Abstract
Study rationale:
Veterinary practitioners often perform geriatric health screening in cats. Unfortunately, scientific information regarding clinical and laboratory abnormalities and normal blood pressure values in elderly cats is scarce. This prospective study evaluated routine health screening tests in apparently healthy middle-aged and old cats.
Protocol:
One hundred cats of 6 years and older underwent blood pressure measurement, physical examination, blood and urine analysis, indirect fundoscopy and bilateral Schirmer tear tests.
Findings:
Mean systolic blood pressure (SBP) was 133.6 ± 21.5 mmHg. Increased SBP (>160 mmHg) was observed in eight cats, submandibular lymphadenopathy in 32, gingivitis in 72, heart murmur in 11, thyroid goitre in 20, increased creatinine in 29, hyperglycaemia in 25, increased total thyroxine in three, feline immunodeficiency virus positivity in 14, crystalluria in 41, borderline proteinuria in 25 and overt proteinuria in two. Mean tear production was very similar for both eyes and none of the cats had ocular lesions secondary to hypertension.
Clinical significance:
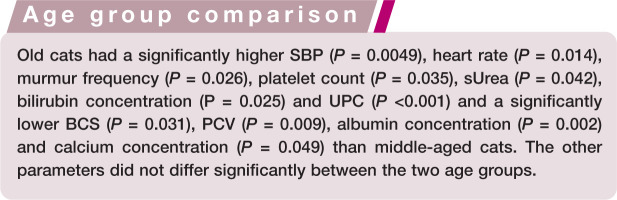
Old cats (>10 years) had significantly higher SBP, heart rate, murmur frequency, thrombocyte count, urine protein:creatinine ratio and serum urea and bilirubin concentrations, and significantly lower body condition score, haematocrit, albumin and total calcium concentrations than middle-aged cats (6–10 years). The common occurrence of physical examination and laboratory abnormalities in apparently healthy old cats underlines the need for regular health checks and the development of age-dependent laboratory reference intervals.
In the past few decades, the expected life span of pet cats in Europe and the United States has increased and the population of senior and geriatric cats has grown concurrently. 1 Old cats are susceptible to many chronic diseases and senior care guidelines have been developed to improve early disease detection and promote longevity and quality of life. Veterinary clinics are increasingly offering ‘geriatric health care packages’, and these should consist of a thorough history (preferably by detailed owner questionnaire), physical examination (including oral cavity examination and thyroid palpation), blood pressure (BP) measurement, ophthalmic examination and laboratory tests.2–4 Unfortunately, the interpretation of results is difficult because scientific information regarding clinical and laboratory abnormalities in older animals is scarce.
With ageing, several physiological changes can be expected, resulting in age-related but clinically insignificant changes. 5 Thus, specific reference intervals (RIs) for senior or geriatric animals may be warranted. 6 Although routine BP monitoring in middle-aged and old cats is advised, 7 normal BP values in elderly cats have not been reported. Several studies have evaluated indirect BP measurements in healthy adult cats.8–11 However, only small numbers of old cats were included and these studies yielded conflicting results regarding the association between age and systolic blood pressure (SBP). The Schirmer tear test (STT) to evaluate tear production can also be part of the minimum database for senior pets. 3 STT results are, however, poorly documented in older cats. Several studies have described STT results in healthy cats, but most included young or middle-aged cats.12–14 In normal dogs, tear production decreases with age, 15 but a similar age effect has not been examined in cats.
Furthermore, the age at which cats are defined as geriatric varies widely, from 8 years and older, 16 to 14 years 17 or even 15 years2,4,18 and older. In general, it is accepted that a geriatric animal is one that has reached 75% of its expected life span, which obviously depends on species and breed. 19
All these questions make interpretation of clinical and laboratory parameters complex in healthy and diseased old cats. As geriatric medicine is forming an increasing part of the case load of first opinion and referral practices, 20 improved scientific data on this age group would help veterinarians to manage and treat senior or geriatric cats.
This prospective study aimed to evaluate the presence of abnormal findings on SBP measurement, and physical, ophthalmic and routine blood and urine examinations in middle-aged and old cats that were apparently healthy according to their owners.
Materials and methods
Study population
One hundred healthy cats aged 6 years and older were included. To evaluate an age effect, the cats were allocated to two groups: group 1 (middle-aged cats) between 6 and 10 years and group 2 (old cats) older than 10 years. We aimed to include an equal number of cats in both groups. All cats were fasted for 12 h, and water was offered ad libitum.
Health was defined as ‘being healthy for the owner’; namely, showing no changes in general behaviour or habits, normal eating and drinking behaviour, stable body weight and absence of clinical signs. Cats needed to be free of medication (except preventive medication) for at least 2 months before inclusion. Preventive medication was allowed, except in the week leading up to the study. To check health status, owners completed a questionnaire covering their cat’s health, living environment, daily activity, feeding practices, vaccination status, parasite control and disease history.
The study was completed at the Department of Small Animal Medicine and Clinical Biology, Ghent University, between May and October 2010. All cats were privately owned, the owners provided signed consent and the study was approved by the local and national ethical committees (EC2010/27).
Procedures
All procedures were performed in the same order by the same author (GV) without using sedation or anaesthesia.
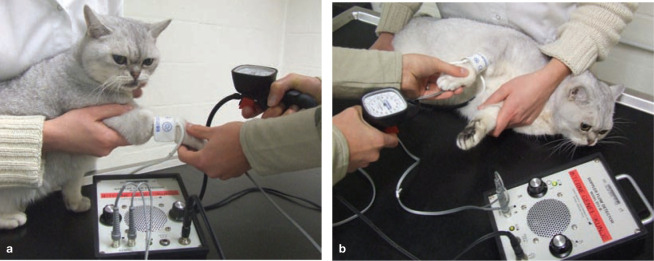
First, SBP was measured using the Doppler ultrasonic technique (Figure 1) and a standardised procedure following the ACVIM consensus statement. 21 Hypertension was defined as SBP >160 mmHg and hypotension as SBP <80 mmHg.21–23
Figure 1.
SBP measurement using the Doppler ultrasonic technique in (a) the sitting position and (b) lateral recumbency. The cat is restrained gently in a comfortable position with the cuff held at the level of the heart base. In cats, we always use headphones to avoid stress hypertension due to the sounds of the Doppler machine and to improve audibility of the Doppler sounds to the clinician
A standard physical examination was performed that included evaluation of general demeanour, body condition score (BCS) on a nine-point scale (Figure 2), 24 rectal temperature, colour and capillary refill time of the mucous membranes, visual oral inspection, peripheral lymph node palpation, respiratory rate (RR), heart rate (HR), cardiac and pulmonary auscultation (Figure 3), abdominal palpation and thyroid gland palpation (Figure 4). The thyroid glands were palpated as previously described,25,26 using the classic technique 27 and semiquantitative scoring system initially proposed by Norsworthy et al. 28
Figure 2.

Top view of a cat with ideal body condition (body condition score 5/9). The cat is well proportioned and the waist behind the ribs is visible
Figure 3.
Auscultation of the right ventral thorax. Standing behind the cat to restrain it gently in a comfortable position allows auscultation in almost all cats
Figure 4.
