellular potassium and restoration of acid–base balance.
✜ An IV bolus of a short-acting insulin such as regular insulin 0.5 U/kg, plus an iV bolus of 25% dextrose at 2 g/U of insulin administered; this promotes intracellular uptake of potassium. Following the initial insulin and dextrose boluses, dextrose can be continued as a CRI.
For urinary catheter placement sedation may be needed; butorphanol 0.1–0.2 mg/kg (IM or IV) alone may be sufficient but additional sedation or general anesthesia may be required. A sacrococcygeal epidural block with a local anesthetic agent is easy to perform and highly recommended to provide analgesia and increase the success of urethral catheterization. 56
Critical patient emergencies57–59
Evaluation of the critical feline patient ideally begins before the patient arrives at the hospital through the telephone conversation between the owner and a team member. The team member asks the owner specific questions that will help the team to proactively prepare for the cat’s arrival. The cat’s medical history should be obtained to verify that there are no pre-existing illnesses that will impact treatment. A hospital standard operating procedure (SoP) that details how the team receives and assesses the emergency feline patient is an extremely important tool that every team member should be familiar with. in addition, all team members should receive hands-on simulated training using different patient scenarios, along with education about feline-specific emergency characteristics. By doing this everyone knows what to do when an emergency case comes through the door.
The following are important facts to keep in mind when presented with an emergency feline case:
✜ The average feline thorax can contain 300 ml of fluid before respiratory distress is seen.
✜ The lung is the shock organ of the cat and the blood volume is less than that of dogs on a ml/kg basis; therefore, fluid therapy must be closely monitored.
✜ In cats, shock typically results in bradycardia.
✜ Cats have a limited ability to contract their spleen during hemorrhage.
✜ With poor perfusion and lack of nutritional support, cats can quickly develop a catabolic state, which predisposes them to the development of hepatic lipidosis.
✜ Cats presenting with seizures need to be evaluated for extracranial and intracranial causes because idiopathic epilepsy is uncommon in the cat.
✜ Cardiac emergencies typically present with respiratory distress, syncope and/or hindlimb paresis.
Due to the cat’s inherent ability to mask disease, many cats that present as emergency cases are already in a critical condition and additional stress or handling can result in sudden death.
Upon arrival at the hospital, the cat should be immediately assessed for life-threatening conditions. The primary survey focuses on the respiratory, cardiovascular, neurological and urinary systems. The respiratory and cardio-vascular systems should be evaluated first. Cats presenting with open-mouth breathing and an increased respiratory rate and effort typically have severe respiratory compromise and have a high risk of sudden death just from handling during the physical examination. Therefore, these cats should be immediately placed in an oxygen cage, if available, or be provided with flow-by oxygen with minimal handling. During this time, a dedicated technician should observe the cat and, once respiratory signs improve, alert the veterinarian so that the primary survey can be completed. if perfusion is significantly compromised and neurological deficits are noted during the physical examination, the perfusion deficits should be corrected first, and the neurological system re-evaluated.
Once the cat is stabilized, a secondary survey is performed. During this time, a complete physical examination is conducted that evaluates the patient from head to toe. Depending upon what is discovered during this examination, additional diagnostic testing may be performed. Communication between the team and owner regarding patient status is very important during this entire process.

If a life-threatening condition develops that requires immediate surgery, every effort should be made to stabilize the patient first, especially if there is concurrent heart failure, intracranial hypertension, pneumothorax and/or pulmonary edema present. Preoperative assessment is extremely challenging in these critically ill cats, so checklists have been developed to help ensure that all systems are evaluated and monitored for change. Although ‘Kirby’s Rule of 20’ was developed primarily to monitor small animal septic patients, the checklist has been used by emergency clinicians as a tool to monitor the critical patient so that, in the event the patient’s condition worsens, treatment can be instituted immediately. 59
Anesthesia and sedation
If needed, behavioral modification and/or pre-hospital sedation should be utilized to decrease anxiety and fear prior to a medical procedure. However, some cats will require sedation before they can be handled.
Procedural sedation
Procedural sedation uses agents such as tranquilizers, sedatives and analgesics (Table 7), often with the addition of local anesthetics, so that some medical procedures can be performed without loss of consciousness; examples include bandage changes and diagnostics such as radiography and ultrasound examination. Benefits of procedural sedation include less physical restraint, decreased stress, simpler airway management, avoidance of inhalants and rapid recovery.
Table 7.
Drugs used for procedural sedation
| Sedative | Dose (mg/kg) | Route(s)* | Comments | References |
|---|---|---|---|---|
| Opioids | ||||
| Butorphanol † | 0.2–0.5 | IM, IV | ||
| Methadone | 0.05–0.6 | IM, IV, OTM | ||
| Morphine | 0.05–0.3 | IM, IV | Not recommended for use as a sole agent | |
| Hydromorphone | 0.05–0.1 | IM, IV | Higher doses are more likely to produce dysphoria | |
| Buprenorphine | 0.005–0.04 | IM, IV, OTM | ||
| Alpha2-adrenergic agonists | Emesis is common with administration of all alpha(α&x41;2- adrenergic agonists | |||
| Dexmedetomidine | 0.0025–0.04 | IM, IV | Preferred over other α2-adrenergic agonists if available | |
| Medetomidine | 0.01–0.04 | IM, IV | ||
| Xylazine | 0.1–1 | IM, IV | ||
| Others | ||||
| Tiletamine/ zolazepam | 2–3 | IM, IV, SC | IV will produce profound sedation/anesthesia; IM is preferred. Better sedation achieved in combination with opioid ± α2-adrenergic agonist | 60 |
| Alfaxalone | 2–3 | IM, IV, SC | IV doses as low as 0.5 mg/kg may provide significant sedation | |
| Combination examples ‡ | ||||
|
Opioid
+ dexmedetomidine |
0.0025–0.005 | IM, IV | 61 | |
|
Opioid
+ alfaxalone |
2–3 | IM, IV | ||
|
Opioid
+ dexmedetomidine + ketamine |
0.0025–0.005
2–3 |
IM, IV | If the opioid and the dexmedetomidine are reversed there will be minimal residual effect with the low dose of ketamine | 62 |
|
Opioid
+ acepromazine |
0.05–0.1 | IM, IV | ||
|
Opioid
+ dexmedetomidine + midazolam |
0.0025–0.005
0.05–0.2 |
IM, IV | Each of these drugs is reversible if it is necessary to remove the sedative effects | 63 |
|
Opioid
+ dexmedetomidine + alfaxalone |
0.0025–0.005
1–2 |
IM, IV | The high end of the dose range may anesthetize the cat | 64 |
|
Alfaxalone
+ dexmedetomidine |
1–2
0.0025–0.005 |
IM, IV | ||
Note: subcutaneous administration results in unpredictable uptake and effect, and some opioids given by this route produce more nausea and vomiting than when given by the IV or IM route
Generally provides better quality of sedation than other opioids when used as a sole agent
This is not a complete list of all possible combinations, but rather a series of examples of commonly used drug combinations. Doses and individual drugs will need to be tailored to the patient’s and clinician’s needs
IM = intramuscular; IV = intravenous; OTM = oral transmucosal – under the tongue or buccally (place in the cheek pouch); SC = subcutaneous
Two approaches are recommended:
✜ Use sedative drugs such as dexmedetomidine with the expectation of a ceiling effect; consider whether this is sufficient for the required procedure.
✜ Incorporate an anesthetic (eg, ketamine, alfaxalone, tiletamine/zolazepam) into the mixture to provide good sedation at a low dose but which will anesthetize the animal at higher doses if required.
Opioids, benzodiazepines and α2-adrenergic agonists are reversible, allowing rapid return to an awake state.63,65
Cats should always be provided a place to hide, as this will often alleviate fear, anxiety and frustration.66–68 Cages can be furnished with cardboard boxes, high-sided cat beds or ‘igloo’ beds; the cat can then be removed from the cage while still in its hiding place and injected.
Other techniques for injecting cats that are difficult to handle include transferring the cat from its carrier or cage to a humane trap (Figure 8), or using a squeeze cage (Figure 9) for iM injection; alternatively, if the cat is in a soft-sided mesh carrier, it is often possible to inject through this.
Figure 8.
A cat can be transferred from a carrier to a humane trap for IM injection. (a) The door of the carrier has been removed and the carrier placed next to the humane trap. When the cat moves from one to the other, the acrylic door is slid into place. For injection, an injection guard (pictured, with holes and a handle, in [b]) is used to squeeze the cat against the back of the cage, and the IM injection can be made through one of the holes. Courtesy of Sheilah Robertson
Figure 9.

When ready to inject, one person pulls the handles (red) towards themselves so the cat cannot turn around and is gently held in place; another person can then inject the cat IM. Squeeze cages are available with both top and end doors. Courtesy of Sheilah Robertson
Premedication
Drug dosages used for premedication should be tailored to the individual cat. opioids are useful for premedication and as part of procedural sedation (Table 8). Morphine and buprenorphine undergo hepatic metabolism with glucuronidation, so the duration of action of these tends to be longer because of the lack of functional glucuronyl transferase in cats.69,70 At the low doses of opioids generally used for premedication, the heart rate may decrease slightly. Anticholinergics can be used to reverse this effect if deemed necessary. Atropine (0.02 mg/kg) and glycopyrrolate (0.01 mg/kg) SC may increase heart rate by about 10% in combination with an opioid (P Pascoe, unpublished observations).
Table 8.
Opioids used for premedication
| Drug | Dose | Route(s) | Comments |
|---|---|---|---|
| Buprenorphine | 0.01–0.02 mg/kg | IM, IV | May antagonize the effects of other mu-opioid agonists
Longer duration of action (4–6 h) |
| Butorphanol | 0.1–0.4 mg/kg | IM, IV | May antagonize the effects of other mu-opioid agonists
Moderate to good sedation, short duration (60–90 mins) |
| Meperidine | 2–5 mg/kg | IM | Short duration (60–90 mins) with minimal sedation
Not to be given IV |
| Fentanyl | 2–5 µg/kg | IV | Short duration with minimal sedation |
| Hydromorphone | 0.02–0.1 mg/kg | IM, IV | Moderate sedation with duration of 2–4 h |
| Methadone | 0.2–0.5 mg/kg | IM, IV | Moderate sedation with duration of 2–4 h |
| Morphine | 0.1–0.3 mg/kg | IM, IV | Moderate sedation with duration of 2–4 h |
| Oxymorphone* | 0.02–0.1 mg/kg | IM, IV | Moderate sedation with duration of 2–4 h |
Note: Opioids may need to be given more or less frequently and at higher or lower doses than listed above based on pain assessment
Currently unavailable and future availability is unknown. Note: at the time of press the availability of several opioids is variable in the United States and future shortages are predicted
IM = intramuscular; IV = intravenous

Acepromazine has an inconsistent effect in cats and can make intraoperative hypotension more difficult to treat. 71 it is, however, anesthetic-sparing and is still widely used, often combined with other drugs such as opioids. Recommended doses range from 0.01–0.05 mg/kg (SC or IM); time to peak sedation may be up to 20–30 mins and the effects last 4–6 h. Acepromazine should not be used in the face of pre-existing hypotension, hypovolemia or dehydration.
The benzodiazepines do not appear to be suitable drugs for premedication alone or in combination with opioids in young or adult healthy cats due to unreliable outcomes including excitation and dysphoria.19,72 in geriatric or very sick patients, the benzodiazepines may produce more reliable sedation.
α2-adrenergic agonists have been widely used in cats. Currently the most commonly used agents in this class are medetomidine and dexmedetomidine. These drugs provide reliable dose-dependent sedation. Cardiovascular effects include vasoconstriction, decreased heart rate (by 40%) and cardiac output (by 60%) and increased systemic vascular resistance (300%) with minimal change in blood pressure. 73 Following high doses, cats are profoundly sedated and have pale to blue mucous membranes but breathe well, maintain normal oxygenation and are eucapnic. 73 If α -adrenergic agonists are used for premedication, the decreased cardiac output may slow the onset of subsequent intravenous induction drugs. Anticholinergics should not be used with α2-adrenergic agonists because of the significant hypertension that may ensue. 74 Many cats vomit following administration of dexmedetomidine, a side effect that occurs more often with higher doses. The combination of dexmedeto-midine with ondansetron or butorphanol reduces the incidence of vomiting, as does pretreatment with maropitant.75–79 The addition of other classes or types of sedatives (Table 7) allows the use of lower doses of α2-adrenergic agonists. 19
Induction
Induction of anesthesia may be achieved by SC, IM or IV administration of injectable drugs (Table 9) or by chamber or mask induction with inhalant anesthetics. The SC and IM routes are very useful for cats that are difficult to restrain for IV catheter placement. Sometimes a drug is administered at doses that chemically restrain the animal for catheter placement and then the same drug is titrated iV to achieve unconsciousness.
