Abstract
Practical relevance:
Many veterinary practices have invested in quality automated hematology instruments for use in-house. However, regardless of the specific choice of analyzer, there are important hematology findings that can only be determined by microscopic examination of stained blood films. For this reason, and also for the purpose of quality control for the analyzer, a quick blood film review should be performed alongside every automated complete blood count. Even those practices that submit their blood samples to outside diagnostic laboratories for evaluation, still require the capability to examine stained blood films in emergency situations.
Series outline:
This is the first of a two-part article series that aims to familiarize the practitioner with normal findings on feline blood films, with a particular focus on unique features in the cat, as well as to assist with interpretation of common abnormalities. Part 1 focuses on how to prepare and examine blood films in order to maximize the reliability of the information they convey, and describes the morphology of feline erythrocytes in health and disease.
Evidence base:
The information and guidance offered is based on the published literature and the author’s own extensive clinical pathology research.
Blood film examination – we have a hematology analyzer so why the need?
Many private veterinary practices have quality automated hematology instruments in-house that rapidly provide complete blood count (CBC) results. Some analyzers provide automated leukocyte differential results, but others require a manual differential count to be performed using a stained blood film. Regardless of the analyzer used, there are important additional hematology findings that can only be determined by microscopic examination of stained blood films.1–3 These include:
Erythrocyte abnormalities – abnormal shapes, inclusions and parasites;
Leukocyte abnormalities – neutrophilic left shifts and hypersegmentation, neutrophil cytoplasmic toxicity, abnormal granules, inclusions and infectious agents;
Platelet abnormalities – megaplatelets and platelet aggregates;
Miscellaneous cells – for example, presence of nucleated erythrocytes, neoplastic cells and mast cells;
Platelet numbers – most automated hematology analyzers cannot perform platelet counts in cats because there is an overlap in size between feline platelets and erythrocytes. Consequently, platelet numbers must generally be estimated from a stained blood film, or a manual count must be performed.
The scanning of stained blood films also provides a measure of quality control for the hematology analyzer (see box). For this, and all of the above reasons, a quick blood film review should be performed alongside each automated CBC.

Some veterinary practices submit their blood samples to outside diagnostic laboratories for evaluation, but these practices nevertheless require the capability to examine stained blood films in emergency situations. Hematocrits can be determined by microhematocrit centrifugation, and leukocyte and platelet counts can be estimated from blood films.
Blood film preparation
Blood films should be prepared as soon as possible after blood sample collection to minimize artefactual changes that can distort blood cell morphology.
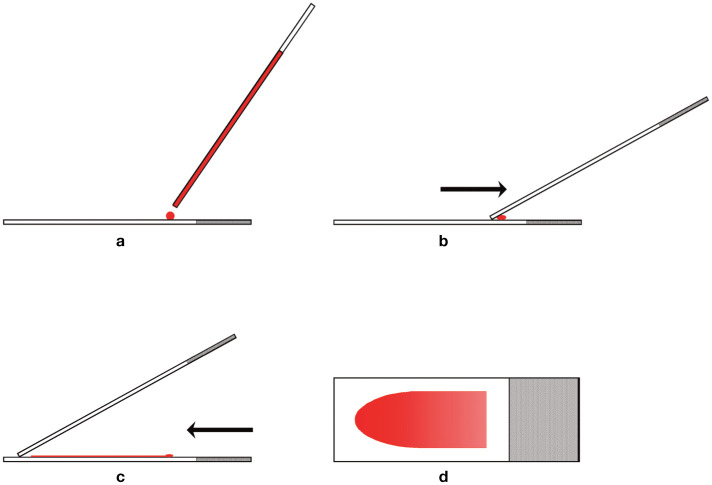
The most common method of preparation is the wedge method, which requires two clean glass slides (Figure 1). 1 The blood sample is mixed either using a blood tube rotator or by hand for at least 1 min, and a microhematocrit tube is used to collect blood from the vacuum tube by capillary action. A glass slide is placed on a flat surface and a small drop of blood from the hematocrit tube is placed on one end of the slide. This slide is held in place with one hand and a second glass slide (spreader slide) is placed on the first slide and held between the thumb and forefinger with the other hand at about a 30–45° angle in front of the drop of blood. The spreader slide is then backed into the drop of blood, and as soon as the blood flows along the back side of the spreader slide, the spreader slide is rapidly pushed forward.