Thyroid gland palpation in a cat using the classic technique. The cat is restrained in a sitting position with the neck extended. The clinician’s thumb and forefinger are placed on each side of the trachea and swept downwards from the larynx to the sternal manubrium
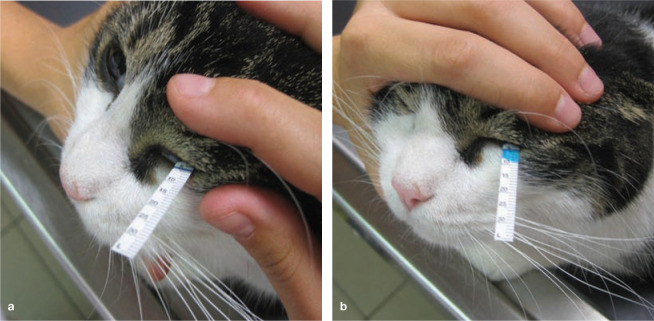
All cats underwent an ophthalmic examination. Tear production was evaluated by placing a STT strip in the ventral conjunctival sac for 60 s (Figure 5) and was recorded in mm/min for both eyes. Afterwards, indirect fundoscopy was performed, paying special attention for signs indicating systemic hypertension, such as retinal oedema, vascular tortuosity, haemorrhage or detachment and papilloedema.
Figure 5.
One of the study cats just after (a) and 60 s after (b) placement of a Schirmer tear test strip in the ventral conjunctival sac of the left eye. The tear production for the left eye is 9 mm/min
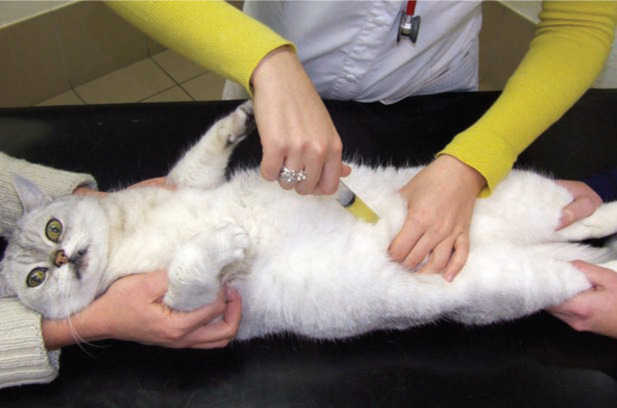
Blood (5 ml) was taken from the jugular vein (Figure 6) and 10 ml of urine was collected by cystocentesis (Figure 7). To obtain serum, the coagulated tubes were centrifuged within 30 mins at 2431 x g. All samples were preserved at 4°C and analysed on the day of collection (Medvet Algemeen Medisch Laboratorium, Antwerp, Belgium).
Figure 6.
Blood sampling from the jugular vein. By keeping the neck and front legs extended, the jugular vein becomes visible and/or easily palpable in most cats
Figure 7.
For cystocentesis the cat is restrained in dorsal recumbency and the bladder is palpated and immobilised during aspiration of urine
A complete blood count (Advia 2120, Siemens) and serum biochemistry profile (Architect C16000, Abbott, Wiesbaden, Germany) were performed and the total thyroxine (TT4) concentration was measured using a previously validated immunoassay (Immulite 2000 system, Siemens). 29 All cats were screened for infection with feline immunodeficiency virus (FIV) and feline leukaemia virus (FeLV) using an in-house test (ELISA, Snap Combo Plus, Idexx Europe).
Urinalysis consisted of a urinary dipstick test, measurement of urine specific gravity (USG) with a manual refractometer, urinary pH and urine protein:creatinine ratio (UPC) (Iricell IQ, Instrumentation Laboratory, Zaventem, Belgium), sediment examination and urine bacterial culture (BioMerieux Media Square, Brussels, Belgium). The sediment was prepared by centrifugation of 5 ml of urine in a conical-tipped tube for 3 mins at 447 x g, decanting the supernatant to leave an equal amount of sediment and urine. This sediment was resuspended by flicking the tube several times and one unstained drop was placed on a clean glass slide and covered with a coverslip. 30 The sediment (cells, casts, crystals) was evaluated under the microscope within 30 mins of collection. Crystalluria was evaluated semiquantitatively and expressed per low-power field (lpf, 10x objective) as mild (<1/lpf), moderate (1–3/lpf) or severe (>3/lpf).
Statistical methods
All statistical analyses were performed with the statistical software package SAS (version 9.2, SAS Institute, USA). Different response variables were compared between two groups (FIV+ versus FIV–, middle-aged versus old cats, normal versus abnormal UPC). The response variables that were normally distributed were analysed by the t-test. For binary response variables, such as UPC (normal = UPC <0.2, abnormal = UPC <0.2), Fisher’s exact test was used. For discrete response variables with more than two outcomes, such as BCS (underweight [BCS <5], ideal [BCS = 5], overweight [BCS >5]), the Wilcoxon rank sum test was used. The Pearson’s correlation coefficient was used to evaluate the correlation between continuous variables. The significance level was set at 0.05.
Results
Study population
One hundred cats, 56 middle-aged and 44 old-aged, were included. The population consisted of 44 males (five intact, 39 neutered) and 56 females (seven intact, 49 neutered). Most (n = 93) were domestic short- or longhair cats; seven were pure breed cats (two British Shorthair, two Ragdoll, one Norwegian Forest cat, one Maine Coon, one Persian). The majority of cats had both indoor and outdoor access (n = 61); some were strictly indoor (n = 19) or strictly outdoor (n = 20) cats. The age and body weight of the total population, and by age group, are presented in Table 1.
Table 1.
Descriptive statistics for cats in the study population (mean ± standard deviation)
| Global population (n = 100) | Group 1 (n = 56) | Group 2 (n = 44) | Unit | |
|---|---|---|---|---|
| Age | 9.9 ± 2.7 | 7.9 ± 1.4 | 12.5 ± 1.5 | Years |
| Body weight | 4.6 ± 1.2 | 4.7 ± 1.2 | 4.4 ± 1.2 | kg |
| SBP | 133.6 ± 21.5 | 128.3 ± 16.7 | 140.4 ± 25.0 | mmHg |
| Heart rate | 191.4 ± 17.4 | 187.6 ± 18.4 | 196.2 ± 14.8 | bpm |
| Respiratory rate | 49.6 ± 16.2 | 48.0 ± 15.9 | 51.6 ± 16.6 | /min |
| Body temperature | 38.2 ± 0.5 | 38.3 ± 0.5 | 38.1 ± 0.5 | °C |
| STT: left eye right eye | 13.4 ± 5.5 14.0 ± 5.7 |
12.8 ± 5.1 14.1 ± 5.7 |
14.2 ± 6.0 14.0 ± 5.9 |
mm/min |
| PCV | 36.8 ± 4.6 | 37.9 ± 4.7 | 35.5 ± 4.2 % | |
| Leukocytes | 9828 ± 4575 | 9162 ± 4048 | 10,677 ± 5091 | /μl |
| Thrombocytes | 293,140 ± 144,685 | 266,179 ± 120,856 | 327,455 ± 165,416 | /μl |
| Sodium | 152.9 ± 1.9 152.9 ± 1.9 |
153.0 ± 1.8 153.0 ± 1.8 |
152.8 ± 2.0 152.8 ± 2.0 l |
mmol/ mEq/l |
| Potassium | 4.42 ± 0.47 4.42 ± 0.47 |
4.4 ± 0.4 4.4 ± 0.4 |
4.5 ± 0.5 4.5 ± 0.5 |
mmol/l mEq/l |
| Total calcium | 2.38 ± 0.11 9.52 ± 0.44 |
2.4 ± 0.1 9.6 ± 0.4 |
2.4 ± 0.1 9.6 ± 0.4 |
mmol/l mg/dl |
| Phosphorus | 1.42 ± 0.26 4.40 ± 0.80 |
1.38 ± 0.22 4.28 ± 0.68 |
1.46 ± 0.30 4.53 ± 0.93 |
mmol/l mg/dl |
| Urea | 9.19 ± 2.35 57.0 ± 14.1 |
9.06 ± 1.70 54.4 ± 10.2 |
10.02 ± 2.90 60.2 ± 17.4 |
mmol/l mg/dl |
| Creatinine | 122.7 ± 30.8 1.35 ± 0.34 |
126.0 ± 26.0 1.39 ± 0.29 |
118.5 ± 35.9 1.30 ± 0.39 |
μmol/l mg/dl |
| Total protein | 73.7 ± 6. 1 7.37 ± 0.61 |
73.2 ± 5.8 7.32 ± 0.58 |
74.4 ± 6.4 7.44 ± 0.64 |
g/l g/dl |
| Albumin | 37.5 ± 3.5 3.75 ± 0.35 |
38.4 ± 3.3 3.84 ± 0.33 |
36.3 ± 3.4 3.63 ± 0.34 |
g/l g/dl |
| Glucose | 5.4 ± 2.1 97.2 ± 37.8 |
5.4 ± 2.1 97.2 ± 37.8 |
5.4 ± 2.2 97.2 ± 39.6 |
mmol/l mg/dl |
| ALT | 40.2 ± 33.9 | 36.0 ± 14.6 | 45.5 ± 48.2 | IU/l |
| AST | 19.7 ± 10.5 | 18.3 ± 8.3 | 21.4 ± 12.7 | IU/l |
| Total thyroxine | 29.1 ± 18.4 2.27 ± 1.44 |
28.0 ± 8.0 2.18 ± 0.62 |
30.6 ± 27.3 2.39 ± 2.13 |
nmol/l μg/dl |
| Urinary pH | 6.7 ± 0.6 | 6.8 ± 0.6 | 6.6 ± 0.4 | |
| USG | 1.046 ± 0.01 | 1.047 ± 0.010 | 1.044 ± 0.012 | |
| UPC | 0.18 ± 0.11 | 0.13 ± 0.05 | 0.21 ± 0.15 | |
Group 1 = middle-aged cats (6–10 years), group 2 = old cats (older than 10 years), n = number of cats, SBP = systolic blood pressure, STT = Schirmer tear test, PCV = packed cell volume, ALT = alanine aminotransferase, AST = aspartate aminotransferase, USG = urinary specific gravity, UPC = urine protein:creatinine ratio
Blood pressure measurement, and physical and ophthalmic examination
The descriptive statistics for the SBP measurements and physical examination findings for the total population, and by age group, are summarised in Table 1.