Table 9.
Induction drugs
| Drugs | Dose (mg/kg) | Route(s) | Comments |
|---|---|---|---|
|
Ketamine
+ midazolam |
2–5
0.2–0.3 |
IV, SC, IM | *Stings on injection SC or IM: expect the cat to react! |
| Tiletamine/zolazepam | 2–5 | IV, SC, IM | *Stings on injection SC or IM: expect the cat to react! |
| Thiopental† | 5–10 | Only IV | Co-induction with midazolam will reduce the dose needed |
| Propofol | 4–8 | Only IV | Co-induction with midazolam or ketamine will reduce the dose needed. Propofol containing 2% benzyl alcohol (PropoFlo 28 [Abbott Laboratories], PropoFlo Plus [Zoetis UK]) may be used for induction of anesthesia in cats |
| Alfaxalone | 1–4 | IV, IM | Co-induction with midazolam will reduce the dose needed. For IM injection the volume of drug may need to be split between two sites |
| Etomidate | 1.5–4 | IV | High osmolality, contains propylene glycol, irritant to veins – dilute or inject with fluids running |
Note: Doses may be significantly lower when premedicant agents have been given
For drugs that are known to sting due to their pH, use a small gauge needle and, if possible, inject slowly
Currently unavailable in the USA
IV = intravenous; SC = subcutaneous; IM = intramuscular
All induction drugs have the potential to cause significant respiratory depression, so preoxygenation should be standard practice (see later section on airway management). With most induction drugs, there is a possibility of excitation with low doses, so the initial bolus should be large enough to avoid this effect but small enough to minimize adverse cardiopulmonary events. However, titration to effect is important; after the initial dose, the cat should be observed, and the drug given time to take effect. if subsequent doses are required to reach the desired endpoint, they are given in small increments.
Chamber inductions
Chamber inductions should never be routine but rather a ‘last resort’ and only when other approaches have failed. Transferring the cat to an anesthetic induction chamber (many cats can be transferred by placing the open end of the carrier over the top of the chamber) allows the administration of an inhalant anesthetic without having to touch the cat.
Chamber induction in unpremedicated, agitated cats is the least desirable technique described in these Guidelines, since an agitated cat will require more inhalant anesthetic to achieve the desired endpoint. This increased inhalant anesthetic requirement results in severe depression of the cardiovascular system. Additionally, there is an increased release of catecholamines that predispose the cat to the development of cardiac arrhythmias.
If chamber inductions are performed, the cat must be visible within the chamber; for example, use a commercially available impact-resistant plastic (eg, polycarbonate) or acrylic box with appropriate inflow and outflow openings and leak-proof seals. The cat must be observed at all times while in the chamber. Covering a cat carrier with a plastic bag to create a ‘chamber’ is not appropriate for the safety of the cat or personnel.
If time permits, spray the chamber with feline facial pheromone (Feliway; Ceva Animal Health) 15–20 mins prior to use, or place a pretreated towel in the chamber. if a chamber induction is performed, the oxygen flow rate should be high and the vaporizer should be turned to the maximum setting. The cat should be removed from the chamber as soon as the righting reflex has been lost (tested by rolling the chamber from side to side), and a tight-fitting face mask applied with the oxygen flow and vaporizer settings decreased. A disadvantage of this technique is the unavoidable release of anesthetic gases and exposure of personnel when the cat is removed from the chamber.
Maintenance
If an injectable anesthetic does not provide enough time to complete the procedure, anesthesia is most commonly maintained with an inhalant agent. The dose for inhalant anesthetics is higher in cats than in many other species. 80 isoflurane and sevoflurane have profound effects on the cardiovascular system, with cardiac output and blood pressure decreasing by 50–70% at concentrations required for surgery. Since this effect is partially reversed by noxious stimulation, it may not be apparent in a cat that is anesthetized and undergoes surgery within a few minutes of induction. However, in cats where there is minimal stimulation during non-invasive procedures (eg, clipping and skin preparation), the blood pressure should be monitored, and the vaporizer setting decreased if the cat becomes hypotensive. Settings as low as 0.5% isoflurane or 1% sevoflurane may be enough to maintain anesthesia and blood pressure during this phase.
Opioid CRIs allow a reduction in the inhalant concentration and minimally affect the cardiovascular system (Table 10). intraoperative opioids allow for a decreased inhalant concentration (up to 30%), while also decreasing changes in blood pressure and heart rate associated with the procedure. Ketamine also decreases anesthetic requirements. 81 Both lidocaine and dexmedetomidine can reduce the minimum alveolar concentration (MAC) but show no cardiovascular benefit when used as anesthetic adjuncts.82,83
Table 10.
Adjunct agents used to decrease inhalant requirements
| Drug | IV loading dose (µg/kg) | Constant rate infusion (CRI) (µg/kg/min) | Comments |
|---|---|---|---|
| Fentanyl | 3–5 | 0.1–0.4 | Plasma concentrations may increase over time |
| Alfentanil | 10–15 | 0.5–0.8 | Rapid onset/offset |
| Remifentanil | Not needed | 0.1–0.4 | Very short half-life and may lead to increased postoperative pain.
Ensure adequate opioid dose at end of the procedure |
| Sufentanil | 0.3–0.5 | 0.01–0.04 | Very potent but little accumulation over time |
| Morphine | 100–200 | 1–3 | Very slow changes of concentration in the brain |
| Ketamine | 500–1000 | 10–40 | The high infusion rate may decrease MAC by ~60% |
MAC = mean alveolar concentration; IV = intravenous
Propofol and alfaxalone have been used for total intravenous anesthesia. Since propofol is conjugated in the liver to glucuronide, the recovery time will vary depending upon the dose and duration of the infusion. if the propofol dosage is kept low (0.1–0.15 mg/kg/min), the initial part of the recovery (time to extubation) is not affected. With longer infusion times full recovery (walking without ataxia) is delayed. 84 if higher doses are used, the cat may be anesthetized for several hours after the termination of the infusion. it is recommended to limit propofol infusions to <30 mins. While propofol containing 2% benzyl alcohol is safe and appropriate for induction of anesthesia in cats, this formula should not be used as an infusion due to the potential for accumulation of benzyl alcohol. 85 Recoveries following maintenance of anesthesia with alfaxalone infusions tend to be prolonged (1–2 h to standing) and occasionally the hyperesthesia associated with this drug can lead to a difficult recovery with side effects such as myoclonus, opisthotonus and vocalization. 86
Airway management
Proper airway management is essential for safe anesthesia in cats. General anesthesia is associated with a loss of protective airway reflexes and respiratory depression. Thus, it is important to maintain a patent airway (ie, open and clear), prevent aspiration, and be able to deliver oxygen and inhalant anesthetics and support ventilation when required. Fluids and solid material pose aspiration risks and include gastrointestinal contents, mucus, saliva, blood, flushing fluids and debris from dental and other oral procedures.
Published data highlight airway-related problems as being a significant cause of anesthetic-related death and morbidity in cats.2,87 Most anesthetic deaths occur soon after anesthesia ends (within the first 3 h) and are commonly caused by airway obstruction. 2 The cat’s airway is small and delicate, and the larynx is susceptible to spasm when stimulated and therefore easily damaged. Tracheal tears are also reported in cats, with outcomes ranging from full recovery with medical or surgical intervention to death or euthanasia.88,89 Use of a cuffed ETT is currently the most common technique for airway management. The appropriate techniques discussed in this section are necessary for atraumatic intubation.
Necessary equipment, materials and methods
✜ A laryngoscope to facilitate visualization and atraumatic intubation.
✜ An ETT of appropriate diameter. Most adult cats will require a 3.5–5.0 mm (internal) diameter but a range from 2.0–5.5 mm should be available.
✜ The ETT should reach from the incisors to the tip of the shoulder (thoracic inlet) and be premeasured in length (Figure 10). The ETT can be cut to the correct length to minimize equipment dead space. if the ETT is pushed beyond the thoracic inlet, endobronchial intubation is possible. intrathoracic tracheal tears have a poor prognosis (Figure 11).
Figure 10.
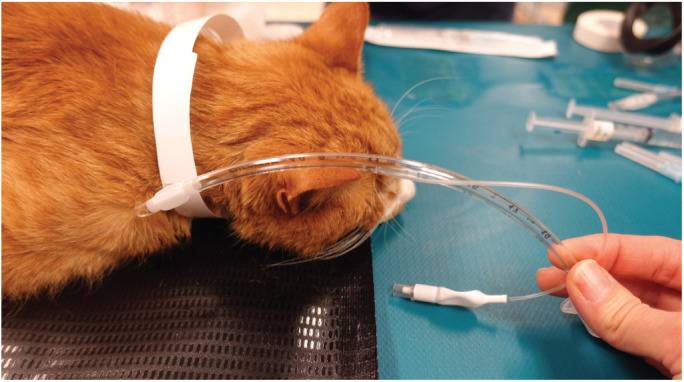
The ETT is measured before placement; the tip should lie at the point of the shoulder when inserted. Mark (eg, with tape) or note the number on the tube that lines up with the incisors, and when intubating stop at this point. The tube can be cut to shorten it. Courtesy of Sheilah Robertson
Figure 11.
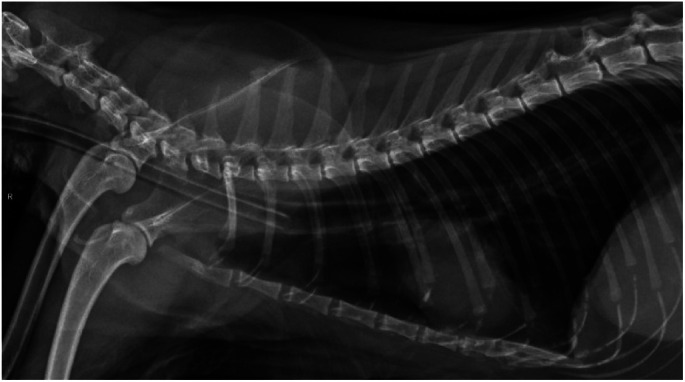
The tip of the ETT should lie near the point of the shoulder. Proper placement can be assured by measuring the tube, as described in Figure 10. Tracheal tears can occur in cats when the cuff is overinflated and carry a poorer prognosis if they are intrathoracic (for example at the location of the ETT cuff shown in this radiograph). Courtesy of Sheilah Robertson
✜ Sterile water-soluble lubricant. This can enhance the airway seal at a given pressure within the cuff. Lubricate the cuff only (avoid the Murphy eye) immediately prior to starting preoxygenation or iV induction (see below).
✜ 2% lidocaine (without epinephrine/adrenaline), 0.2 ml in a 1 ml syringe (without a needle or catheter, since these can separate from the syringe and enter the airway), for topical application. Care should be taken with products that use a nozzle for delivery as high expulsion pressures may damage delicate laryngeal mucosa. in addition, the nozzle must be cleaned between each patient.
✜ Cotton-tipped applicators to clear mucus and saliva.
Note that stylettes are rarely needed for routine endotracheal intubation but can be used to stiffen or alter the shape of the ETT. If stylettes are used, the tip should be blunt and remain within the lumen of the endotracheal tube.
Technique for appropriate endotracheal tube placement
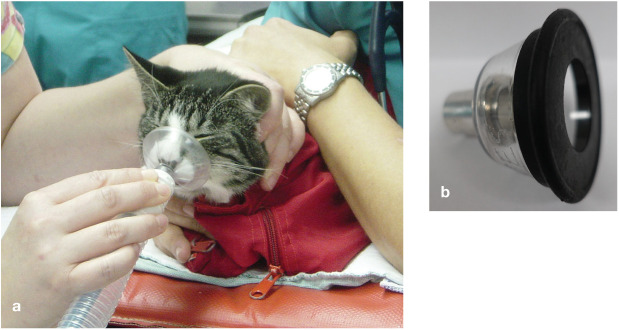
✜ If the cat is tolerant, preoxygenate for 3 mins using a face mask (Figure 12a) or flow-by technique (Figure 12b).
✜ Administer the IV induction drug.
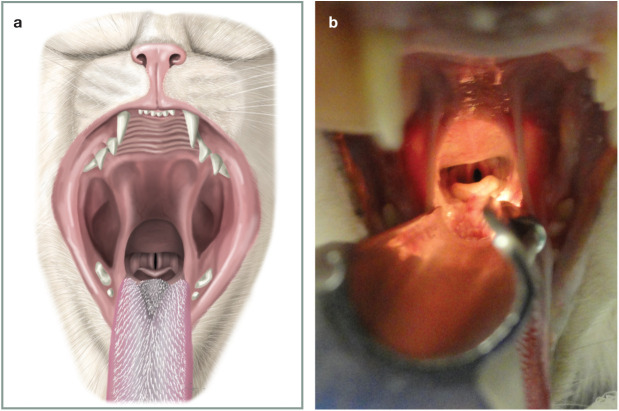
✜ Position the cat in sternal recumbency and check for general muscle relaxation before checking jaw tone.