Figure 1.
Blood film preparation. (a) A glass slide is placed on a flat surface and a small drop of well-mixed blood is placed on one end of the slide using a microhematocrit tube. (b) A second glass slide (spreader slide) is placed on the first slide at about a 30–45° angle in front of the drop of blood. The spreader slide is then backed into the drop of blood. (c) As soon as the blood flows along the back edge of the spreader slide, the spreader slide is rapidly pushed forward. (d) The blood film produced is thick at the back of the slide, where the drop of blood was placed, and thin at the front (feathered) edge of the slide. Reproduced from Harvey (2012), 1 with permission
The thickness of the smear depends on a number of factors, including the size of the drop of blood, the viscosity of the sample (which is greater as the hematocrit increases), the angle of the spreader slide and the speed of spreading. A greater angle and faster speed of spreading results in thicker and shorter blood films. 8 The drop of blood should be of sufficient size to prepare a smear that occupies at least half the length of the slide. Large cells and clumps of cells tend to be pushed to the front (feathered) end of the smear. If the drop of blood is too large, some of the blood will be pushed off the end of the slide, and large cells and clumps of cells will not be available for examination.
Once prepared, the slide is immediately dried by waving it in the air or by holding it in front of a hair dryer set on a warm (not hot) setting.
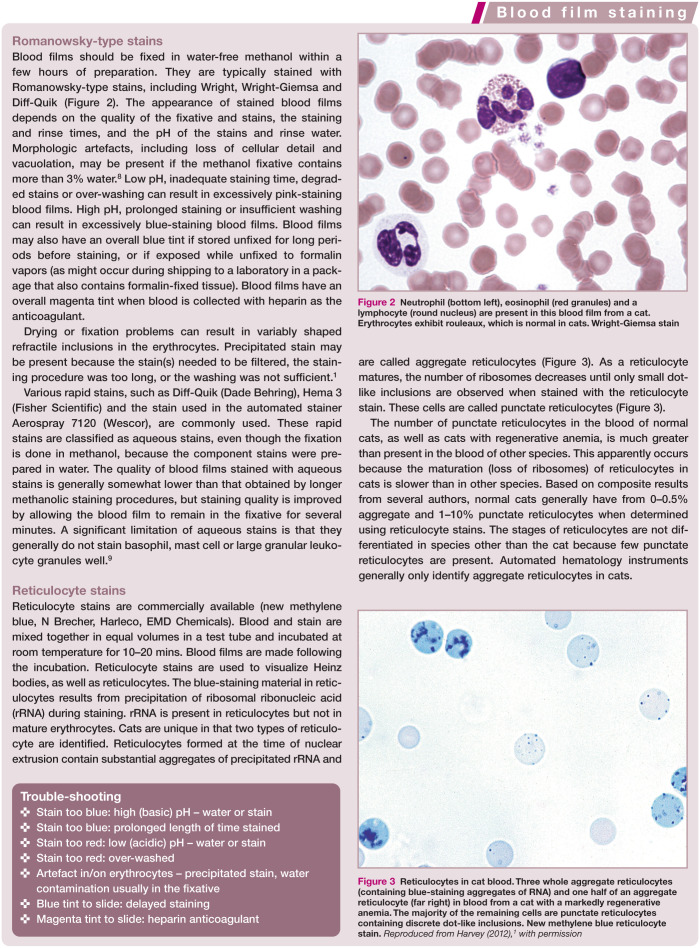
Figure 3.
Reticulocytes in cat blood. Three whole aggregate reticulocytes (containing blue-staining aggregates of RNA) and one half of an aggregate reticulocyte (far right) in blood from a cat with a markedly regenerative anemia. The majority of the remaining cells are punctate reticulocytes containing discrete dot-like inclusions. New methylene blue reticulocyte stain. Reproduced from Harvey (2012), 1 with permission
Method to examine stained blood films
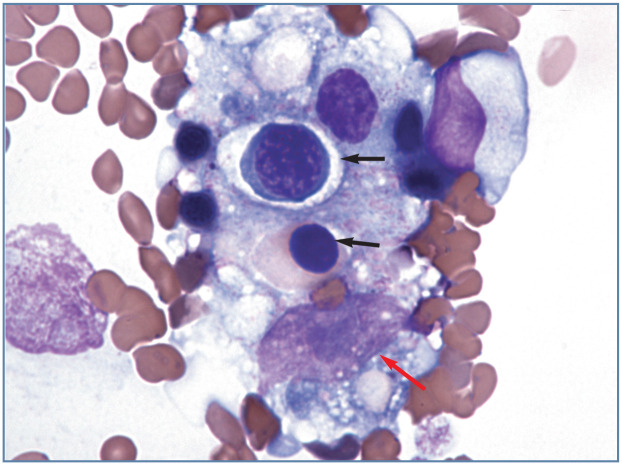
Initial low-power survey Stained blood films should first be scanned using a low-power objective to look for the presence of erythrocyte autoagglutination, leukocyte aggregates, platelet aggregates, microfilaria and abnormal cells that might be missed during the differential leukocyte count. It is particularly important that the feathered end of blood films made on glass slides is examined because large cells and aggregates of cells (especially platelets) may be concentrated in this area (Figure 4).