Eight cats had a mean SBP exceeding 160 mmHg. One of these cats had tachypnoea, which was thought to be stress-related, and one had mild leukocytosis, but none of these cats had hyperglycaemia or glucosuria. No significant correlation between SBP and parameters that could be influenced by stress, namely RR (r = −0.144), HR (r = −0.118), serum glucose concentration (r = −0.127) and total leukocyte count (r = −0.033) was found. SBP ranged from 160–170 mmHg in four cats (two middle-aged, two old cats) and one of these was borderline proteinuric and had a serum creatinine concentration (sCreat) at the upper limit of the RI but with hypersthenuric urine. SBP exceeded 180 mmHg (with a maximum of 237 mmHg) in the other four cats (one middle-aged, three old cats). The middle-aged cat had a grade 3/6 systolic heart murmur and the three old cats were borderline proteinuric (one with USG 1.051 and normal sCreat; one with USG 1.020 and normal sCreat; one with USG 1.027 and mildly increased sCreat). In the other hypertensive cats target organ abnormalities or an underlying cause for the hypertension were not found based on the diagnostic tests carried out in this study.
Forty-nine cats had an ideal BCS, 11 cats (two middle-aged, nine old cats) were too thin (BCS <5) and 40 cats (24 middle-aged, 16 old cats) too heavy (BCS >5). Most cats (n = 96) had a body temperature between 37.5° and 39.3°C. Four old cats had a body temperature below 37.5°C (minimum 37°C). The mucous membranes were moist and pink with normal capillary refill time in all cats. Mild local lymphadenopathy was detected in 34 cats: 32/34 had submandibular and 2/34 popliteal lymphadenopathy. Thirty cats with submandibular lymphadenopathy had gingivitis. In total, 72/100 cats had gingivitis.
Pathological tachycardia (HR >240 beats per minute [bpm]) or bradycardia (HR <140 bpm) was not observed, but 11 cats had stress-related tachypnoea. One cat even exhibited open-mouth breathing initially, but calmed down after a prolonged acclimatisation period, allowing further examinations. Auscultation revealed a cardiac murmur in 11 cats (two middle-aged, nine old cats), all systolic and with the following murmur intensity: grade 1/6 (n = 3), 2/6 (n = 4), 3/6 (n = 2), 4/6 (n = 2). These 11 cats were normotensive, normothermic and had a normal packed cell volume (PCV), but one was hyperthyroid. Cardiac arrhythmias or gallop sounds were not heard. Lung auscultation was normal in 98 cats; two cats had a diffuse mild increase in lung sounds. Abdominal palpation did not reveal significant abnormalities in any of the cats. The majority (n = 92) had a soft abdomen; the others had a tensed but not painful abdomen. A thyroid goitre (score >0) was palpated in 20 cats, with a maximum score of 3 (score 1 in 13/20, score 2 in 4/20, score 3 in 3/20).
Mean tear production was 14.0 ± 5.7 mm/ min for the right eye and 13.5 ± 5.7 mm/min for the left eye. A STT result <5 mm/min was found in both eyes of one cat and in the left eye of another cat. One cat showed mild corneal oedema and one had papillary membrane remnants in both eyes. Fundoscopic abnormalities secondary to systemic hypertension were not found in any of the cats.
Laboratory parameters
The descriptive statistics for the laboratory parameters are presented in Table 1 and the number of animals having laboratory parameters within, below or above the RI in Table 2. The distribution of serum phosphorus concentration of the cats across different categories, based on the 2006 Phosphate Round Table Guidelines, 31 is shown in Table 3.
Table 2.
Distribution of the study population below, within and above the RI for certain laboratory parameters
| Parameter | RI | Below RI | Within RI | Above RI | ||
|---|---|---|---|---|---|---|
| n | Minimum | n | n | Maximum | ||
| PCV | 24–45% | 1 | 20.5% | 95 | 4 | 51.8% |
| Leukocytes | 5000–15,000/µl | 5 | 3950/µl | 82 | 13 | 26,990/µl |
| Platelets | 175,000–500,000/µl | 26 | 22,000/µl | 67 | 8 | 909,000/µl |
| Urea | 6.16–10.98 mmol/l 37–66 mg/dl |
1 | 5.99 mmol/l 36 mg/dl |
84 | 15 | 25.64 mmol/l 154 mg/dl |
| Creatinine | 44.2–132.6 µmol/l 0.49–1.46 mg/dl |
0 | N/A | 71 | 29 | 280.2 µmol/l 3.08 mg/dl |
| Total protein | 53–76 g/l 5.3–7.6 g/dl |
1 | 51 g/l 5.1 g/dl |
69 | 30 | 94 g/l 9.4 g/dl |
| Albumin | 25–45 g/l 2.5–4.5 g/dl |
0 | N/A | 98 | 2 | 45.8 g/l 4.58 g/dl |
| Glucose | 3.05–5.55 mmol/l 54.9–99.9 mg/dl |
0 | N/A | 75 | 25 | 18.59 mmol/l 334.62 mg/dl |
| Sodium | 146–154 mmol/l 146–154 mEq/l |
0 | N/A | 80 | 20 | 159 mmol/l 159 mEq/l |
| Potassium | 3.6–5.3 mmol/l 3.6–5.3 mEq/l |
1 | 3.3 mmol/l | 96 | 3 | 6.3 mmol/l 6.3 mEq/l |
|
Total
calcium |
2.15–2.65 mmol/l 8.6–10.6 mg/dl |
2 | 2.06 mmol/l 8.24 mg/dl |
97 | 1 | 2.66 mmol/l 10.64 mg/dl |
| Phosphorus | 1.35–2.97 mmol/l 4.19–9.20 mg/dl |
40 | 0.87 mmol/l 2.70 mg/dl |
60 | 0 | N/A |
| TT4 | 14.19–45.15 nmol/l 1.1–3.5 µg/dl |
4 | 6.45 nmol/l 0.50 µg/dl |
93 | 3 | >193.5 nmol/l 15.09 µg/dl |
| ALT | <70 lU/l | 0 | N/A | 94 | 6 | 333 lU/l |
| AST | <42 lU/l | 0 | N/A | 98 | 2 | 88 lU/l |
| GGT | <4 lU/l | 0 | N/A | 100 | 0 | N/A |
| Bilirubin | <6.8 µmol/l <0.40 mg/dl |
0 | N/A | 99 | 1 | 14.11 µmol/l 0.83 mg/dl |
RI = reference interval, n = number of cats, minimum = minimum value observed for this laboratory parameter, maximum = maximum value observed for this laboratory parameter, PCV = packed cell volume, TT4 = total thyroxine concentration, ALT = alanine aminotransferase, AST = aspartate aminotransferase, GGT =γ-glutamyl transpeptidase, N/A = not applicable
Table 3.