✜ identify the arytenoid cartilages and vocal folds (Figure 13). Using a 1 ml syringe drop ⩽0.2 ml of lidocaine onto the arytenoids and wait 60–90 s (Figure 14). Be extremely careful that the tip of the syringe does not come into contact with the arytenoid cartilages and vocal folds. Continue or start giving oxygen as described in the first step of these instructions.
Figure 12.
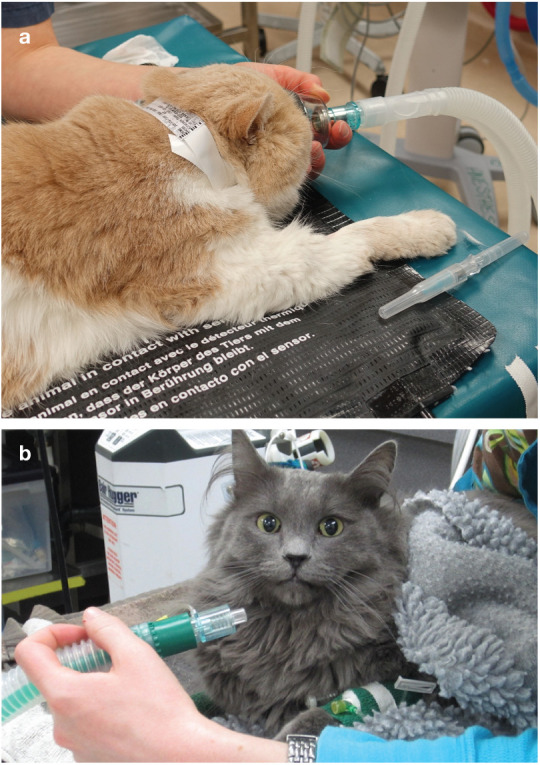
(a) Preoxygenation for 3 mins using a face mask is recommended if tolerated by the cat. (b) If the cat does not tolerate a face mask, a flow-by technique can be used. Courtesy of Sheilah Robertson (a) and Heidi Shafford (b)
Figure 13.
(a) View of cat’s larynx with the mouth wide open and the cat in sternal recumbency. Under normal circumstances the ventral surface of the epiglottis would be dorsal to the soft palate, but opening the mouth and pulling the tongue produces this view. The tip of the laryngoscope should be placed just rostral to the epiglottis and pressed down to bring the opening of the larynx into view. (b) The same view as Figure 13a in a clinical patient. Courtesy of Chrisoula A Toupadakis Skouritakis (a) and Sheilah Robertson (b)
Figure 14.
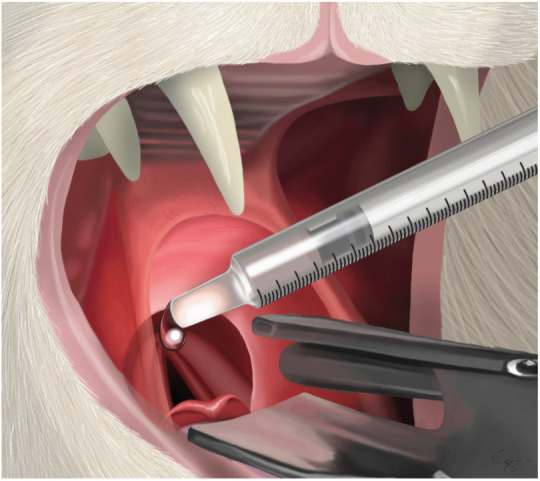
A 1 ml syringe being advanced over the top of the laryngeal opening and 0.2 ml of 2% lidocaine being dropped on to the top of the arytenoids. This will take 60–90 s to work so, once the lidocaine has been applied, the cat should be put back on oxygen until this time has elapsed. Courtesy of Chrisoula A Toupadakis Skouritakis
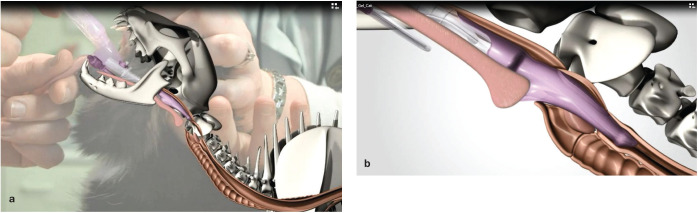
✜ Keep the tip of the ETT close to the glottal opening, with the most distal part of the tip vertical, and advance the tube gently on inspiration (Figure 15).
✜ If the patient coughs or resists, stop and administer additional induction agent.
✜ If laryngospasm develops, stop and administer oxygen. Assess depth of anesthesia and administer additional induction agent if required. Reapplying lidocaine can be considered, ensuring 60–90 s elapse before reattempting intubation. Laryngospasm is usually self-limiting.
✜ After intubation, observe for the presence of arytenoid cartilages on either side of the ETT (confirmation of correct placement). if these cannot be seen on either side, the tube is not in the trachea.
✜ After intubation, secure the ETT (eg, wrap gauze or plastic tubing around the tube and tie behind the ears). Attach the patient to the breathing circuit with oxygen flowing, check for a heartbeat and assess mucous membrane color.
✜ Inflate the cuff using 0.5 ml increments of air from a 3 ml syringe until no leak can be heard when the reservoir (rebreathing) bag is squeezed and the pressure in the breathing circuit is 16–18 cmH2o. ETT cuff pressure measurement devices are also available (eg, Tru-Cuff inflation syringe, http://tru-cuff.com) that will decrease the time taken for this step.
✜ Look for condensation forming and disappearing on the inside of the ETT with each breath along with movement of the reservoir bag. if a capnograph is available, confirm intubation by observing for an appropriate Co2 reading or waveform on expiration. Palpation of the neck should reveal one firm tube (the trachea); if two are felt, the ETT is in the esophagus.
Figure 15.
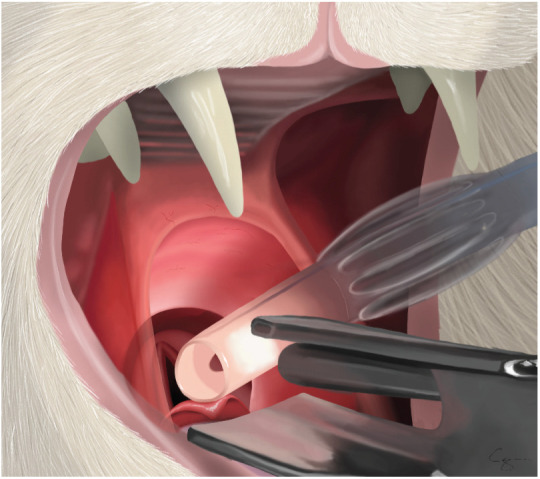
ETT being advanced into the larynx. Note that the bevel is vertical so that the end of the tube can act like a wedge to open up the vocal folds. The tube is advanced to the laryngeal opening and kept as ventral as possible. Advancing the tube on inspiration, when the laryngeal opening is at its widest, will provide the best conditions for ETT placement. Courtesy of Chrisoula A Toupadakis Skouritakis
Alternative airway options
Because of the challenges and risks associated with cuffed ETTs, the ‘pros and cons’ of these and alternatives techniques should be considered, as outlined in Table 11.
Table 11.
Airway management options
| Airway options | Advantages | Disadvantages |
|---|---|---|
|
Endotracheal tube (ETT) cuffed
Two types of cuff are available: Low-volume high-pressure High-volume low-pressure |
Provides a secure airway
No leakage of oxygen or inhalant anesthetic agents when the cuff is correctly inflated Prevents aspiration < Allows mechanical ventilation Inexpensive Placement can be confirmed by visualization of the ETT between the arytenoids |
Requires skill to place atraumatically
Requires a deeper plane of anesthesia for placement than a supraglottic airway device (SGAD) Traumatic intubation leads to laryngeal damage Overinflation of the cuff can lead to tracheal damage, such as necrosis or tearing. This is a greater risk with high-volume cuffs because of the greater dimensions of the inflated cuff May result in pharyngeal/laryngeal discomfort or stridor |
| ETT uncuffed | Provides a patent airway
Reduced risk of tracheal tears Placement can be confirmed by visualization of the ETT between the arytenoids |
Does not protect from aspiration
Inhalant anesthetic agents and oxygen can leak around the outside of the tube* Leaks occur during manual or mechanical ventilation* May provide inadequate tidal volume during assisted ventilation |
|
Supraglottic airway device (SGAD)
(v-gel; Docsinnovent) |
Can be placed with cat in a more superficial plane of <
anesthesia than an ETT < Can be placed faster than an ETT and with fewer attempts < Less pharyngeal/laryngeal discomfort or stridor after removal < Higher food intake after use compared with an ETT Mechanical ventilation possible < Does not enter the trachea; therefore, avoids tracheal irritation May be better tolerated for repeated anesthesia (eg, successive days) |
May take up too much room for oral procedures Easily dislodged with changes in patient position Correct placement cannot be confirmed by visualization
Mechanical ventilation possible, but has only been tested up to 16 cmH2O Higher initial cost but designed as a multi-use device |
|
Face mask
Masks that conform to the cat’s face (Figure 16a) and with removable rubber seals (Figure 16b) are available |
Ideal for preoxygenation <
Can be used to administer oxygen and inhalant < anesthetic agents during anesthesia < Can be used to provide oxygen during procedural < sedation Can be used in an emergency to provide assisted < ventilation Suitable for providing oxygen during short procedures not requiring additional inhalant agent (eg, castration under injectable anesthesia) |
Does not protect the airway from aspiration Airway obstruction can occur with neck flexion Leakage of oxygen and inhalant anesthetic agents* Assisted or mechanical ventilation will result in gastric distension
If too large, rebreathing occurs |
| Nothing | No cost <
< < |
No means of protecting the airway
No ability to provide oxygen or inhalant anesthetics No ability to support ventilation |
Note: The ability to monitor end-tidal CO2 allows verification that ETTs and SGADs are correctly placed
Waste anesthetic gases pose a potential hazard to personnel
Figure 16.
(a) A soft ‘all-in-one’ mask that conforms to the cat’s face (a) and a mask with a removable rubber seal (b). Both are available in several sizes. Courtesy of Sheilah Robertson
Considerations for airway management
The choice of technique for airway management depends on several factors including length of the procedure, patient position, type of anesthetic and procedure, obesity, comorbidities, facial conformation (eg, brachycephalic breeds) and frequency of anesthesia (eg, daily anesthesia for radiation therapy).
A feline-specific SGAD (v-gel; Docsinnovent) is a suitable alternative to an ETT (Figure 17). Studies show that the time to obtain a clinically acceptable capnograph reading was shorter when an SGAD was used compared with an ETT, fewer attempts were needed, and less propofol was required.90,91 The device is suitable for spontaneous ventilation and for controlled mechanical ventilation up to 16 cmH2O 91 . Less airway discomfort and stridor and greater food intake were documented after use of an SGAD compared with an ETT; 90 this may be because the device does not enter the trachea (Figure 18).
Figure 17.
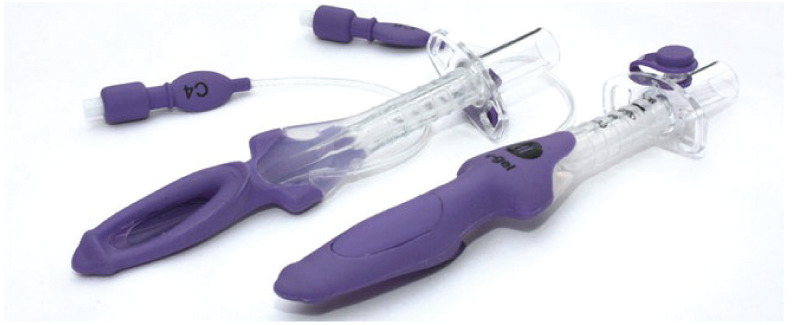
A supraglottic airway device (v-gel) specifically designed for the cat’s pharyngeal and laryngeal anatomy. Courtesy of Docsinnovent, UK
Figure 18.
The tip of the supraglottic airway device is lodged in the esophagus and the opening lies over the laryngeal opening; (a) overall view, (b) close-up view. Courtesy of Docsinnovent, UK
As with ETTs, a capnograph is valuable for verifying placement and monitoring during a procedure.