Leukocyte numbers As a quality control measure if using a hematology analyzer, the number of leukocytes present should be estimated to assure that the number present on the slide is consistent with the total leukocyte count measured. If a x 10 ocular and a x 10 objective are used (x 100 magnification), the total leukocyte count in blood (cells / μl) may be estimated by determining the average number of leukocytes present per field and multiplying by 100. If a x 20 objective is used, the total leukocyte count may be estimated by multiplying the average number of leukocytes per field by 400. The correction factor used may vary, depending on the microscope used. Manual differential leukocyte counts are inherently imprecise, so it is also important to scan the blood film to gain an appreciation of the relative distribution of leukocyte types present prior to performing the differential leukocyte count.
Differential leukocyte count A differential leukocyte count is performed by identifying 200 consecutive leukocytes using a x 40 or x 50 objective. Because monocytes and other large leukocytes are pushed to the sides and ends of wedge-prepared blood films, differential counts are performed by examining cells in a pattern that evaluates both the edges and the center of the smear. 8 After the count is complete, the percentage of each leukocyte type present is calculated and multiplied by the total leukocyte count to determine the absolute number of each cell type present per microliter of blood. it is the absolute number of each leukocyte type that is important. Relative values (percentages) can be misleading when the total leukocyte count is abnormal.
Nucleated red blood cells Nucleated red blood cells (NRBCs) are counted along with the leukocytes by most hematology analyzers. When encountered during blood film examination, the number of NRBCs per 100 leukocytes should be tabulated and the total leukocyte count should be corrected for the number of NRBCs present, unless the instrument can differentiate between NRBCs and lymphocytes, and exclude NRBCs from the total leukocyte count.
Leukocyte morphology The presence of abnormal leukocyte morphology, such as toxic cytoplasm in neutrophils or increased (eg, >5%) reactive lymphocytes, should be recorded on the hematology report form. The frequency of toxic neutrophils is reported as few (5–10%), moderate (11–30%) or many (>30%), and the severity of toxic change is recorded as 1+ to 3+ (Table 1).1,10
Erythrocyte morphology Erythrocyte morphology should be examined and recorded as either normal or abnormal. Observations regarding erythrocyte morphology, such as the degree of polychromasia (presence of polychromatophilic erythrocytes), anisocytosis (variation in size) and poikilocytosis (abnormal shape), should also be made. Abnormal erythrocyte shapes should be classified as specifically as possible, because specific shape abnormalities may help to determine the nature of a disorder that is present. The number of abnormal cells should be reported in a semiquantitative fashion, using a system such as that shown in Table 2. 10
Platelet numbers Platelet numbers should be estimated as being low, normal or increased. Blood smears from cats normally average between 10 and 30 platelets per high power field when examined using a x 10 ocular and x 100 objective (x 1000 magnification). An estimate of the number of platelets / μl of blood may be obtained by multiplying the average number per field by 15,000 to 20,000. If a thrombocytopenia is suspected, it should be confirmed with a platelet count. The presence of platelet aggregates and abnormal platelet morphology (large or hypogranular platelets) should also be recorded on the hematology form.
Figure 4.
Aggregate of cells, which appears to contain a macrophage with phagocytized nucleated erythrocytes (black arrows), located at the feathered end of a blood film from a cat with Mycoplasma haemofelis infection. The large oval magenta structure near the bottom of the image (red arrow) is the nucleus of the macrophage. The nucleus contains a blue-staining nucleolus. Wright-Giemsa stain
Table 1.