Distribution of the study population across four different categories for serum phosphorus concentration*
| Global population (n = 100) | Group 1 (n = 56) | Group 2 (n = 44) | |
|---|---|---|---|
| Phosphorus 0.81 1.45 mmol/l | 56 | 33 (58.9%) | 23 (52.3%) |
| Phosphorus 1.45 1.61 mmol/l | 24 | 14 (25%) | 10 (22.7%) |
| Phosphorus 1.61 1.94 mmol/l | 18 | 9 (16.1%) | 9 (20.5%) |
| Phosphorus >1.94 mmol/l | 2 | 0 (0%) | 2 (4.5%) |
The differential leukocyte count of all five leukopenic cats revealed a decrease in mature neutrophils only. Of the 13 cats with a leukocytosis, none had a left shift or a total leukocyte count exceeding the expected value for cats with steroid leukograms (<30,000/µl). 32 The majority of cats with thrombocytopenia (18/26) showed platelet aggregates on their blood smear, suggesting pseudothrombocytopenia.
Of the 29 cats with sCreat above the RI, two had isosthenuric urine and six a USG between 1.015 and 1.035, six were borderline proteinuric (UPC 0.2–0.4), two were hypertensive, seven had an increased serum urea concentration (sUrea) and one a decreased TT4 concentration. The others had a USG above 1.035, were non-proteinuric (UPC <0.2) and normotensive, and had sUrea and TT4 concentration within the RIs. Both cats with isosthenuric urine were old cats (9 and 13 years) and non-proteinuric. They were diagnosed with chronic kidney disease (CKD) IRIS stage 2 and stage 3, respectively.
Serum glucose exceeded 10 mmol/l in 3/25 cats with hyperglycaemia. Two of these and one other cat (glucose 9.6 mmol/l) also had glucosuria.
The increase in alanine aminotransferase (ALT) was mild (<1.5 times the upper limit of the RI) in 5/6 cats, but severe in one cat (almost five times the upper limit of the RI). This cat (13 years old) was hyperthyroid and also the only cat with a significant elevation in aspartate aminotransferase (twice the upper limit of the RI). The bilirubin concentration was increased in only one cat (11 years). This cat also had a very mildly increased ALT without increase of other liver enzymes and a decreased PCV.
Of the three cats with increased TT4, the concentration was severely elevated in one old cat and just above the upper limit of the RI in two middle-aged cats (6 and 9 years). The hyperthyroid cat and 1/2 cats with mildly increased TT4 had a palpable thyroid gland (score 1).
Fourteen cats were FIV positive (eight middle-aged, six old cats); no cats were FeLV positive. Three FIV positive cats were hypertensive and FIV positive cats had a significantly higher SBP (mean 145.9 ± 29.8 mmHg) than FIV negative cats (mean 131.6 ± 19.4 mmHg; P = 0.02). Three of five cats with leukopenia were FIV positive, and FIV positive cats had significantly lower total leukocyte counts (mean 6899 ± 2068 cells/µl) than FIV negative cats (mean 10,305 ± 4699 cells/µl; P = 0.009).
Urinalysis was performed in all cats except one, which had an empty bladder. Fifteen cats had a USG below 1.035, with isosthenuria detected in three (one middle-aged, two old cats). More detailed information regarding USG is given in Table 4. Urinary pH ranged from 5.1–7.5 in 91/99 cats. The remaining eight had a pH >7.5 (maximum 9) and one of these had a positive urine culture.
Table 4.
Distribution of the study population across three categories for USG
| Global population (n = 99) | Group 1 (n = 56) | Group 2 (n = 43) | |
|---|---|---|---|
| USG <1.035 | 15 | 6 (10.7%) | 9 (20.9%) |
| USG 1.035–1.040 | 8 | 7 (12.5%) | 1 (2.3%) |
| USG >1.040 | 76 | 43 (76.8%) | 33 (76.7%) |
For definitions of group 1, group 2 and n, see Table 1. USG = urine specific gravity
The distribution of cats in proteinuria categories according to the ACVIM consensus statement 33 is shown in Table 5. Of the 27 cats with an abnormal UPC (UPC >0.2), none had overt renal failure, isosthenuria or macroscopic haematuria; four were hypertensive; five had USG <1.035; six an increased sCreat; and the amount of urinary crystals did not differ significantly from cats with UPC <0.2. Of the 25 cats with borderline proteinuria, three had microscopic haematuria and one a positive urine culture. Both cats with UPC >0.4 had normal SBP and normal sUrea and sCreat. One had microscopic haematuria and the other was hyperthyroid.
Table 5.
Distribution of the study population across three categories for proteinuria*
| Global population (n = 99) | Group 1 (n = 56) | Group 2 (n = 43) | |
|---|---|---|---|
| UPC <0.2 | 72 | 50 (89.3%) | 22 (51.2%) |
| UPC 0.2–0.4 | 25 | 6 (10.7%) | 19 (44.2%) |
| UPC >0.4 | 2 | 0 (0%) | 2 (4.7%) |
Casts were not detected on urinary sediment analysis. Crystalluria was present in 41/99 cats and was mild in 28/41, moderate in 8/41 and severe in 5/41. Amorphous crystals were mostly (33/41) detected, with struvite crystals in 5/41 and calcium oxalate crystals in 3/41 cats. For cats with crystalluria, 17/41 mainly received dry food, 1/41 mainly canned food and 15/41 a combination of dry and canned food; for 8/41 the owners did not specify the diet type. For cats without crystalluria, 20/58 mainly received dry food, 2/58 mainly canned food, 20/58 a combination of dry and canned food, and 2/58 a combination of dry and table food; for 12/58 the owners did not specify the diet type. The distribution of the diet type did not significantly differ between cats with and without crystalluria and between cats with different types of urinary crystals.
On urinary dipstick examination a trace of ketonuria, glucose positivity and a trace of urobilinogen were each present in 3/99 cats, 28/99 cats were haemoglobin positive, 98/99 cats were leukocyte esterase positive and no cats were bilirubin or nitrites positive. Only one cat had a positive urine bacterial culture, with an Enterococcus species identified. This was a 9-year-old, intact female, mixed-breed cat that had serum glucose, TT4, sUrea and sCreat within the RIs, with alkaline urine, mild pyuria and moderately concentrated urine (USG 1.020).
Underweight cats
Of the 11 underweight cats, one was diagnosed with CKD IRIS stage 3, one with hyperthyroidism, and one was FIV positive. The sCreat, sUrea, TT4 concentration and UPC did not significantly differ between the three BCS categories (underweight, ideal and overweight). However, underweight cats had a significantly lower USG (mean 1.038 ± 0.016) compared with cats with an ideal BCS (mean 1.045 ± 0.011; P = 0.037) and overweight cats (mean 1.048 ± 0.008; P = 0.007).