Perioperative management
Goals of the preanesthetic period are to minimize patient stress, prepare an individualized anesthesia and analgesia plan, anticipate potential complications and assemble monitoring and support equipment.
Checklists
Checklists aid in consistent preparation and improve patient safety. For example, in humans, the use of checklists for specific procedures and scenarios has been shown to significantly decrease morbidity and mortality. 92 Veterinary checklists are now available and are highly recommended. 93
Veterinary anesthesia checklists are available at: http://ava.eu.com/resources/checklists/ .
IV catheter placement and fluid therapy
In almost all situations, placement of an IV catheter is optimal prior to anesthesia as it allows for administration of emergency medication, additional anesthetic and analgesic delivery, and fluid administration. Patients undergoing very short procedures do not necessarily require fluids, yet still benefit from an IV catheter. To minimize patient stress, perform IV catheterization after administration of a sedative alone or in combination with an anxiolytic.
Fluid therapy considerations in cats include:
✜ A blood volume of 50–60 ml/kg (vs 80–90 ml/kg in a dog).
✜ The potential for occult cardiac disease.
✜ The difficulties of accurate fluid delivery (a challenge in all small patients).
The recommended intraoperative fluid maintenance rate is 3 ml/kg/h of a balanced crystalloid solution in healthy adult cats undergoing routine procedures. 47

Monitoring during anesthesia
Cats are sensitive to the cardiovascular and respiratory depressant effects of inhalant agents; significant decreases in mean arterial pressures have been reported with isoflurane at <1 MAC). 94 Clinical research confirms that adverse events related to anesthesia can be mitigated by monitoring. Brodbelt and colleagues reported that pulse and pulse oximetry monitoring was associated with a lower mortality rate and thus should be performed routinely. 2 Monitoring respiratory function and body temperature are also important to enable early intervention, and there is evidence that having a dedicated anesthetist decreases mortality in veterinary patients. 1 In addition, the importance of frequent intraoperative monitoring and documentation of trends cannot be overemphasized when it comes to patient safety. Effective team communication is paramount.
Critical components of monitoring include:
✜ Physical observation of the patient’s clinical condition;
✜ Circulation;
✜ oxygenation;
✜ Ventilation;
✜ Body temperature.
Patient’s physical condition
Monitoring includes physical observation of the patient and their anesthetic depth while focusing on specific physiologic variables using one’s eyes, ears and hands to provide physical assessment and verification of:
✜ Heart rate and rhythm;
✜ Respiratory rate;
✜ Presence of a pulse;
✜ Mucous membrane color;
✜ Jaw tone;
✜ Palpebral reflexes;
✜ Patient movement;
✜ Response to surgical stimulation.
Monitoring equipment is an extension of the physical senses but is not a substitute for physical assessment of the patient by the anesthetist.

Emergency drug calculations
All patients undergoing anesthesia are at risk of complications. An emergency drug sheet should be calculated for each patient undergoing anesthesia that includes, as a minimum, patient-specific emergency doses and volumes of atropine, epinephrine/ adrenaline, lidocaine, glycopyrrolate, atipamezole and naloxone, and crystalloid and colloid fluid boluses for treatment of anesthetic hypotension (see Table 12).
Table 12.
Emergency anesthetic drugs and dose calculations
| Medication | Feline-specific anesthetic emergency doses* |
|---|---|
| Atropine | 0.04 mg/kg |
| Epinephrine (adrenaline) | 0.01 mg/kg, initial low dose |
| Vasopressin | 0.4–0.8 U/kg |
| Lidocaine | 0.25 mg/kg |
| Glycopyrrolate | 0.005–0.01 mg/kg |
| Atipamezole | 50–100 μg/kg, or atipamezole volume equal to 25–50% of administered dexmedetomidine volume (if a concentration of 500 μg/ml was used) or 5–10% of administered dexmedetomidine volume (if a concentration of 100 μg/ml was used) |
| Naloxone | 0.05 mg/kg |
| Crystalloid fluid bolus | 5–15 ml/kg |
| Colloid fluid bolus | 1–5 ml/kg |
|
Calcium gluconate 10%
Calcium chloride 10% |
0.5–1.5 ml/kg
0.2–0.5 ml/kg |
All drugs are given by the intravenous route
Circulation
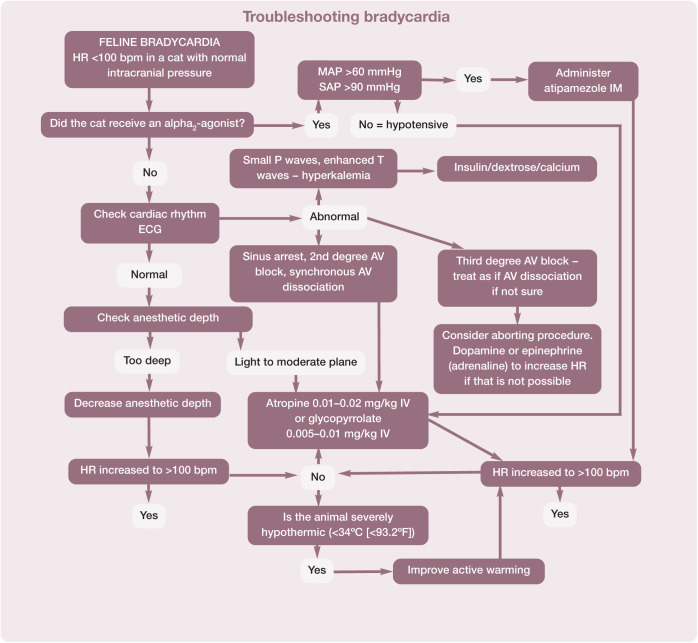
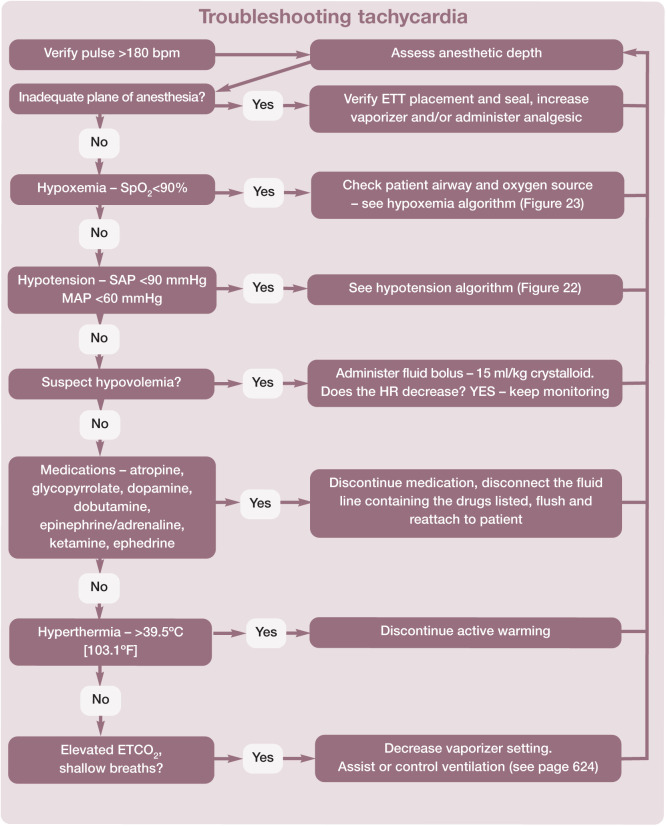
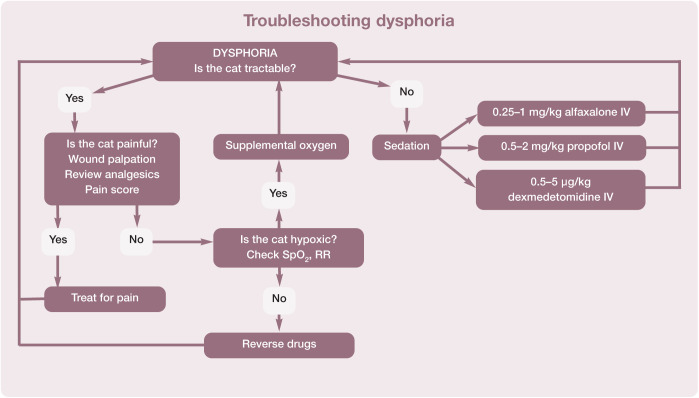
Pulse, heart rate and rhythm, and blood pressure are the basis for assessing the circulatory system. The attributes of different modalities for monitoring heart or pulse rate are shown in Table 13. The normal heart rate in anesthetized cats is 100–180 bpm. Algorithms are helpful to aid troubleshooting and for recommending actions to take if the heart rate falls outside these normal limits (Figures 19 and 20).
Table 13.
Methods for monitoring heart rate during anesthesia
| Modality | Reliability in cats | Comments |
|---|---|---|
| Manual palpation | ++ | Small patient size makes palpation challenging |
| Auscultation with external stethoscope | +++ | Placement of an external stethoscope can be challenging when cat is covered by surgical drapes |
| Auscultation with esophageal stethoscope | ++++ | Tip of esophageal stethoscope is positioned directly over the heart; easy access throughout surgery |
| Doppler ultrasonography | +++ | Audible signal of pulse rate and rhythm |
| Pulse oximeter | ++ | When signal quality is good, pulse rate is accurate; many factors decrease signal quality (see later section on oxygenation) |
| ECG | + | Small patient size often associated with poor ECG signal; ECG rate (electrical activity) is not the same as heart rate (physical contraction/pulse) |
+ = Poor reliability; ++++ = very reliable; ECG = electrocardiography
Figure 19.
Recommended procedure for troubleshooting bradycardia in cats during anesthesia. An HR of <100 bpm may be associated with a decrease in cardiac output even in the face of normal blood pressure. Note that bradycardia may be accompanied by hypotension. The two algorithms (Figures 19 and 22) may need to be used together to achieve the best outcome, but bradycardia should usually be treated first. This algorithm may be downloaded; for details and key to abbreviations, see box on page 628
Figure 20.
Recommended procedure for troubleshooting tachycardia (HR >180 bpm) in cats during anesthesia. This algorithm may be downloaded; for details and key to abbreviations, see box on page 628
Blood pressure also provides information about cardiovascular function. Hypotension is a common anesthetic complication, even in healthy cats undergoing short procedures. The Panel recommends monitoring and documenting trends in blood pressure for all cats undergoing general anesthesia. For Doppler and oscillometric blood pressure monitoring, accuracy is related to cuff size and placement; the ideal cuff width should be approximately 40% of limb or tail circumference at the site of cuff placement (Figure 21). With the Doppler technique, operator error (eg, deflating the sphygmomanometer too quickly) and lack of experience can result in erroneous readings. options for monitoring blood pressure in cats are compared in Table 14.
Figure 21.
Using the correct size of blood pressure cuff is important for accuracy. The cuff width should be 40% of the limb circumference, as shown in (a). The cuff is then placed around the most cylindrical portion of the limb (b). Courtesy of Sheilah Robertson
Table 14.
Methods for monitoring blood pressure
| Modality | Reliability in cats | Comments |
|---|---|---|
| Manual palpation | − | Presence of a pulse does not confer any objective information about blood pressure. Pulse pressure is the difference between SAP and DAP; pulse pressure will be the same if SAP = 120, DAP = 80 (normotensive) and SAP = 70, DAP = 30 (hypotensive) |
| Doppler ultrasonography | +++ | Doppler measures systolic blood pressure and provides an audible signal of pulse rate and rhythm; reliable in patients of all sizes |
| Oscillometry | ++ | Oscillometric monitors less reliable and less accurate in small patients |
| Direct arterial pressure | ++++ | Technically challenging but achievable with practice; rarely performed in routine cases but is used in critical care practice and more often with the increasing availability of multiparameter monitors for veterinary use |
− = Not reliable; + = poor reliability; ++++ = very reliable; SAP = systolic arterial pressure; DAP = diastolic arterial pressure
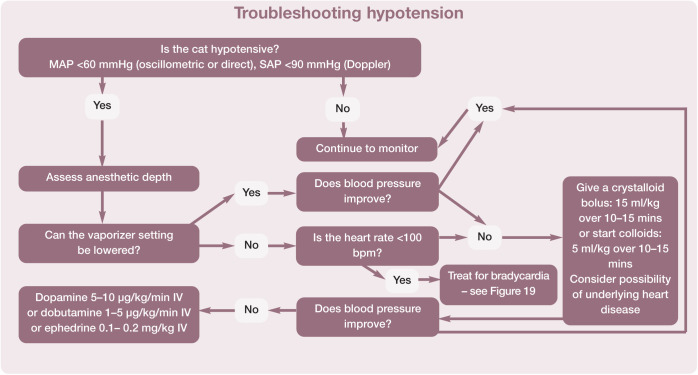
In the opinion of a number of anesthesiologists, hypotension was defined as a systolic arterial pressure (SAP) <87 mmHg and a mean arterial pressure (MAP) <62 mmHg. 95 For the purposes of these Guidelines the Panel suggests an SAP <90 mmHg and an MAP <60 mmHg. Figure 22 presents a practical guide for treatment of intraoperative hypotension. if the patient’s blood pressure is not improving with therapy and the procedure allows, consider discontinuing anesthesia and allowing the patient to recover. Also, consider consulting with an anesthesia expert or modifying the patient’s anesthesia plan for subsequent anesthesia.