Semiquantitative evaluation of toxic changes in the cytoplasm of neutrophils
| Neutrophils with toxic change | |
| Few | 5–10% |
| Moderate | 11–30% |
| Many | >30% |
| Severity of toxic change in cytoplasm | |
| Döhle bodies* | 1+ |
| Mildly basophilic | 1+ |
| Moderately basophilic with Döhle bodies | 2+ |
| Moderately basophilic and foamy | 2+ |
| Dark blue–gray and foamy† | 3+ |
| Basophilic with toxic granules† (not reported in cats) | 3+ |
One or two Döhle bodies are sometimes seen in a few neutrophils from cats that do not exhibit signs of illness
May also contain Döhle bodies
From Harvey (2012), 1 with permission
Table 2.
Semiquantitative evaluation of erythrocyte morphology based on average number of abnormal cells per x 1000 microscopic monolayer field*
| 1+ | 2+ | 3+ | 4+ | |
|---|---|---|---|---|
| Anisocytosis | 5–8 | 9–15 | 16–20 | >20 |
| Polychromasia | 1–2 | 3–8 | 9–15 | >15 |
| Hypochromasia | 1–10 | 11–50 | 51–200 | >200 |
| Poikilocytosis | 3–10 | 11–50 | 51–200 | >200 |
A monolayer field is defined as a field in which erythrocytes are close together, with approximately one-half touching each other. In severely anemic animals, such monolayers may not be present. When erythrocytes are generally not touching (eg, tend to be separated by the distance of one cell diameter), then the number of erythrocytes with morphologic abnormalities is counted for two fields From Weiss (1984), 10 with permission
Erythrocyte morphology – normal and abnormal
Rouleaux
Like horses, blood films from healthy cats often exhibit rouleaux (aggregations of erythrocytes resembling stacks of coins; Figure 2). 1 Rouleaux formation depends on both the nature of the erythrocytes and the composition of plasma. Although difficult to evaluate in cats, rouleaux formation is further increased when plasma globulins are increased, as occurs with inflammation and plasma cell neoplasia. Prominent rouleaux formation results in rapid erythrocyte sedimentation in whole blood samples allowed to stand undisturbed. Consequently, cat blood must be well mixed before hematology measurements are made.
Figure 2.
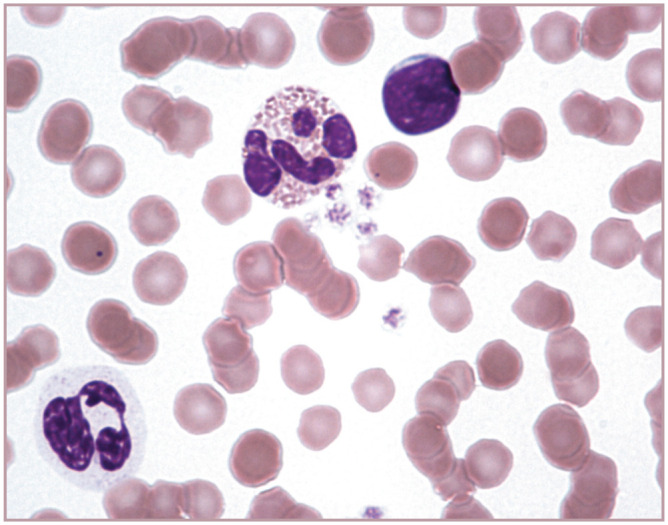
Neutrophil (bottom left), eosinophil (red granules) and a lymphocyte (round nucleus) are present in this blood film from a cat. Erythrocytes exhibit rouleaux, which is normal in cats. Wright-Giemsa stain
Agglutination
The aggregation of erythrocytes together in clusters (not in stacks of coins as in rouleaux) is termed agglutination (Figure 5). Agglutination is caused by the presence of immunoglobulins bound to erythrocyte surfaces. It may be observed in association with both primary and secondary (eg, Mycoplasma haemofelis infection) immune-mediated hemolytic anemia. 1
Figure 5.