Discussion
This study is the first to describe health screening results in apparently healthy cats. The study population was balanced for sex and age, and the breed distribution reflected the general cat population in Belgium. Domestic short- and longhair cats have been the predominant breeds also in other clinical studies performed in Belgium. 34
The mean SBP of our population was 133.6 ± 21.5 mmHg, which is very similar to two earlier reports (mean SBP 133.6 ± 16 mmHg and 131.1 ± 17.8 mmHg).11,35 These studies also evaluated healthy conscious client-owned cats with the indirect Doppler technique, but the sampled cats had a wider age distribution. Eight of our cats had a mean SBP that exceeded the cut-off value above which further diagnostics are advised (160 mmHg).7,11,22 We tried to limit the white-coat effect by measuring the SBP in the presence of the owner after acclimatisation in a quiet room and before performing the physical examination. 36 However, white-coat hypertension cannot be excluded in our cats because the SBP was only measured on a single occasion. 21 SBP above 180 mmHg, as recorded in four of our cats, is less likely to reflect white-coat hypertension.7,36 Also, the SBP did not correlate with other physical and laboratory parameters that can be influenced by stress.
An obvious underlying cause for the hypertension in our cats was not found. Further work-up was advised but declined by the owners. Several cats were borderline proteinuric or had moderately concentrated urine, which can be consequences of hypertension or indicative of early renal insufficiency.21,37 Assessment of the glomerular filtration rate could be helpful in such cats to diagnose non-azotaemic kidney disease, 37 but this was outside the scope of the present study. Several of our hypertensive cats were FIV positive and these cats had a significantly higher SBP than FIV negative cats. In human medicine, seropositivity for human immunodeficiency virus is a known risk factor for hypertension, cardiovascular disease and nephropathy.38–40 To the best of our knowledge, an association between hypertension and FIV infection is not reported and further studies are needed to elucidate if this is an incidental finding or if FIV positive cats are at risk of hypertension.
Based on BCS, which is useful for assessing the body fat percentage of pet cats, 41 less than half of this apparently healthy cat population had an ideal body condition. This indicates that cat owners do not appreciate under- or overweight as a problem. 42 Improved owner awareness of normal feline body condition, and regular nutritional assessments by veterinarians, are important to increase the proportion of cats with an optimal body condition. 43 Forty per cent of our cats were too heavy, which is comparable to a recent UK study (39%) 42 and somewhat higher than in a recent French study (27%). 44 However, all three studies confirm that pet cats are commonly overweight or obese. As reported in another study, 42 underweight cats were significantly older than cats with an ideal or overweight body condition. This may be explained by reduced fat and protein digestion with age in cats. 45 Only a few of our underweight cats were diagnosed with a condition that could explain weight loss. However, we must also consider that our underweight cats could have had occult systemic disease. The significantly lower USG might indicate decreased renal function, at least in some underweight cats. Assessment of the glomerular filtration rate in underweight cats with poorly concentrated urine would have helped identify cats with non-azotaemic kidney disease.
Forty percent of cats were too heavy, underlining the necessity for improved owner awareness of normal feline body condition and regular nutritional assessments by veterinarians.
The most common abnormalities on physical examination were gingivitis, submandibular lymphadenopathy and a cardiac murmur. The majority of our cats (72%) showed gingivitis, which is very similar to the 73.2% prevalence of gingivitis found by Verhaert and Van Wetter in a large cat population. 46 Both studies took place in the same geographic area (Flanders, Belgium) and in both studies few owners (in the present study none) brushed their cats’ teeth. Because we only performed visual oral inspection on conscious cats, we cannot comment on the number of cats with periodontitis, stomatitis or odontoclastic resorptive lesions. The presence of gingivitis did lead to mild submandibular lymphadenopathy in many of our cats.
A heart murmur was auscultated in 11% of our cats, which is lower than the 21% murmur prevalence recorded in a young to middle-aged healthy domestic cat population. 47 In both studies, all murmurs were systolic with an intensity between 1/6 and 4/6. Differences in murmur prevalence between studies may reflect differences in study populations (eg, age), interobserver variation or geographic differences. 47 One of our cats with a murmur was hyperthyroid, but in 10 cats there was no evidence of a systemic condition that could explain the murmur. In these cats the murmur could be caused by subclinical structural heart disease, which has been a common finding in other studies that evaluated apparently healthy cats with a murmur.47–49 Although echocardiography is strongly recommended in healthy cats with a murmur,47–49 clinicians need to be aware that the presence of a murmur has only poor sensitivity for detecting cardiomyopathy in cats. 35
Several euthyroid cats had a palpable goitre. The maximum score of 3 is in line with other studies in which euthyroid cats mostly had small thyroid gland nodules.25,26,28
The mean STT results in the present study are very comparable with the STT results reported for young adult and middle-aged normal cats.12–14 In contrast to dogs, 15 we did not detect decreased tear production with increasing age. Only two cats had a STT <5 mm/min in one or both eyes, which could be consistent with keratoconjunctivitis sicca. 50
Major findings on blood examination were leukocytosis, thrombocytopenia, increased sUrea, increased sCreat, hyperproteinaemia, hyperglycaemia, hypernatraemia and hypophosphataemia. The leukocytosis and hyperglycaemia were probably stress related and the thrombocytopenia was probably pseudothrombocytopenia in the majority of cases. The 25% of cats with hyperglycaemia is lower than recently published percentages for ill cats at admission to an emergency service (40%) 51 or during hospitalisation (64%). 52 For the other laboratory parameters where many cats were outside the RI (Table 2), some of the abnormal values could truly be the result of occult disease, but we should also bear in mind that the RI may not be appropriate.
One of the most striking findings was the increased sCreat in almost one third of cats. Although some cats could have had early CKD, the increase was probably not clinically relevant in the majority of cases because most cats only had a mildly increased sCreat and hypersthenuric urine. The most likely explanation for the high proportion of cats with an increased sCreat is the laboratory RI. RIs for sCreat can vary markedly between laboratories, influencing the classification of samples as normal or abnormal.53,54 Finally, some cats could have had an increased creatinine production rate, resulting in mild azotaemia despite normal renal function. 55
Many cats also had mild hyperproteinaemia, hypophosphataemia or hypernatraemia, which questions the appropriateness of these RIs.
Also many cats had mild hyperproteinaemia, hypophosphataemia or hypernatraemia, which questions the appropriateness of these RIs. To avoid misinterpretation of clinical data, RIs need to reflect the population for which they are used. 56 The laboratory that analysed our samples used healthy young cats to calculate RIs, which is not representative for our study population. An obvious example of an inappropriate RI in our study is the serum phosphorus concentration. In the veterinary literature, typically the RI for serum phosphorus concentration in cats is between 0.81 and 1.94 mmol/l.57,58 In contrast, the lower limit of our RI was 1.35 mmol/l, which resulted in a high proportion (40%) of cats with hypophosphataemia. All but two of our cats had serum phosphorus within the RI if the published RI is used (Table 3). In addition, the majority of our cats had a serum phosphorus concentration in the lower half of the published RI and many of these cats were incorrectly classified as hypophosphataemic. This indicates the need to calculate age-dependent RIs to improve the interpretation of laboratory parameters in all age categories.