Figure 22.
Recommended method for the treatment of hypotension during anesthesia. This algorithm may be downloaded; for details and key to abbreviations, see box on page 628
Arrhythmias in cats under anesthesia warrant further investigation and this is discussed in the later section on perioperative complications.
Oxygenation
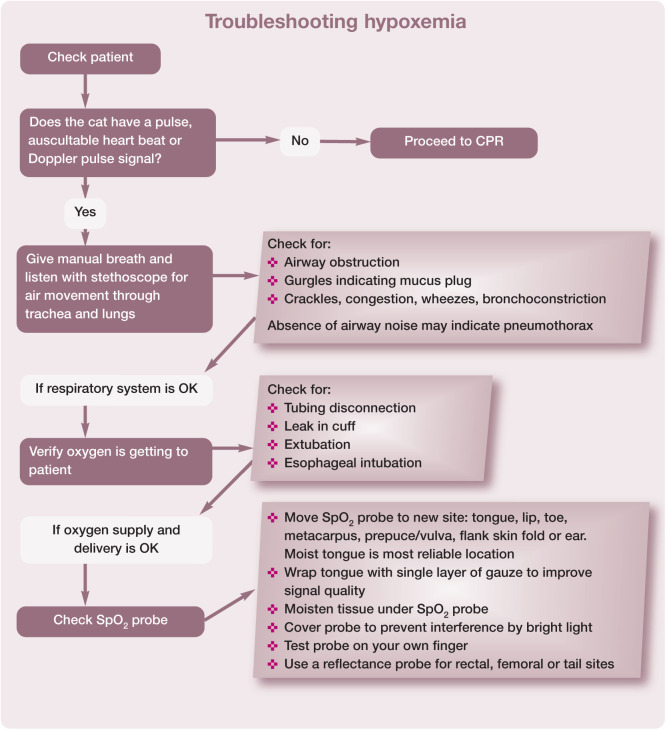
While many hospitals rely on pulse oximetry to determine oxygenation, it is important to understand the limitations of this approach. Patients breathing 100% oxygen are typically protected from hypoxemia, so when the pulse oximeter reading is <90%, the patient is in serious trouble. For this reason, the pulse oximeter is often considered a crisis monitor. The pulse oximeter can be a very finicky monitor (eg, affected by minor movement and fluorescent lighting) and, as such, its alarms are often ignored. it is recommended to use a pulse oximeter with a waveform display because, if the waveform is steady, this indicates a good signal quality; causes of a low reading then need to be evaluated quickly.
Figure 23 details the appropriate response to a low pulse oximeter reading and includes tips for troubleshooting abnormal readings.
Figure 23.
Recommended responses in the face of hypoxemia (SpO2 <90%). This algorithm may be downloaded; for details and key to abbreviations, see box on page 628
Ventilation
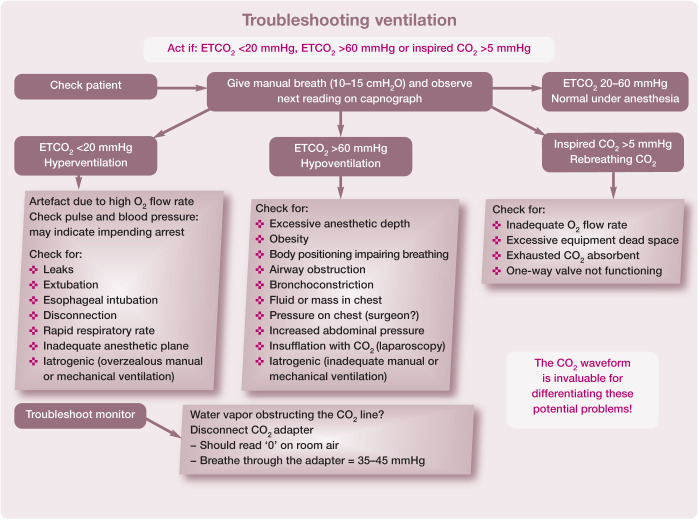
Although the gold standard for monitoring ventilation is measuring arterial blood gases, it can be difficult to obtain a sample and equipment for analysis is not available in many hospitals. Even though the respiratory rate is a poor indicator of respiratory function some assessment of the patient’s tidal volume can be made by looking at the reservoir bag; however, due to the small tidal volume of cats, this is challenging. A clinically valuable tool that evaluates respiratory and equipment function is capnography with a visual waveform equipped with a pediatric or low dead space capnograph adapter (Figure 4).
Hypoventilation and subsequent hypercapnia are common during anesthesia; 87 Figure 24 provides a guide to ascertaining the cause and how to resolve it. Table 15 summarizes modalities for monitoring the respiratory system in cats.
Figure 24.
Recommendations for responding to abnormal capnograph readings during anesthesia. This algorithm may be downloaded; for details and key to abbreviations, see box on page 628
Table 15.
Details on modalities and reliability for monitoring the respiratory system
| Modality | Reliability in cats | Comments |
|---|---|---|
| Respiratory rate | ± | Respiratory rate is not a good indicator of efficiency of air movement into and out of the lungs. Respiratory rate does not indicate that patient is inspiring or absorbing adequate oxygen |
| Mucous membrane color | − | Human eyes cannot detect meaningful changes in oxygenation until patient becomes cyanotic (SpO2 = ~70–80%) |
| Tidal volume | + | Difficult to determine breath volume by visually assessing reservoir bag or chest movement due to small patient size |
| Pulse oximeter without waveform | + | Without a waveform it is difficult to assess signal quality. Does not indicate patient is breathing |
| Pulse oximeter with waveform | ++ | A steady waveform is indicative of good signal quality; many factors affect signal quality including exposure to ambient light, tissue compression, drying of tissue (See Figure 23 for SpO2 troubleshooting tips). Does not indicate patient is breathing |
| Capnometer (no waveform) | + | Difficult to assess accuracy of monitor without waveform |
| Capnograph with waveform | +++ | Waveform analysis provides invaluable insight into respiratory function and equipment malfunction (eg, stuck unidirectional valves in a circle system) |
| Arterial blood gases | ++++ | Gold standard for evaluating ventilation. Obtaining sample can be difficult; requires specialized equipment. Rarely used in clinical practice |
− = Not reliable; + = poor reliability; ++++ = very reliable; SpO2 = oxygen saturation of hemoglobin in arterial blood
Body temperature
Patients can develop hypothermia following administration of preanesthetic medications. The consequences of hypothermia may include reduced drug clearance and metabolism. Procedural and environmental factors also promote heat loss, such as water in the mouth (eg, during dental procedures), an open body cavity, contact with cool surfaces and exposure to a cold environment. Changes in body temperature are discussed further in the later section on perioperative complications.
Anesthesia record
A completed anesthesia record documents vital signs and aids in identifying trends. The Panel recommends recording parameters at least every 15 mins, though greater frequency (ie, every 5 mins) allows better assessment of changes in patient status. The dose and volume of medications administered, placement of IV catheter (size and site), size of ETT (or other airway management device), and quality of sedation, induction and recovery are recorded. Fluid rate and total volume of fluid administered are also recorded. Graphic records display symbols representing vital signs over time and assist in evaluating patient trends. The American Animal Hospital Association has developed a concise and user-friendly anesthesia record for purchase (search for ‘AAHA Anesthesia and Sedation Record’ at www.aaha.org ).
The Association of Veterinary Anesthetists has several anesthetic record formats which are free to download at: http://ava.eu.com/resources/checklists/ .
Perioperative complications
Hypotension
Significant myocardial depression and decrease in cardiac output is common in cats when inhalant anesthetics are used in the absence of a noxious stimulus, resulting in hypotension (SAP <90 mmHg, MAP <60 mmHg). The first approach to treatment is to decrease the concentration of the inhalant, based on an evaluation of anesthetic depth (eg, jaw tone, heart rate, blood pressure). During patient preparation prior to surgical stimulation, this may mean reducing the isoflurane or sevoflurane vaporizer setting to 0.5% or 1%, respectively. These concentrations are usually insufficient for a surgical procedure and the need to increase the concentration at the start of surgery should be anticipated. if the blood pressure remains low, then a fluid bolus (balanced electrolyte solution) should be administered at 3–10 ml/kg, but up to 15 ml/kg may be required, over 5–15 mins. In cats with known cardiomyopathy or anuric renal failure a fluid bolus may be contraindicated. If anesthetic depth is deemed appropriate, provision of supplemental drugs should be considered so that the vaporizer setting can be reduced (Table 10).
With persistent hypotension a positive inotrope should be administered. A CRI of dopamine is recommended, starting at 5 µg/kg/min. 96 This should increase cardiac output and blood pressure. if the initial infusion rate is not effective the dose should be increased within 5 mins. Typically, the dosage is increased in 2.5 µg/kg/min increments, but smaller or larger changes may be needed depending on the cat’s response. Dobutamine or ephedrine have also been used to treat hypotension in this setting. Dobutamine should only be administered as a CRI, at a rate of 1–5 µg/kg/min. Ephedrine (0.03–0.2 mg/kg IV) is diluted in 5.0 ml of a balanced electrolyte solution and then administered in small IV boluses. Dobutamine and ephedrine may be less effective at increasing blood pressure. As with other sympathomimetics, ephedrine may promote arrhythmias.
Hypothermia
Cats are susceptible to hypothermia due to their high surface area to body mass ratio. Shivering during recovery increases oxygen consumption and discomfort. Hypothermia depresses immune function and has been linked to an increased incidence of wound infections and delayed healing time.97,98 Heat is lost mainly by radiation, with contributions from evaporation and conduction. Because the most effective method for preventing heat loss is to increase room temperature or surround the animal with a warm environment, methods to maintain body temperature should be initiated in the patient-holding area before premedication, and continued during anesthesia and recovery. Maintaining core body temperature using an active warming approach, such as forced warm air devices and medical grade electric and circulating warm water blankets, will be more effective than passive heat-retaining methods such as blankets, towels and bubble wrap, although insulation should be promoted over doing nothing. Limiting the clipped area, using warmed scrub solutions and keeping the cat dry are also important. The thermal costs of fluid therapy and dry cold gases from the breathing circuit are minor, but providing warm fluids and warm humidified gas may decrease heat loss. 99
Temperature monitoring should continue postoperatively to prevent hypo- or hyperthermia.
Hyperthermia
Rebound hyperthermia has been reported in cats after general anesthesia or sedation, with recorded temperatures as high as 41.1–42.2°C (106–108°F). While hydromorphone administration was first reported to be associated with hyperthermia, several other opioids and ketamine can result in an increase in body temperature.100–102 However, this is not a reason to withhold opioid analgesics. The magnitude of hyperthermia may be related to the degree of hypothermia during anesthesia. Posner and colleagues reported that patients with the lowest core body temperatures at the end of anesthesia became significantly more hyperthermic during recovery. 101
Treatment for hyperthermia is generally supportive, including measures such as sedation with acepromazine (vasodilation), removing heat sources, wetting the animal with lukewarm water and using a fan. Non-steroidal anti-inflammatory drugs (NSAiDs) do not appear to decrease core body temperature in this scenario but small doses of nalox-one (1–5 µg/kg IV) have been anecdotally shown to be effective at restoring temperature to a normal range. The temperature of most patients will return to normal in a matter of hours without intervention, so the benefit of naloxone should be carefully considered in the overall pain management plan.
Cardiac arrhythmias
Cardiac arrhythmias are uncommon in cats, but this does not preclude the use of ECG monitoring. The most commonly encountered arrhythmia is synchronous atrioventricular (AV) dissociation, a type of idioventricular rhythm where the ventricular depolarization rate closely approximates the atrial rate, but normal AV conduction is absent (Figure 25). This is typically caused by a sinus bradycardia that is slightly slower than the depolarization rate of a ventricular focus. This arrhythmia generally resolves spontaneously and may not require treatment if the heart rate is adequate. This bradyarrhythmia may respond to administration of an anticholinergic (atropine or glycopyrrolate).
Figure 25.