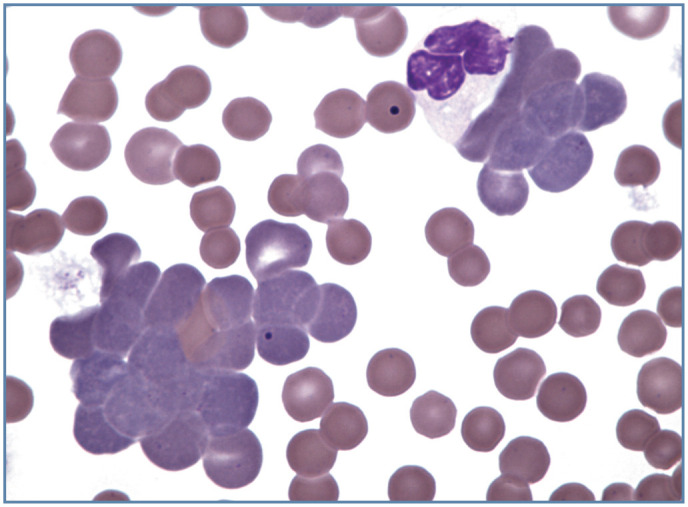
Agglutination of polychromatophilic erythrocytes (aggregate reticulocytes) in blood from a cat with immune-mediated hemolytic anemia. Many mature erythrocytes appear to be spherocytes. Wright-Giemsa stain
Polychromasia
Aggregate reticulocytes in cats appear as polychromatophilic (bluish-red) erythrocytes in routinely stained blood films (Figures 5 and 6). 11 This coloration results from the presence of blue-staining RNA in cells that are synthesizing red-staining hemoglobin. Slight polychromasia may be present in normal cats, but normal cats may also exhibit no polychromasia in stained blood films. Punctate reticulocytes in cats do not contain sufficient RNA for these cells to appear bluish. The presence of increased polychromasia in cat blood films indicates that an aggregate reticulocytosis is present, as expected in response to hemorrhage or increased erythrocyte destruction (hemolysis).
Figure 6.
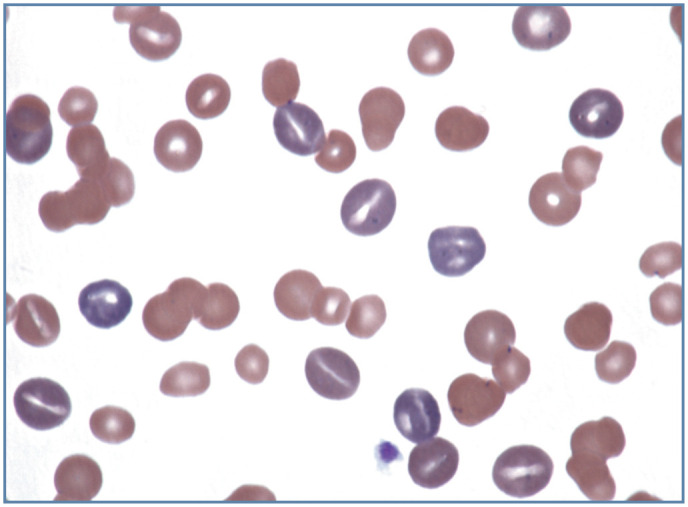
Increased polychromasia and anisocytosis in a Mycoplasma haemofelis-infected cat with a regenerative anemia. Most polychromatophilic erythrocytes have a stomatocyte appearance. Wright-Giemsa stain
Anisocytosis
Anisocytosis refers to the variation in erythrocyte diameters in stained blood films. Increased anisocytosis may be observed when substantial numbers of larger than normal cells are present, as occurs when increased numbers of reticulocytes are produced. Consequently, increased anisocytosis is often a feature of regenerative anemia (Figure 6). However, large erythrocytes may also be produced during dyserythropoiesis in some cats with non-regenerative anemia (Figure 7).
Figure 7.