We found a 14% FIV seroprevalence. Recent FIV seroprevalences vary greatly (2.5–33.9%), depending on area and the cat population studied.59–65 Our cats were all client-owned cats living in and around Ghent. Urban stray cats in the same area had a seroprevalence of 11.2%. 66 This means that the FIV seroprevalence has not decreased despite stray cat programmess having been running for years in this area. In contrast, FeLV antigen was detected in 3.8% of stray cats, 66 but not in our cats. Possible explanations are different study populations (stray versus client-owned cats) and different ages, as FeLV mainly affects young cats. 61
On urinalysis, borderline proteinuria and crystalluria were common. The majority of cats with borderline proteinuria (except one with a positive culture) had an inactive sediment, and the amount of crystals was similar in cats with a normal (<0.2) versus abnormal (≥0.2) UPC. As the urine was collected by cystocentesis, this borderline proteinuria was probably of renal origin. 33 Although none of these cats showed overt renal failure, early CKD cannot be ruled out. Borderline proteinuria could also be associated with occult or subclinical systemic disease because further diagnostics were not performed in any of the cats.67,68 Finally, because the UPC was measured only once, we cannot determine if these cats were transiently or persistently borderline proteinuric. 33 As in Whittemore et al’s study, 68 an age effect on the degree of urinary protein excretion was found, with a significantly higher UPC in old versus middle-aged cats. Although older cats may be more prone to early CKD or occult systemic disease, the 0.2 cut-off value may also not be appropriate for UPC evaluation in old cats. Further research to explain borderline proteinuria in old cats is definitely warranted.
Crystalluria was detected in almost half of our cats and was mostly mild and caused by amorphous crystals. All types of crystals that we detected (amorphous, struvite, calcium oxalate) can occur in normal urine samples. Although it is generally accepted that crystals are commonly present in feline urine, 69 we are not aware of scientific studies assessing how often crystalluria affects client-owned healthy cats. Although we cannot rule out urolithiasis, the crystalluria was probably non-pathogenic in the majority because none of our cats had lower urinary tract signs. Veterinarians should be aware that crystalluria is common in healthy cats and not a sufficient reason to start feeding a calculolytic diet. In a previous study in specific pathogen-free cats, struvite crystals were found more commonly in cats fed a mixed diet compared with cats fed solely canned food. 70 This contrasts with the lack of association between diet type and the presence or type of urinary crystals in our study. However, the absence of an association must be interpreted cautiously because dietary information was not available for several of our cats and because we only found low number of cats with anything other than amorphous crystals.
Another remarkable finding on urinalysis was the positive leukocyte-esterase dipstick test in all but one of the cats. This confirms that this test is non-specific and cannot replace microscopic urine sediment examination. 71
One cat was diagnosed with an occult bacterial urinary tract infection (UTI). Spontaneous bacterial UTIs occur most frequently in older cats,72–74 mainly because common metabolic diseases such as CKD, diabetes mellitus and hyperthyroidism predispose to UTIs.72,73 However, a recent study found that cats of all ages were equally affected by UTIs. 75 Cats with UTIs often do not show lower urinary tract signs (ie, occult bacterial UTI).72,73,75,76 A recent study revealed that occult bacterial UTIs particularly affect older female cats, mostly involve Enterococcus faecalis and can occur in cats without a history or clinical signs of a predisposing disease, as was the case in our cat. 77 Decreased USG, which was present in our cat, was recently found to be associated with UTI in cats. 75
Comparison of age groups revealed significant differences for several parameters. A wider spread of UPC values in old versus middle-aged cats was obvious. For the other parameters, there was moderate to substantial overlap between both groups, which limits the clinical relevance of these differences. The significantly higher SBP, HR and murmur frequency in the old cats may be consequences of cardiovascular changes that occur with ageing. 19
Summary
Based on the screening we performed in 100 cats, we diagnosed FIV infection in 14 cats, CKD in two cats, hyperthyroidism in one cat and a UTI in one cat. In addition, several cats had a suboptimal SBP or BCS, gingivitis, heart murmur or laboratory abnormalities (eg, increased sCreat, increased bilirubin, increased ALT, increased TT4, borderline proteinuria, glucosuria) for which further diagnostic investigation, treatment and/or follow-up were indicated. A minor limitation was that our cats were only evaluated at a single time point. Also further diagnostic tests to explain abnormal SBP, physical examination and laboratory parameters were not always performed, although recommended to the owners.
Nevertheless, it can be concluded that physical examination and laboratory abnormalities are common in apparently healthy older cats, which emphasises the need for regular health checks and age-dependent laboratory RIs.
Acknowledgments
This study was presented in part at the 21st ECVIM-CA congress in Seville, Spain, in September 2011, and published as an abstract in the 21st ECVIM-CA Congress Proceedings (pages 210–211) and in J Vet Intern Med (2011; 25: 1499). Special thanks go to MSD Animal Health, Boxmeer, the Netherlands, for financial support for the study and to Dr Linda Horspool for her assistance with the manuscript. We are also particularly grateful to all owners of the participating cats, as they have made this study possible.
Footnotes
Funding: This work was supported by MSD Animal Health, Boxmeer, the Netherlands.
The authors declare that there is no conflict of interest.
Date accepted: 17 September 2012
Key Points
This study clearly indicates the need for and value of regular health checks of apparently healthy cats to improve early disease detection and allow early therapeutic intervention.
This health screening should involve a comprehensive history and thorough physical examination, including BCS assessment and oral inspection.
Because most relevant abnormal laboratory findings were observed in middle-aged (group 2) cats (except FIV seropositivity), we recommend FIV/FeLV testing in middle-aged cats with outdoor access and complete blood and urine examinations in old cats (above 10 years).
Our findings support the widely accepted recommendation of monitoring the BP of cats that are ?10 years of age.
To improve the interpretation of geriatric screening, small animal laboratories should make efforts to develop age-dependent RIs for certain parameters.
Further research is warranted to examine the clinical significance of proteinuria in the borderline range.
References
- 1. Gunn-Moore DA. Considering older cats. J Small Anim Pract 2006; 47: 430–431. [DOI] [PubMed] [Google Scholar]
- 2. Feline Advisory Bureau. WellCat for life. A guide to engaging your clients in a lifelong partnership. In: WellCat veterinary handbook. 1st ed. Tisbury: FAB, 2008, pp 1–30. [Google Scholar]
- 3. Senior Care Guidelines Task Force, AAHA, Epstein M, Kuehn NF, Landsberg G, Lascelles BD, Marks SL, et al. AAHA senior care guidelines for dogs and cats. J Am Anim Hosp Assoc 2005; 41: 81–91. [DOI] [PubMed] [Google Scholar]