(a) ECG tracing from an anesthetized cat demonstrating synchronous atrioventricular dissociation. Note the largely negative QRS complexes that are junctional in origin, as well as the smaller, normally conducted QRS complexes (solid arrows). The P waves (open arrows) are seen to be masked by junctional escape beats, indicating a slightly faster rate of junctional depolarization as compared with sinus node depolarization. (b) ECG tracing from the same patient after administration of an anticholinergic agent (glycopyrrolate 0.01 mg/kg IV). Note the normal sinus rhythm, with slightly increased rate of sinus node depolarization and lack of junctional escape beats. ECG tracings in (a) and (b) were recorded at a paper speed of 25 mm/s. Courtesy of Gregg Griffenhagen
Other arrhythmias, including ventricular and atrial premature complexes and other ventricular tachyarrhythmias, are rare but should prompt the clinician to discuss pursuing further cardiac work-up with the client due to the high likelihood of structural cardiac disease. 103 In the anesthetized cat, ventricular arrhythmias may respond to lidocaine (0.25–0.5 mg/kg IV) and supraventricular arrhythmias to esmolol (0.1–0.5 mg/kg IV). if no positive response is noted, consideration should be given to terminating anesthesia if possible and pursuing further diagnostic testing.
Anesthesia recovery
Sixty percent of all anesthetic-related cat deaths occur during the recovery period, especially the first 3 h. 2 Therefore, monitoring during this time should be maintained with the same vigilance as during anesthesia. if the patient’s urinary bladder is very full or surgery/disease will interfere with the patient’s ability to use a litter box, consider carefully expressing the bladder prior to return of consciousness to help decrease post-operative discomfort.
Patient parameters that should be monitored in recovery include heart rate, pulse quality and rate, respiratory rate and pattern, oxygenation (pulse oximetry), blood pressure and body temperature, continuing until they have returned to within normal reference intervals. intravenous catheters should remain in place until the patient’s vital signs return to normal and the patient is in sternal recumbency.
A dark quiet recovery area with warm bedding will help reduce patient anxiety. Direct visualization of the patient should be maintained, and the litter box and food bowl should not be placed in the recovery cage until the cat is sternal and alert.
Optimal recovery includes regaining consciousness and extubation (if applicable) within 15–30 mins of the end of anesthesia (depending on maintenance technique and patient status), maintaining normal temperature, heart rate and respiratory rate, no coughing or upper respiratory noise, no paddling or muscle rigidity, no agitation or vocalization and regaining sternal recumbency.
Dose-dependent respiratory depression can occur with anesthetic agents that leads to a decrease in respiratory rate and an increase in ETCo2. A capnograph should be kept on the end of the endotracheal tube until the patient is extubated to help assess ventilation status.
Common complications in the immediate postanesthetic period include delayed recovery, dysphoria and emergence delirium. Delayed recovery is generally multifactorial, but often attributed to a combination of hypothermia, hypovolemia and impaired intrinsic drug metabolism. Treatment for delayed recovery is generally supportive and focused on thermal support and tissue oxygen delivery (eg, iV fluids, active warming devices and oxygen supplementation). if residual drug effects are thought to be contributing to delayed recovery, titration of specific reversal agents should be considered, with the understanding that reversal may impact analgesia. Naloxone, nalbuphine or butorphanol may be used to reverse opioids; these can be diluted 1:10 with a balanced electrolyte solution and slowly titrated (0.1 ml increments every 15–30 s) until the cat shows signs of arousal, stopping at that point and only continuing or repeating if necessary. Flumazenil is a specific reversal agent for benzodiazepines and atipamezole is used to reverse α2-adrenergic agonists. Figure 26 provides guidance in the event of a prolonged recovery.
Figure 26.
Steps that should be followed if the cat’s recovery is prolonged. This algorithm may be downloaded; for details and key to abbreviations, see box below
Emergence delirium usually manifests as thrashing, rolling and uncoordinated efforts to get away from perceived restraint or handlers. Pain is the most common differential for this behavior and should be managed with opioids and/or an NSAiD if these have not already been given or, after assessment, are considered inadequate. Continued attempts at restraint will likely result in escalation of the cat’s behavior. if there are no contraindications, a small dose of dexmedetomidine (0.5–5 µg/kg IV) or medetomidine (1–10 µg/kg IV) can be titrated to effect and will provide 15–30 mins of sedation, to allow time for metabolism and elimination of other anesthetic agents, and generally providing for a smoother, less excited recovery.
Dysphoric recoveries can be attributed to pain, drug administration (opioids, ketamine, alfaxalone, benzodiazepines) and hypoxemia. Antagonism of benzodiazepines or opioids (if administered) may alleviate dysphoria, provided that adequate analgesia is maintained. Airway obstruction, bronchoconstriction and laryngospasm can all contribute to hypoxemia. if after extubation the cat cannot maintain normal respiration, it may panic. in such cases, administer a sedative, give supplemental oxygen and assess the respiratory function of the patient; in some cases, the cat may need to be re-anesthetized and intubated. Figure 27 provides guidance for troubleshooting dysphoria during recovery.
Figure 27.
Recommended treatment steps for dealing with a dysphoric cat. This algorithm may be downloaded; for details and key to abbreviations, see box above

Summary Points
✜ General anesthesia is an essential component of feline practice, without which surgery and certain other treatment modalities and diagnostic procedures would be impossible.
✜ Due to their unique physiology and small size, cats undergoing anesthesia are at relatively greater risk of complications and mortality than many other species. Thus, relying on a standardized, evidence-based approach for administering anesthesia is especially useful for ensuring the patient’s safe and predictable perioperative response and recovery.
✜ Reliability of anesthesia is dependent on properly functioning anesthesia equipment. It is important that anesthesia breathing circuits are leak tested prior to each usage.
✜ A thorough physical examination and careful patient history can reveal physiologic deficits or age-related conditions that could increase the risk of anesthesia. A preanesthetic screening and evaluation is particularly important for feline patients because of the cat’s tendency to avoid showing overt clinical signs of disease. The American Society of Anesthesiologists’ physical status classification is a useful means of categorizing preanesthetic health.
✜ Preanesthetic sedation is advisable in cats that are anxious or stressed. The choice and dosages of premedication drugs listed in the Guidelines should be based on the cat’s health and temperament.
✜ Induction drugs have the potential to cause significant respiratory depression, so preoxygenation should be a standard practice whenever possible.
✜ The dose of inhalant anesthetics is higher in cats than in most other species, resulting in the potential for significant cardiovascular depression, and prolonged or dysphoric recovery.
✜ Most feline anesthetic deaths are due to obstruction of the cat’s small and delicate airway, so equipment and techniques for maintaining and monitoring a patent airway during and following anesthesia are critical, along with close observation during recovery.
✜ The Guidelines include resources designed to minimize risks associated with anesthesia; namely, checklists that ensure consistent preanesthetic preparation, and protocol algorithms for monitoring the patient’s condition and physiological parameters and responding to complications during anesthesia. Perioperative management techniques described in the Guidelines will minimize complications during anesthesia and improve the likelihood of a smooth and uneventful recovery.
Supplemental Material
Supplemental material, Endotracheal_tube_placement for AAFP Feline Anesthesia Guidelines by Sheilah A Robertson, Susan M Gogolski, Peter Pascoe, Heidi L Shafford, Jennifer Sager and Gregg M Griffenhagen in Journal of Feline Medicine and Surgery
Supplemental material, Feline_anesthesia_Client_brochure for AAFP Feline Anesthesia Guidelines by Sheilah A Robertson, Susan M Gogolski, Peter Pascoe, Heidi L Shafford, Jennifer Sager and Gregg M Griffenhagen in Journal of Feline Medicine and Surgery
Supplemental material, Troubleshooting_bradycardia for AAFP Feline Anesthesia Guidelines by Sheilah A Robertson, Susan M Gogolski, Peter Pascoe, Heidi L Shafford, Jennifer Sager and Gregg M Griffenhagen in Journal of Feline Medicine and Surgery
Supplemental material, Troubleshooting_dysphoria for AAFP Feline Anesthesia Guidelines by Sheilah A Robertson, Susan M Gogolski, Peter Pascoe, Heidi L Shafford, Jennifer Sager and Gregg M Griffenhagen in Journal of Feline Medicine and Surgery
Supplemental material, Troubleshooting_hypotension for AAFP Feline Anesthesia Guidelines by Sheilah A Robertson, Susan M Gogolski, Peter Pascoe, Heidi L Shafford, Jennifer Sager and Gregg M Griffenhagen in Journal of Feline Medicine and Surgery
Supplemental material, Troubleshooting_hypoxemia for AAFP Feline Anesthesia Guidelines by Sheilah A Robertson, Susan M Gogolski, Peter Pascoe, Heidi L Shafford, Jennifer Sager and Gregg M Griffenhagen in Journal of Feline Medicine and Surgery
Supplemental material, Troubleshooting_prolonged_recovery for AAFP Feline Anesthesia Guidelines by Sheilah A Robertson, Susan M Gogolski, Peter Pascoe, Heidi L Shafford, Jennifer Sager and Gregg M Griffenhagen in Journal of Feline Medicine and Surgery
Supplemental material, Troubleshooting_tachycardia for AAFP Feline Anesthesia Guidelines by Sheilah A Robertson, Susan M Gogolski, Peter Pascoe, Heidi L Shafford, Jennifer Sager and Gregg M Griffenhagen in Journal of Feline Medicine and Surgery
Supplemental material, Troubleshooting_ventilation for AAFP Feline Anesthesia Guidelines by Sheilah A Robertson, Susan M Gogolski, Peter Pascoe, Heidi L Shafford, Jennifer Sager and Gregg M Griffenhagen in Journal of Feline Medicine and Surgery
Acknowledgments
The authors gratefully acknowledge the contributions of Mark Dana and Dr Edward W Kanara of the Kanara Consulting Group, LLC in the preparation of the Guidelines. The artistic skills of Chrisoula A Toupadakis Skouritakis are greatly appreciated for the creation of the line drawings used in the Guidelines.
Appendix : Client brochure
Footnotes
Funding: The Panel members received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article.
The Panel members have no conflicts of interest to declare.
Contributor Information
Sheilah A Robertson, Lap of Love Veterinary Hospice, 1780 N US Highway 41, Lutz, FL 33549, USA.
Susan M Gogolski, AMEDDC&S Department of Veterinary Science, 3630 Stanley Rd, Bldg 2618, Fort Sam Houston, TX 78234, USA.
Peter Pascoe, Emeritus Professor, University of California, 1536 Notre Dame Drive, Davis, CA 95616, USA.
Heidi L Shafford, Veterinary Anesthesia Specialists, PO Box 418, Clackamas, OR 97015, USA.
Jennifer Sager, University of Florida Veterinary Hospital, College of Veterinary Medicine, 2015 SW 16th Avenue, Gainesville, FL 32610, USA.
Gregg M Griffenhagen, Colorado State University Veterinary Teaching Hospital, 300 W Drake Rd, Fort Collins, CO 80523, USA.