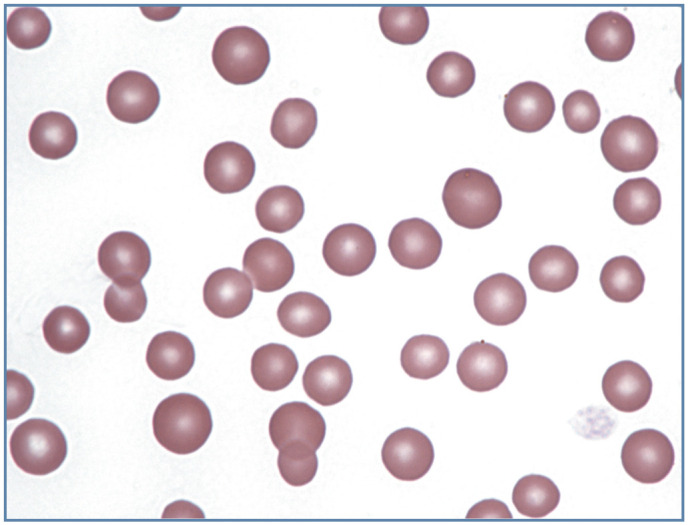
Increased anisocytosis without polychromasia in blood from a feline immunodeficiency virus-infected cat with a non-regenerative anemia. Wright-Giemsa stain
Increased anisocytosis may also be seen when substantial numbers of smaller than normal cells are present, as commonly occurs in iron deficiency anemia in dogs secondarily to chronic blood loss. With the exception of nursing kittens, iron deficiency anemia is rarely recognized in cats (Figure 8). 1
Figure 8.
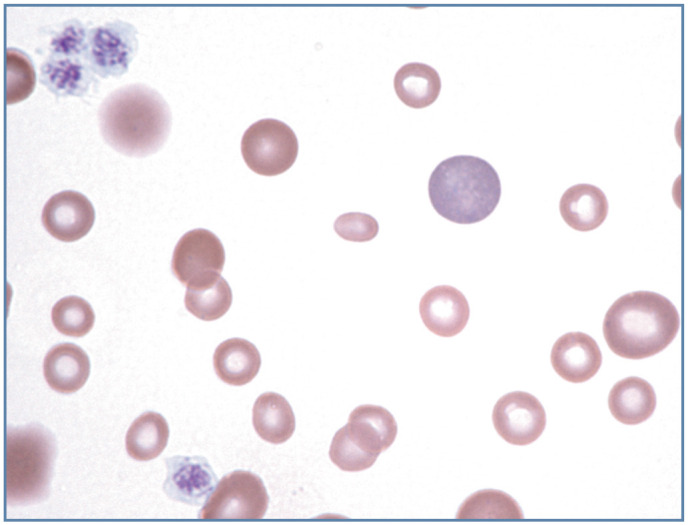
Marked anisocytosis in blood from a 6-week-old kitten after a blood transfusion. The kitten presented with severe iron deficiency anemia and marked lipemia. The small, hypochromic erythrocytes are from the kitten, and the larger erythrocytes are from the blood donor cat. A large polychromatophilic erythrocyte and two lysed erythrocytes (red smudges on the left side of the image) are present. The lipemia promoted erythrocyte lysis during blood film preparation. Wright-Giemsa stain
Hypochromasia
Hypochromasia refers to the presence of erythrocytes with decreased hemoglobin concentration and increased central pallor. Increased hypochromasia is commonly observed in dogs with chronic iron deficiency anemia. With the exception of severe iron deficiency anemia in kittens (Figure 8), hypochromasia is generally not apparent in iron-deficient cats. 1
Erythrocyte shapes
Cat erythrocytes are anucleated biconcave discs (discocytes) that are smaller than dog erythrocytes, and similar in size to horse erythrocytes. The biconcavity of feline erythrocytes is less pronounced than that of their canine counterparts, resulting in less central pallor of erythrocytes in feline blood films compared with canine blood films.
Poikilocytosis
The general term used to describe the presence of abnormally shaped erythrocytes is poikilocytosis (Figure 9). Although specific terminology is used for certain abnormal shapes, it may be less important to quantify each type of shape change than it is to determine the cause of the shape change. Poikilocyte formation occurs in various disorders associated with erythrocyte fragmentation including disseminated intravascular coagulation (DIC), liver disease, cardiomyopathy, myeloid neoplasms, renal failure and doxorubicin toxicity.1,12–14 Poikilocytes can also form when oxidant injury results in Heinz body formation and / or membrane injury (eccentrocytes).
Figure 9.