- 4. Pittari J, Rodan I, Beekman G, Gunn-Moore D, Polzin D, Taboada D, et al. American Association of Feline Practitioners: senior care guidelines. J Feline Med Surg 2009; 11: 763–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dowling PM. Geriatric pharmacology. Vet Clin North Am Small Anim Pract 2005; 35: 557–569. [DOI] [PubMed] [Google Scholar]
- 6. Gunn RG, Alleman AR. Clinical pathology in veterinary geriatrics. Vet Clin North Am Small Anim Pract 2005; 35: 537–556. [DOI] [PubMed] [Google Scholar]
- 7. Stepien RL. Feline systemic hypertension. Diagnosis and management. J Feline Med Surg 2011; 13: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bodey AR, Sansom J. Epidemiological study of blood pressure in domestic cats. J Small Anim Pract 1998; 39: 567–573. [DOI] [PubMed] [Google Scholar]
- 9. Sparkes AH, Caney SM, King MC, Gruffydd-Jones TJ. Inter- and intraindividual variation in Doppler ultrasonic indirect blood pressure measurements in healthy cats. J Vet Intern Med 1999; 13: 314–318. [DOI] [PubMed] [Google Scholar]
- 10. Sansom J, Rogers K, Wood JLN. Blood pressure assessment in healthy cats and cats with hypertensive retinopathy. Am J Vet Res 2004; 65: 245–252. [DOI] [PubMed] [Google Scholar]
- 11. Lin CH, Yan CJ, Lien YH, Huang HP. Systolic blood pressure of clinically normal and conscious cats determined by an indirect Doppler method in a clinical setting. J Vet Med Sci 2006; 68: 827–832. [DOI] [PubMed] [Google Scholar]
- 12. Margadant DL, Kirkby K, Andrew SE, Gelatt KN. Effect of topical tropicamide on tear production as measured by Schirmer’s tear test in normal dogs and cats. Vet Ophthalmol 2003; 6: 315–320. [DOI] [PubMed] [Google Scholar]
- 13. Cullen CL, Lim C, Sykes J. Tear film breakup times in young healthy cats before and after anesthesia. Vet Ophthalmol 2005; 8: 159–165. [DOI] [PubMed] [Google Scholar]
- 14. Ghaffari MS, Malmasi A, Bokaie S. Effect of acepromazine or xylazine on tear production as measured by Schirmer tear test in normal cats. Vet Ophthalmol 2010; 13: 1–3. [DOI] [PubMed] [Google Scholar]
- 15. Hartley C, Willimans DL, Adams VJ. Effect of age, gender, weight, and time of day on tear production in normal dogs. Vet Ophthalmol 2006; 9: 53–57. [DOI] [PubMed] [Google Scholar]
- 16. Metzger FL. Senior and geriatric care programs for veterinarians. Vet Clin North Am Small Anim Pract 2005; 35: 743–753. [DOI] [PubMed] [Google Scholar]
- 17. Vogt AH, Rodan I, Brown M, Brown S, Buffington CAT, Forman MJL, et al. AAFP–AAHA Feline life stage guidelines. J Feline Med Surg 2010; 12: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simpson KE, Gunn-Moore DA, Shaw DJ, French AT, Dukes-McEwan J, Moran CM, et al. Pulsed-wave Doppler tissue imaging velocities in normal geriatric cats and geriatric cats with primary or systemic diseases linked to specific cardiomyopathies in humans, and the influence of age and heart rate upon these velocities. J Feline Med Surg 2009; 11: 293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carpenter RE, Pettifer GR, Tranquilli WJ. Anesthesia for geriatric patients. Vet Clin North Am Small Anim Pract 2005; 35: 571–580. [DOI] [PubMed] [Google Scholar]
- 20. Caney S. Weight loss in the elderly cat. Appetite is fine, and everything looks normal… J Feline Med Surg 2009; 11: 738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown S, Atkins C, Bagley R, Carr A, Cowgill L, Davidson M, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007; 21: 542–558. [DOI] [PubMed] [Google Scholar]
- 22. Stepien RL. Pathophysiology of systemic hypertension and blood pressure assessment. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 7th ed. Missouri: Elsevier Saunders, 2010, pp 577–582. [Google Scholar]
- 23. Waddell LS. Hypotension. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 7th ed. Missouri: Elsevier Saunders, 2010, pp 585–588. [Google Scholar]
- 24. Laflamme DP. Development and validation of a body condition score system for cats: a clinical tool. Feline Pract 1997; 25: 13–18. [Google Scholar]
- 25. Paepe D, Smets P, van Hoek I, Saunders J, Duchateau L, Daminet S. Within- and between-examiner agreement for two thyroid palpation techniques in healthy and hyperthyroid cats. J Feline Med Surg 2008; 10: 558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boretti FS, Sieber-Ruckstuhl NS, Gerber B, Laluha P, Baumgartner C, Lutz H, et al. Thyroid enlargement and its relationship to clinicopathological parameters and T4 status in suspected hyperthyroid cats. J Feline Med Surg 2009; 11: 286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feldman EC, Nelson RW. Feline hyperthyroidism (thyrotoxicosis). In: Feldman EC, Nelson RW. (eds). Canine and feline endocrinology and reproduction. 3rd ed. St Louis: Elsevier Saunders, 2004, pp 152–218. [Google Scholar]
- 28. Norsworthy GD, Adams VJ, McElhaney MR, Milios JA. Relationship between semi-quantitative thyroid palpation and total thyroxine concentration in cats with and without hyperthyroidism. J Feline Med Surg 2002; 4: 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singh AK, Jiang Y, White T, Spassova D. Validation of non-radioactive chemiluminescent immunoassay methods for the analysis of thyroxine and cortisol in blood samples obtained from dogs, cats, and horses. J Vet Diagn Invest 1997; 9: 261–268. [DOI] [PubMed] [Google Scholar]
- 30. Meyer DJ. Microscopic examination of the urinary sediment. In: Raskin RE, Meyer DJ. (eds). Atlas of canine and feline cytology. 1st ed. Philadelphia: WB Saunders, 2001, pp 261–276. [Google Scholar]
- 31. Elliott J. Hyperphosphataemia and chronic kidney disease – outcomes of the 2006 round table in Louisville, KY (USA). In: State of the art in renal disease in cats and dogs, Congress Proceedings; 2007 Dec 2–4; Nice, France, pp 12–17. [Google Scholar]
- 32. Stockham SL, Scott MA. (eds). Leukocytes. In: Fundamentals of veterinary clinical pathology. 2nd ed. Oxford: Blackwell Publishing, 2008, pp 53–106. [Google Scholar]
- 33. Lees GE, Brown SA, Elliott J, Grauer GE, Vaden SL; American College of Veterinary Internal Medicine. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM forum consensus statement (small animal). J Vet Intern Med 2005; 19: 377–385. [DOI] [PubMed] [Google Scholar]
- 34. Defauw PAM, Van de Maele I, Duchateau L, Polis IE, Saunders JH, Daminet S. Risk factors and clinical presentation of cats with feline idiopathic cystitis. J Feline Med Surg 2011; 13: 967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paige CF, Abbott JA, Elvinger F, Pyle RL. Prevalence of cardiomyopathy in apparently healthy cats. J Am Vet Med Assoc 2009; 234: 1398–1403. [DOI] [PubMed] [Google Scholar]
- 36. Belew AM, Barlett T, Brown SA. Evaluation of the white-coat effect in cats. J Vet Intern Med 1999; 13: 134–142. [DOI] [PubMed] [Google Scholar]
- 37. Jepson R. Feline systemic hypertension. Classification and pathogenesis. J Feline Med Surg 2011; 13: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weiner NJ, Goodman JW, Kimmel PL. The HIV-associated renal diseases: current insight into pathogenesis and treatment. Kidney Int 2003; 63: 1618–1631. [DOI] [PubMed] [Google Scholar]
- 39. Jung O, Bickel M, Ditting T, Rickerts V, Welk T, Helm EB, et al. Hypertension in HIV-1-infected patients and its impact on renal and cardiovascular integrity. Nephrol Dial Transplant 2004; 19: 2250–2258. [DOI] [PubMed] [Google Scholar]
- 40. Bloomfield GS, Hogan JW, Keter A, Sang E, Carter EJ, Velazquez EJ, et al. Hypertension and obesity as cardiovascular risk factors among HIV seropositive patients in Western Kenya. PloS ONE 2011; 6: e22288. DOI: 10.1371/journal.pone.0022288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bjornvad CR, Nielsen DH, Armstrong PJ, McEvoy F, Hoelmkjaer KM, Jensen KS, et al. Evaluation of a nine-point body condition scoring system in physically inactive pet cats. Am J Vet Res 2011; 72: 433–437. [DOI] [PubMed] [Google Scholar]