References
- 1. Dyson DH, Maxie MG, Schnurr D. Morbidity and mortality associated with anesthetic management in small animal veterinary practice in Ontario. J Am Anim Hosp Assoc 1998; 34: 325–335. [DOI] [PubMed] [Google Scholar]
- 2. Brodbelt DC, Pfeiffer DU, Young LE, et al. Risk factors for anaesthetic-related death in cats: results from the confidential enquiry into perioperative small animal fatalities (CEP-SAF). Br J Anaesth 2007; 99: 617–623. [DOI] [PubMed] [Google Scholar]
- 3. Epstein ME, Rodan I, Griffenhagen G, et al. AAHA/AAFP pain management guidelines for dogs and cats. J Feline Med Surg 2015; 17: 251–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paepe D, Verjans G, Duchateau L, et al. Routine health screening: findings in apparently healthy middle-aged and old cats. J Feline Med Surg 2013; 15: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vogt AH, Rodan I, Brown M, et al. AAFP–AAHA feline life stage guidelines. J Am Anim Hosp Assoc 2010; 46: 70–85. [DOI] [PubMed] [Google Scholar]
- 6. Quimby JM, Smith ML, Lunn KF. Evaluation of the effects of hospital visit stress on physiologic parameters in the cat. J Feline Med Surg 2011; 13: 733–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carney HC, Little S, Brownlee-Tomasso D, et al. AAFP and ISFM feline-friendly nursing care guidelines. J Feline Med Surg 2012; 14: 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodan I, Sundahl E, Carney H, et al. AAFP and ISFM feline-friendly handling guidelines. J Feline Med Surg 2011; 13: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pankratz KE, Ferris KK, Griffith EH, et al. Use of single-dose oral gabapentin to attenuate fear responses in cage-trap confined community cats: a double-blind, placebo-controlled field trial. J Feline Med Surg 2018; 20: 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Haaften KA, Forsythe LRE, Stelow EA, et al. Effects of a single preappointment dose of gabapentin on signs of stress in cats during transportation and veterinary examination. J Am Vet Med Assoc 2017; 251: 1175–1181. [DOI] [PubMed] [Google Scholar]
- 11. Ellis SLH. Recognising and assessing feline emotions during the consultation: history, body language and behaviour. J Feline Med Surg 2018; 20: 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davies M, Kawaguchi S. Pregeneral anaesthetic blood screening of dogs and cats attending a UK practice. Vet Rec 2014; 174: 506. [DOI] [PubMed] [Google Scholar]
- 13. Rodan I, Sparkes AH. Preventive health care for cats. In: Little SE. (ed). The cat: clinical medicine and management. St Louis, MO: Elsevier, 2012, pp 151–180. [Google Scholar]
- 14. Pittari J, Rodan I, Beekman G, et al. American Association of Feline Practitioners: senior care guidelines. J Feline Med Surg 2009; 11: 763–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Binder J, Ommen SR, Chen HH, et al. Usefulness of brain natriuretic peptide levels in the clinical evaluation of patients with hypertrophic cardiomyopathy. Am J Cardiol 2007; 100: 712–714. [DOI] [PubMed] [Google Scholar]
- 16. Machen MC, Oyama MA, Gordon SG, et al. Multi-centered investigation of a point-of-care NT-proBNP ELISA assay to detect moderate to severe occult (pre-clinical) feline heart disease in cats referred for cardiac evaluation. J Vet Cardiol 2014; 16: 245–255. [DOI] [PubMed] [Google Scholar]
- 17. Bednarski R, Grimm K, Harvey R, et al. AAHA anesthesia guidelines for dogs and cats. J Am Anim Hosp Assoc 2011; 47: 377–385. [DOI] [PubMed] [Google Scholar]
- 18. Honkavaara J, Pypendop B, Ilkiw J. The impact of MK-467 on sedation, heart rate and arterial blood pressure after intramuscular coadministration with dexmedetomidine in conscious cats. Vet Anaesth Analg 2017; 44: 811–822. [DOI] [PubMed] [Google Scholar]
- 19. Biermann K, Hungerbuhler S, Mischke R, et al. Sedative, cardiovascular, haematologic and biochemical effects of four different drug combinations administered intramuscularly in cats. Vet Anaesth Analg 2012; 39: 137–150. [DOI] [PubMed] [Google Scholar]
- 20. Peterson M. Hyperthyroidism in cats: what’s causing this epidemic of thyroid disease and can we prevent it? J Feline Med Surg 2012; 14: 804–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carney HC, Ward CR, Bailey SJ, et al. AAFP guidelines for the management of feline hyperthyroidism. J Feline Med Surg 2016; 18: 400–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rishniw M. Cardiovascular diseases. In: Little SE. (ed). The cat: clinical medicine and management. St Louis, MO: Elsevier, 2012, pp 300–328. [Google Scholar]
- 23. Robertson SA. Anesthetic-related morbidity and mortality in cats. In: Little SE. (ed). August’s consultations in feline internal medicine. St Louis, MO: Elsevier, 2016, pp 752–760. [Google Scholar]
- 24. Fox PR, Rush JE, Reynolds CA, et al. Multicenter evaluation of plasma N-terminal probrain natriuretic peptide (NT-pro BNP) as a biochemical screening test for asymptomatic (occult) cardiomyopathy in cats. J Vet Intern Med 2011; 25: 1010–1016. [DOI] [PubMed] [Google Scholar]
- 25. Oyama MA. Cardiac blood tests. In: Little SE. (ed). August’s consultations in feline internal medicine. Volume 7. St Louis, MO: Elsevier, 2016, pp 363–368. [Google Scholar]
- 26. Fox PR, Oyama MA, Reynolds C, et al. Utility of plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) to distinguish between congestive heart failure and non-cardiac causes of acute dyspnea in cats. J Vet Cardiol 2009; 11 Suppl 1: S51–61. [DOI] [PubMed] [Google Scholar]
- 27. Savvas I, Raptopoulos D, Rallis T. A ‘light meal’ three hours preoperatively decreases the incidence of gastro-esophageal reflux in dogs. J Am Anim Hosp Assoc 2016; 52: 357–363. [DOI] [PubMed] [Google Scholar]
- 28. Viskjer S, Sjostrom L. Effect of the duration of food with holding prior to anesthesia on gastroesophageal reflux and regurgitation in healthy dogs undergoing elective orthopedic surgery. Am J Vet Res 2017; 78: 144–150. [DOI] [PubMed] [Google Scholar]
- 29. Levy JK, Bard KM, Tucker SJ, et al. Perioperative mortality in cats and dogs undergoing spay or castration at a high volume clinic. Vet J 2017; 224: 11–15. [DOI] [PubMed] [Google Scholar]
- 30. Apfelbaum JL, Connis RT, Nickinovich DG, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology 2012; 116: 522–538. [DOI] [PubMed] [Google Scholar]
- 31. Webb JA, Kirby GM, Nykamp SG, et al. Ultrasonographic and laboratory screening in clinically normal mature golden retriever dogs. Can Vet J 2012; 53: 626–630. [PMC free article] [PubMed] [Google Scholar]
- 32. Hosgood G, Scholl DT. Evaluation of age and American Society of Anesthesiologists (ASA) physical status as risk factors for perianesthetic morbidity and mortality in the cat. J Vet Emerg Crit Care 2002; 12: 9–15. [Google Scholar]
- 33. Pypendop BH, Ilkiw JE. Anesthesia and perioperative care. In: Little SE. (ed). The cat: clinical medicine and management. St Louis, MO: Elsevier, 2012, pp 112–150. [Google Scholar]
- 34. Grundy SA. Clinically relevant physiology of the neonate. Vet Clin North Am Small Anim Pract 2006; 36: 443–459, v. [DOI] [PubMed] [Google Scholar]
- 35. Grubb TL, Perez Jimenez TE, Pettifer GR. Neonatal and pediatric patients. In: Grimm KA, Lamont LA, Tranquilli WJ, et al. (eds). Veterinary anesthesia and analgesia. 5th ed. Ames, Iowa: John Wiley & Sons, 2015, pp 983–987. [Google Scholar]
- 36. Kushner LI. Anesthetic protocols for systemically healthy cats. In: Drobatz KJ, Costello MF. (eds). Feline emergency and critical care medicine. Ames, Iowa: Blackwell, 2010, pp 53–61. [Google Scholar]
- 37. Grubb TL, Perez Jimenez TE, Pettifer GR. Senior and geriatric patients. In: Grimm KA, Lamont LA, Tranquilli WJ, et al. (eds). Veterinary anesthesia and analgesia. 5th ed. Ames, Iowa: John Wiley & Sons, 2015, pp 988–992. [Google Scholar]
- 38. Grubb T. Anesthesia for the aging cat. In: Little SE. (ed). August’s consultations in feline internal medicine. Volume 7. St Louis, MO: Elsevier, 2016, pp 986–994. [Google Scholar]
- 39. Kushner LI. Guidelines for anesthesia in critically ill feline patients. In: Drobatz KJ, Costello MF. (eds). Feline emergency and critical care medicine. Ames, Iowa: Blackwell, 2010, pp 39–51. [Google Scholar]
- 40. Perkowski SZ, Oyama MA. Pathophysiology and anesthetic management of patients with cardiovascular disease. In: Grimm K, Lamont LA, Tranquilli WJ, et al. (eds). Veterinary anesthesia and analgesia. 5th ed. Ames, Iowa: John Wiley & Sons, 2015, pp 496–510. [Google Scholar]
- 41. Lamont LA, Bulmer BJ, Sisson DD, et al. Doppler echocardiographic effects of medetomidine on dynamic left ventricular outflow tract obstruction in cats. J Am Vet Med Assoc 2002; 221: 1276–1281. [DOI] [PubMed] [Google Scholar]
- 42. Zwicker LA, Matthews AR, Cote E, et al. The effect of dexmedetomidine on radiographic cardiac silhouette size in healthy cats. Vet Radiol Ultrasound 2016; 57: 230–236. [DOI] [PubMed] [Google Scholar]
- 43. Johard E, Tidholm A, Ljungvall I, et al. Effects of sedation with dexmedetomidine and buprenorphine on echocardiographic variables, blood pressure and heart rate in healthy cats. J Feline Med Surg 2018; 20: 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ribas T, Bublot I, Junot S, et al. Effects of intramuscular sedation with alfaxalone and butorphanol on echocardiographic measurements in healthy cats. J Feline Med Surg 2015; 17: 530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ward CR. Thyroid storm. In: Silverstein DC, Hopper K. (eds). Small animal critical care medicine. 2nd ed. St Louis, MO: Elsevier, 2015, pp 364–367. [Google Scholar]
- 46. Riesen SC, Schober KE, Smith DN, et al. Effects of ivabradine on heart rate and left ventricular function in healthy cats and cats with hypertrophic cardiomyopathy. Am J Vet Res 2012; 73: 202–212. [DOI] [PubMed] [Google Scholar]
- 47. Davis H, Jensen T, Johnson A, et al. AAHA/AAFP fluid therapy guidelines for dogs and cats. J Am Anim Hosp Assoc 2013; 49: 149–159. [DOI] [PubMed] [Google Scholar]
- 48. Coleman AE, Shepard MK, Schmiedt CW, et al. Effects of orally administered enalapril on blood pressure and hemodynamic response to vasopressors during isoflurane anesthesia in healthy dogs. Vet Anaesth Analg 2016; 43: 482–494. [DOI] [PubMed] [Google Scholar]
- 49. Veres-Nyeki KO. Endocrine diseases. In: Duke-Novakovski T, de Vries M, Seymour C. (eds). BSAVA manual of canine and feline anaesthesia and analgesia. 3rd ed. Quedgeley, Gloucester: British Small Animal Veterinary Association, 2016, pp 376–391. [Google Scholar]
- 50. Johnson LR, Drazenovich TL. Flexible bronchoscopy and bronchoalveolar lavage in 68 cats (2001–2006). J Vet Intern Med 2007; 21: 219–225. [DOI] [PubMed] [Google Scholar]
- 51. Courcier EA, O’Higgins R, Mellor DJ, et al. Prevalence and risk factors for feline obesity in a first opinion practice in Glasgow, Scotland. J Feline Med Surg 2010; 12: 746–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vasan RS. Cardiac function and obesity. Heart 2003; 89: 1127–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Love L, Cline MG. Perioperative physiology and pharmacology in the obese small animal patient. Vet Anaesth Analg 2015; 42: 119–132. [DOI] [PubMed] [Google Scholar]
- 54. Marino CL, Lascelles BD, Vaden SL, et al. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg 2014; 16: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Finco DR, Cornelius LM. Characterization and treatment of water, electrolyte, and acid-base imbalances of induced urethral obstruction in the cat. Am J Vet Res 1977; 38: 823–830. [PubMed] [Google Scholar]
- 56. O’Hearn AK, Wright BD. Coccygeal epidural with local anesthetic for catheterization and pain management in the treatment of feline urethral obstruction. J Vet Emerg Crit Care (San Antonio) 2011; 21: 50–52. [DOI] [PubMed] [Google Scholar]