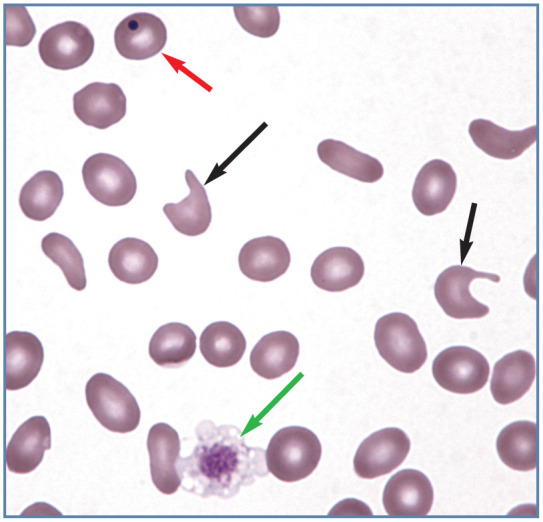
Poikilocytosis in blood from an anemic cat with a portosystemic shunt. Multiple elliptocytes (ovalocytes) and two keratocytes with ruptured vacuoles (black arrows) are present. A discocyte containing a Howell-Jolly body (red arrow) and a large platelet (green arrow) are also present. Wright-Giemsa stain
Echinocytes
Spiculated erythrocytes in which the spicules are relatively evenly spaced and of similar size are classified as echinocytes. When observed in stained blood films, echinocytes are usually an artefact resulting from excess EDTA, improper blood film preparation, or prolonged sample storage before blood film preparation. Echinocytosis (previously referred to as crenation) has been reported in uremic cats (Figure 10a). 15
Figure 10.
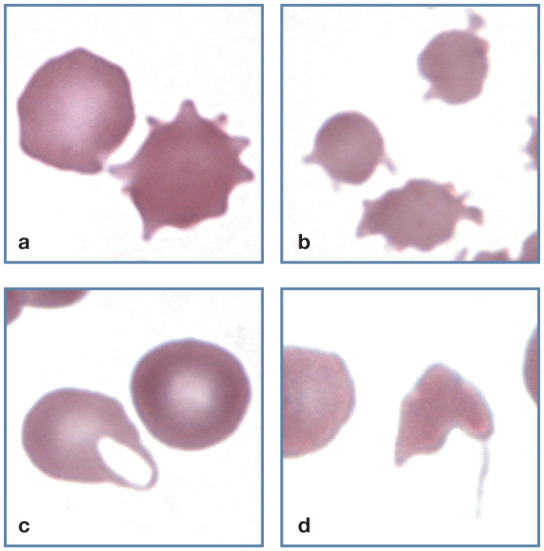
Abnormal erythrocyte shapes. (a) A normal discocyte (left) and an echinocyte (right) in blood from a cat with uremia. (b) Three acanthocytes in blood from a cat with hepatic lipidosis. (c) A keratocyte with intact vacuole (left) and a discocyte (right) in blood from a cat with a portosystemic shunt. (d) A schistocyte in blood from a cat with diabetes mellitus, interstitial lung disease and erythrocyte fragmentation. Wright-Giemsa stain
Acanthocytes
When spiculated erythrocytes have irregularly spaced, variably sized spicules they are classified as acanthocytes (Figure 10b). They have been recognized in cats with liver disease and in cats with disorders that result in erythrocyte fragmentation such as DIC and congestive heart failure.12,14,16
Keratocytes
Erythrocytes containing what appear to be one or more intact or ruptured vesicles are called keratocytes (Figure 10c). The rupture of a vesicle results in the formation of one or two projections. Keratocytes have been observed in various disorders in cats, including liver disease, portosystemic shunts, DIC, heart disease and doxorubicin toxicity.12,13,16,17
Schistocytes
Erythrocyte fragments with pointed extremities are called schistocytes (or schizocytes) (Figure l0d). They are smaller than normal discocytes. Schistocytes have been described in cats with liver disease, DIC, heart disease and doxorubicin toxicity.12,13,16,18
Spherocytes
Spherical erythrocytes that result from cell swelling and/or loss of cell membrane are referred to as spherocytes (Figure 11a). Spherocytes lack central pallor and have smaller diameters than normal on stained blood films. By comparison, flattened erythrocytes present in the feathered end of blood films lack central pallor, but have large diameters.
Figure 11.