- 42. Courcier EA, O’Higgins R, Mellor DJ, Yam PS. Prevalence and risk factors for feline obesity in a first opinion practice in Glasgow, Scotland. J Feline Med Surg 2010; 12: 746–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Freeman L, Becvarova I, Cave N, MacKay C, Nguyen P, Rama B, et al. WSAVA nutritional assessment guidelines. J Feline Med Surg 2011; 13: 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Colliard L, Paragon BM, Lemuet B, Bénet JJ, Blanchard G. Prevalence and risk factors of obesity in an urban population of healthy cats. J Feline Med Surg 2009; 11: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Laflamme DP. Nutrition for aging cats and dogs and the importance of body condition. Vet Clin North Am Small Anim Pract 2005; 35: 713–742. [DOI] [PubMed] [Google Scholar]
- 46. Verhaert L, Van Wetter C. Survey of oral diseases in cats in Flanders. Vlaams Diergen Tijds 2004; 73: 331–341. [Google Scholar]
- 47. Côté E, Manning AM, Emerson D, Laste NJ, Malakoff RL, Harpster NK. Assessment of the prevalence of heart murmurs in overtly healthy cats. J Am Vet Med Assoc 2004; 225: 384–388. [DOI] [PubMed] [Google Scholar]
- 48. Dirven MJ, Cornelissen JM, Barendse MA, van Mook MC, Sterenborg JA. Cause of heart murmurs in 57 apparently healthy cats. Tijdschr Diergeneeskd 2010; 135: 840–847. [PubMed] [Google Scholar]
- 49. Nakamura RK, Rishniw M, King MK, Sammarco CD. Prevalence of echocardiographic evidence of cardiac disease in apparently healthy cats with murmurs. J Feline Med Surg 2011; 13: 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moore CP. Keratoconjunctivitis sicca. In: Bonagura JD. (ed). Kirk’s current veterinary therapy. 13th ed. Philadelphia: WB Saunders, 2000, pp 1061–1066. [Google Scholar]
- 51. Chan DL, Freeman LM, Rozanski EA, Rush JE. Prevalence of hyperglycemia in cats presented to the emergency service. J Vet Emerg Crit Care 2002; 12: 199. [Google Scholar]
- 52. Ray CC, Callahan-Clark J, Beckel NF, Walters PC. The prevalence and significance of hyperglycemia in hospitalized cats. J Vet Emerg Crit Care 2009; 19: 347–351. [DOI] [PubMed] [Google Scholar]
- 53. Boozer L, Cartier L, Heldon S, et al. Lack of utility of laboratory ‘normal’ ranges for serum creatinine concentration for the diagnosis of feline chronic renal insufficiency. J Vet Intern Med 2002; 16: 354. [Google Scholar]
- 54. Ulleberg T, Robben J, Nordahl KM, Ulleberg T, Heiene R. Plasma creatinine in dogs: intra- and inter-laboratory variation in 10 European veterinary laboratories. Acta Vet Scand 2011; 53: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Le Garreres A, Laroute V, De La Farge F, Boudet KG, Lefebvre HP. Disposition of plasma creatinine in non-azotaemic and moderately azotaemic cats. J Feline Med Surg 2007; 9: 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Friedrichs KR. Reference intervals: an essential, expanding, and occasionally equivocal standard. Vet Clin Pathol 2010; 39: 131–132. [DOI] [PubMed] [Google Scholar]
- 57. Bates JA. Phosphorus: a quick reference. Vet Clin North Am Small Anim Pract 2008; 38: 471–475. [DOI] [PubMed] [Google Scholar]
- 58. Kidder A, Chew D. Treatment options for hyperphosphatemia in feline CKD. What’s out there? J Feline Med Surg 2009; 11: 913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Levy JK, Scott HM, Lachtara JL, Crawford PC. Seroprevalence of feline leukemia virus and feline immuno deficiency virus infection among cats in North America and risk factors for seropositivity. J Am Vet Med Assoc 2006; 228: 371–376. [DOI] [PubMed] [Google Scholar]
- 60. Norris JM, Bell ET, Hales L, Toribio JA, White JD, Wigney DI, et al. Prevalence of feline immunodeficiency virus infection in domesticated and feral cats in eastern Australia. J Feline Med Surg 2007; 9: 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gleich SE, Krieger S, Hartmann K. Prevalence of feline immunodeficiency virus and feline leukaemia virus among client-owned cats and risk factors for infection in Germany. J Feline Med Surg 2009; 11: 985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Little S, Sears W, Lachtara J, Bienzle D. Seroprevalence of feline leukemia virus and feline immunodeficiency virus infection among cats in Canada. Can Vet J 2009; 50: 644–648. [PMC free article] [PubMed] [Google Scholar]
- 63. Duarte A, Castro I, Pereira da Fonseca IM, Almeida V, Madeira de Carvalho LM, Meireles J, et al. Survey of infectious and parasitic diseases in stray cats at the Lisbon Metropolitan Area, Portugal. J Feline Med Surg 2010; 12: 441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nakamura Y, Nakamura Y, Ura A, Hirata M, Sakuma M, Sakata Y, et al. An updated nation-wide epidemiological survey of feline immunodeficiency virus (FIV) infection in Japan. J Vet Med Sci 2010; 72: 1051–1056. [DOI] [PubMed] [Google Scholar]
- 65. Al-Kappany YM, Lappin MR, Kwok OCH, Abu-Elwafa SA, Hilali M, Dubey JP. Seroprevalence of Toxoplasma gondii and concurrent Bartonella spp, feline immunodeficiency virus, feline leukemia virus and Dirofilaria immitis infections in Egyptian cats. J Parasitol 2011; 97: 256–258. [DOI] [PubMed] [Google Scholar]
- 66. Dorny P, Speybroeck N, Verstraete S, Baeke M, De Becker A, Berkvens D, et al. Serological survey of Toxoplasma gondii, feline immunodeficiency virus and feline leukaemia virus in urban stray cats in Belgium. Vet Rec 2002; 151: 626–629. [DOI] [PubMed] [Google Scholar]
- 67. Mardell EJ, Sparkes AH. Evaluation of a commercial in-house test kit for the semi-quantitative assessment of microalbuminuria in cats. J Feline Med Surg 2006; 8: 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Whittemore JC, Miyoshi Z, Jensen WA, Radecki SV, Lappin MR. Association of microalbuminuria and the urine albumin-to-creatinine ratio with systemic diseases in cats. J Am Vet Med Assoc 2007; 230: 1165–1169. [DOI] [PubMed] [Google Scholar]
- 69. DiBartola SP. Clinical approach and laboratory evaluation of renal disease. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 7th ed. Missouri: Elsevier Saunders, 2010, pp 1955–1969. [Google Scholar]
- 70. Sturgess CP, Hesford A, Owen H, Privett R. An investigation into the effects of storage on the diagnosis of crystalluria in cats. J Feline Med Surg 2001; 3: 81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Holan KM, Kruger JM, Gibbons SN, Swenson CL. Clinical evaluation of a leukocyte esterase test-strip for detection of feline pyuria. Vet Clin Pathol 1997; 26: 126–131. [DOI] [PubMed] [Google Scholar]
- 72. Mayer-Roenne B, Goldstein RE, Erb HN. Urinary tract infections in cats with hyperthyroidism, diabetes mellitus and chronic kidney disease. J Feline Med Surg 2007; 9: 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bailiff NL, Westropp JL, Nelson RW, Sykes JE, Owens SD, Kass PH. Evaluation of urine specific gravity and urine sediment as risk factors for urinary tract infections in cats. Vet Clin Pathol 2008; 37: 317–322. [DOI] [PubMed] [Google Scholar]
- 74. Lekcharoensuk C, Osborne CA, Lulich JP. Epidemiologic study of risk factors for lower urinary tract diseases in cats. J Am Vet Med Assoc 2001; 218: 1429–1435. [DOI] [PubMed] [Google Scholar]
- 75. Martinez-Ruzafa I, Kruger JM, Miller RA, Swenson CL, Bolin CA, Kaneene JB. Clinical features and risk factors for development of urinary tract infections in cats. J Feline Med Surg 2012; 14: 729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bailiff NL, Nelson RW, Feldman EC, Westropp JL, Ling GV, Jang SS, et al. Frequency and risk factors for urinary tract infection in cats with diabetes mellitus. J Vet Intern Med 2006; 20: 850–855. [DOI] [PubMed] [Google Scholar]
- 77. Litster A, Moss S, Platell J, Trott DJ. Occult bacterial lower urinary tract infections in cats – urinalysis and culture findings. Vet Microbiol 2009; 136: 130–134. [DOI] [PubMed] [Google Scholar]