- 57. Drobatz KJ. Approach to the critically ill cat. In: Drobatz KJ, Costello MF. (eds). Feline emergency and critical care medicine. Ames, Iowa: Blackwell, 2010, pp 3–12. [Google Scholar]
- 58. Reineke KJ. Evaluation and triage of the critically ill patient. In: Silverstein DC, Hopper K. (eds). Small animal critical care medcine. 2nd ed. St Louis, MO: Elsevier, 2015, pp 1–5. [Google Scholar]
- 59. Hackett TB. Physical examination and daily assessment of the critically ill patient. In: Silverstein DC, Hopper K. (eds). Small animal critical care medicine. 2nd ed. St Louis, MO: Elsevier, 2015, pp 6–10. [Google Scholar]
- 60. Ko JC, Abbo LA, Weil AB, et al. A comparison of anesthetic and cardiorespiratory effects of tiletamine-zolazepam-butorphanol and tiletamine-zolazepam-butorphanol-medetomidine in cats. Vet Ther 2007; 8: 164–176. [PubMed] [Google Scholar]
- 61. Nagore L, Soler C, Gil L, et al. Sedative effects of dexmedeto-midine, dexmedetomidine-pethidine and dexmedetomidine-butorphanol in cats. J Vet Pharmacol Ther 2013; 36: 222–228. [DOI] [PubMed] [Google Scholar]
- 62. Volpato J, Mattoso CR, Beier SL, et al. Sedative, hematologic and hemostatic effects of dexmedetomidine-butorphanol alone or in combination with ketamine in cats. J Feline Med Surg 2015; 17: 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ebner J, Wehr U, Baumgartner C, et al. Partial antagonization of midazolam-medetomidine-ketamine in cats – atipamezole versus combined atipamezole and flumazenil. J Vet Med A Physiol Pathol Clin Med 2007; 54: 518–521. [DOI] [PubMed] [Google Scholar]
- 64. Deutsch J, Jolliffe C, Archer E, et al. Intramuscular injection of alfaxalone in combination with butorphanol for sedation in cats. Vet Anaesth Analg 2017; 44: 794–802. [DOI] [PubMed] [Google Scholar]
- 65. Ueoka N, Hikasa Y. Antagonistic effects of atipamezole, flumazenil and 4-aminopyridine against anaesthesia with medetomidine, midazolam and ketamine combination in cats. J Feline Med Surg 2008; 10: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Carlstead K, Brown JL, Strawn W. Behavioral and physiological correlates of stress in laboratory cats. Appl Anim Behav Sci 1993; 38: 143–158. [Google Scholar]
- 67. Kry K, Casey R. The effect of hiding enrichment on stress levels and behaviour of domestic cats (Felis sylvestris catus) in a shelter setting and the implications for adoption potential. Anim Welfare 2007; 16: 375–383. [Google Scholar]
- 68. Vinke CM, Godijn LM, van der Leij WJR. Will a hiding box provide stress reduction for shelter cats? Appl Anim Behav Sci 2014; 166: 86–93. [Google Scholar]
- 69. Taylor PM, Robertson SA, Dixon MJ, et al. Morphine, pethidine and buprenorphine disposition in the cat. J Vet Pharmacol Ther 2001; 24: 391–398. [DOI] [PubMed] [Google Scholar]
- 70. van Beusekom CD, Fink-Gremmels J, Schrickx JA. Comparing the glucuronidation capacity of the feline liver with substrate-specific glucuronidation in dogs. J Vet Pharmacol Ther 2014; 37: 18–24. [DOI] [PubMed] [Google Scholar]
- 71. Sinclair MD, Dyson DH. The impact of acepromazine on the efficacy of crystalloid, dextran or ephedrine treatment in hypotensive dogs under isoflurane anesthesia. Vet Anaesth Analg 2012; 39: 563–573. [DOI] [PubMed] [Google Scholar]
- 72. ilkiw JE, Suter CM, Farver TB, et al. The behaviour of healthy awake cats following intravenous and intramuscular administration of midazolam. J Vet Pharmacol Ther 1996; 19: 205–216. [DOI] [PubMed] [Google Scholar]
- 73. Lamont LA, Bulmer BJ, Grimm KA, et al. Cardiopulmonary evaluation of the use of medetomidine hydrochloride in cats. Am J Vet Res 2001; 62: 1745–1749. [DOI] [PubMed] [Google Scholar]
- 74. Monteiro ER, Campagnol D, Parrilha LR, et al. Evaluation of cardiorespiratory effects of combinations of dexmedetomidine and atropine in cats. J Feline Med Surg 2009; 11: 783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Santos LC, Ludders JW, Erb HN, et al. A randomized, blinded, controlled trial of the antiemetic effect of ondansetron on dexmedetomidine-induced emesis in cats. Vet Anaesth Analg 2011; 38: 320–327. [DOI] [PubMed] [Google Scholar]
- 76. Papastefanou AK, Galatos AD, Pappa E, et al. The effect of butorphanol on the incidence of dexmedetomidine-induced emesis in cats. Vet Anaesth Analg 2015; 42: 608–613. [DOI] [PubMed] [Google Scholar]
- 77. Martin-Flores M, Mastrocco A, Lorenzutti AM, et al. Maropitant administered orally 2–2.5 h prior to morphine and dexmedetomidine reduces the incidence of emesis in cats. J Feline Med Surg 2017; 19: 876–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Martin-Flores M, Sakai DM, Learn MM, et al. Effects of maro-pitant in cats receiving dexmedetomidine and morphine.J Am Vet Med Assoc 2016; 248: 1257–1261. [DOI] [PubMed] [Google Scholar]
- 79. Martin-Flores M, Sakai DM, Mastrocco A, et al. Evaluation of oral maropitant as an antiemetic in cats receiving morphine and dexmedetomidine. J Feline Med Surg 2016; 18: 921–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shaughnessy MR, Hofmeister EH. A systematic review of sevoflurane and isoflurane minimum alveolar concentration in domestic cats. Vet Anaesth Analg 2014; 41: 1–13. [DOI] [PubMed] [Google Scholar]
- 81. Pascoe PJ, ilkiw JE, Craig C, et al. The effects of ketamine on the minimum alveolar concentration of isoflurane in cats. Vet Anaesth Analg 2007; 34: 31–39. [DOI] [PubMed] [Google Scholar]
- 82. Pypendop BH, ilkiw JE. Assessment of the hemodynam-ic effects of lidocaine administered IV in isoflurane-anesthetized cats. Am J Vet Res 2005; 66: 661–668. [DOI] [PubMed] [Google Scholar]
- 83. Pypendop BH, Barter LS, Stanley SD, et al. Hemodynamic effects of dexmedetomidine in isoflurane-anesthetized cats. Vet Anaesth Analg 2011; 38: 555–567. [DOI] [PubMed] [Google Scholar]
- 84. Pascoe PJ, Ilkiw JE, Frischmeyer KJ. The effect of the duration of propofol administration on recovery from anesthesia in cats. Vet Anaesth Analg 2006; 33: 2–7. [DOI] [PubMed] [Google Scholar]
- 85. Taylor PM, Chengelis CP, Miller WR, et al. Evaluation of propofol containing 2% benzyl alcohol preservative in cats. J Feline Med Surg 2012; 14: 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schwarz A, Kalchofner K, Palm J, et al. Minimum infusion rate of alfaxalone for total intravenous anaesthesia after sedation with acepromazine or medetomidine in cats under-going ovariohysterectomy. Vet Anaesth Analg 2014; 41: 480–490. [DOI] [PubMed] [Google Scholar]
- 87. McMillan M, Darcy H. Adverse event surveillance in small animal anaesthesia: an intervention-based, voluntary reporting audit. Vet Anaesth Analg 2016; 43: 128–135. [DOI] [PubMed] [Google Scholar]
- 88. Hardie EM, Spodnick GJ, Gilson SD, et al. Tracheal rupture in cats: 16 cases (1983–1998). J Am Vet Med Assoc 1999; 214: 508–512. [PubMed] [Google Scholar]
- 89. Mitchell SL, McCarthy R, Rudloff E, et al. Tracheal rupture associated with intubation in cats: 20 cases (1996–1998). J Am Vet Med Assoc 2000; 216: 1592–1595. [DOI] [PubMed] [Google Scholar]
- 90. Barletta M, Kleine SA, Quandt JE. Assessment of v-gel supraglottic airway device placement in cats performed by inexperienced veterinary students. Vet Rec 2015; 177: 523. [DOI] [PubMed] [Google Scholar]
- 91. Prasse SA, Schrack J, Wenger S, et al. Clinical evaluation of the v-gel supraglottic airway device in comparison with a classical laryngeal mask and endotracheal intubation in cats during spontaneous and controlled mechanical ventilation. Vet Anaesth Analg 2016; 43: 55–62. [DOI] [PubMed] [Google Scholar]
- 92. Haynes AB, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med 2009; 360: 491–499. [DOI] [PubMed] [Google Scholar]
- 93. Thieman Mankin K. Checklists: an answer to avoiding mistakes. Ask the expert. Clinician’s Brief, May 2016, pp 24–29. [Google Scholar]
- 94. Poterack KA, Kampine JP, Schmeling WT. Effects of isoflurane, midazolam, and etomidate on cardiovascular responses to stimulation of central nervous system pressor sites in chronically instrumented cats. Anesth Analg 1991; 73: 64–75. [DOI] [PubMed] [Google Scholar]
- 95. Ruffato M, Novello L, Clark L. What is the definition of intraoperative hypotension in dogs? Results from a survey of diplomates of the ACVAA and ECVAA. Vet Anaesth Analg 2015; 42: 55–64. [DOI] [PubMed] [Google Scholar]
- 96. Pascoe PJ, Ilkiw JE, Pypendop BH. Effects of increasing infusion rates of dopamine, dobutamine, epinephrine, and phenylephrine in healthy anesthetized cats. Am J Vet Res 2006; 67: 1491–1499. [DOI] [PubMed] [Google Scholar]
- 97. Reynolds L, Beckmann J, Kurz A. Perioperative complications of hypothermia. Best Pract Res Clin Anaesthesiol 2008; 22: 645–657. [DOI] [PubMed] [Google Scholar]
- 98. Clark-Price S. Inadvertent perianesthetic hypothermia in small animal patients. Vet Clin North Am Small Anim Pract 2015; 45: 983–994. [DOI] [PubMed] [Google Scholar]
- 99. Haskins SC, Patz JD. Effect of inspired-air warming and humidification in the prevention of hypothermia during general anesthesia in cats. Am J Vet Res 1980; 41: 1669–1673. [PubMed] [Google Scholar]
- 100. Niedfeldt RL, Robertson SA. Postanesthetic hyperthermia in cats: a retrospective comparison between hydromorphone and buprenorphine. Vet Anaesth Analg 2006; 33: 381–389. [DOI] [PubMed] [Google Scholar]
- 101. Posner LP, Gleed RD, Erb HN, et al. Post-anesthetic hyperthermia in cats. Vet Anaesth Analg 2007; 34: 40–47. [DOI] [PubMed] [Google Scholar]
- 102. Posner LP, Pavuk AA, Rokshar JL, et al. Effects of opioids and anesthetic drugs on body temperature in cats. Vet Anaesth Analg 2010; 37: 35–43. [DOI] [PubMed] [Google Scholar]
- 103. Cote E, Jaeger R. Ventricular tachyarrhythmias in 106 cats: associated structural cardiac disorders. J Vet Intern Med 2008; 22: 1444–1446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Endotracheal_tube_placement for AAFP Feline Anesthesia Guidelines by Sheilah A Robertson, Susan M Gogolski, Peter Pascoe, Heidi L Shafford, Jennifer Sager and Gregg M Griffenhagen in Journal of Feline Medicine and Surgery
Supplemental material, Feline_anesthesia_Client_brochure for AAFP Feline Anesthesia Guidelines by Sheilah A Robertson, Susan M Gogolski, Peter Pascoe, Heidi L Shafford, Jennifer Sager and Gregg M Griffenhagen in Journal of Feline Medicine and Surgery
Supplemental material, Troubleshooting_bradycardia for AAFP Feline Anesthesia Guidelines by Sheilah A Robertson, Susan M Gogolski, Peter Pascoe, Heidi L Shafford, Jennifer Sager and Gregg M Griffenhagen in Journal of Feline Medicine and Surgery
Supplemental material, Troubleshooting_dysphoria for AAFP Feline Anesthesia Guidelines by Sheilah A Robertson, Susan M Gogolski, Peter Pascoe, Heidi L Shafford, Jennifer Sager and Gregg M Griffenhagen in Journal of Feline Medicine and Surgery
Supplemental material, Troubleshooting_hypotension for AAFP Feline Anesthesia Guidelines by Sheilah A Robertson, Susan M Gogolski, Peter Pascoe, Heidi L Shafford, Jennifer Sager and Gregg M Griffenhagen in Journal of Feline Medicine and Surgery
Supplemental material, Troubleshooting_hypoxemia for AAFP Feline Anesthesia Guidelines by Sheilah A Robertson, Susan M Gogolski, Peter Pascoe, Heidi L Shafford, Jennifer Sager and Gregg M Griffenhagen in Journal of Feline Medicine and Surgery
Supplemental material, Troubleshooting_prolonged_recovery for AAFP Feline Anesthesia Guidelines by Sheilah A Robertson, Susan M Gogolski, Peter Pascoe, Heidi L Shafford, Jennifer Sager and Gregg M Griffenhagen in Journal of Feline Medicine and Surgery
Supplemental material, Troubleshooting_tachycardia for AAFP Feline Anesthesia Guidelines by Sheilah A Robertson, Susan M Gogolski, Peter Pascoe, Heidi L Shafford, Jennifer Sager and Gregg M Griffenhagen in Journal of Feline Medicine and Surgery
Supplemental material, Troubleshooting_ventilation for AAFP Feline Anesthesia Guidelines by Sheilah A Robertson, Susan M Gogolski, Peter Pascoe, Heidi L Shafford, Jennifer Sager and Gregg M Griffenhagen in Journal of Feline Medicine and Surgery