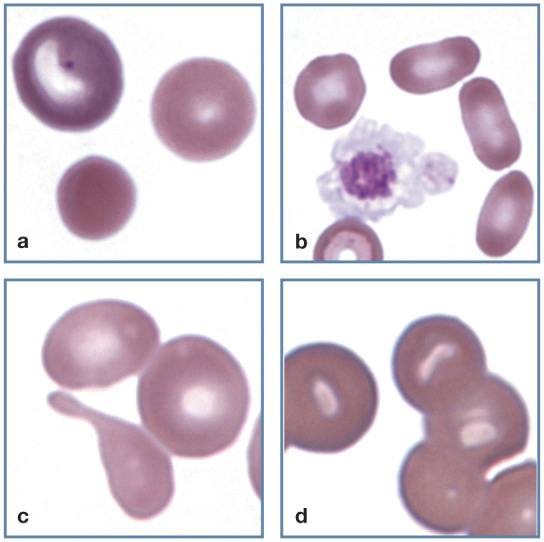
Abnormal erythrocyte shapes. (a) A polychromatophilic erythrocyte (top left), discocyte (top right) and spherocyte (bottom) in blood from a cat with immunemediated hemolytic anemia. (b) Three elliptocytes (right) in blood from a cat with a portosystemic shunt. A large activated platelet (center) with aggregated granules that may be confused with a nucleus is also present. (c) A tear dropshaped dacryocyte appears below two discocytes, each of which contains a pale, barely visible Heinz body in blood from a cat with a portosystemic shunt. (d) Stomatocytes in the thick portion of a feline blood film. This shape was not present in thinner areas of the film. Wright-Giemsa stain
Spherocytes are difficult to recognize with certainty in cats because of the small size and limited central pallor of feline erythrocytes. Consequently, a description of spherocytosis should be assigned cautiously in cats, particularly when being used as a criterion for diagnosing immune-mediated hemolytic anemia (Figure 5). Other potential causes of spherocyte formation include erythrocyte parasites, oxidant damage and transfusion of stored blood. Microspherocytes may result from erythrocyte fragmentation. 1
Elliptocytes
Erythrocytes that are elliptical or oval in shape are classified as elliptocytes (or ovalocytes). They have been recognized in cats with myeloid neoplasms, hepatic lipidosis, portosystemic shunts (Figure 11b) and doxorubicin toxicity.12,13,16,17
Dacryocytes
Tear-drop shaped erythrocytes are called dacryocytes (Figure 11c). They are commonly seen in people with myelofibrosis, but not in animals. They have been observed in cats with portosystemic shunts and myeloid neoplasia. 1
Stomatocytes
Cup-shaped erythrocytes that have oval or elongated areas of central pallor when viewed in stained blood films are called stomatocytes (Figure 11d). They generally occur as artefacts in thick blood film preparations in cats. 1
Eccentrocytes
Eccentrocytes form when oxidant damage to erythrocyte membranes results in the adhesion of opposing areas of the cytoplasmic faces of the membrane. As a result, hemoglobin is eccentrically localized to part of the cell, leaving a hemoglobin-poor area visible in the remainder of the cell (Figure 12a). Eccentrocytes have often been recognized in dogs secondarily to exogenous (eg, onions) and endogenous (eg, ketoacidotic diabetes) oxidants. Eccentrocytes may be present in cats with oxidant damage resulting from acetaminophen administration. 1
Figure 12.
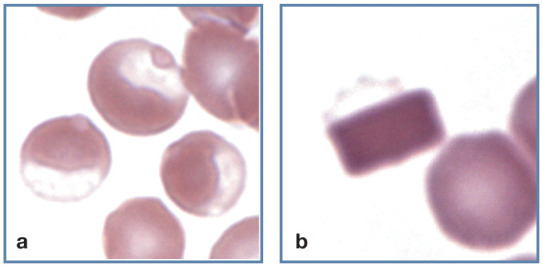
Abnormal erythrocyte shapes. (a) Three eccentrocytes in blood from a cat with acetaminophen toxicity. Pale Heinz bodies are also visible in two of the eccentrocytes (left and top). (b) A hemoglobin crystal is present within an erythrocyte. The faintly stained cell membrane (top) is visible. Wright-Giemsa stain
Hemoglobin crystals
Crystallized hemoglobin is occasionally observed within erythrocytes in blood films from cats (Figure 12b). Crystals may be eccentrically located in the cells and resemble eccentrocytes. No hemoglobin abnormalities have been recognized by hemoglobin electrophoresis in cats with hemoglobin crystals. 1
Nucleated erythrocytes
Low numbers of rubricytes and metarubricytes (see box) occasionally occur in the blood of normal cats. When present in blood these cells are usually described simply as NRBCs. Increased numbers of NRBCs are often seen in blood in association with regenerative anemia (Figure 13); however, their presence does not necessarily indicate a regenerative response. Nucleated erythrocytes may be seen in cats with lead poisoning, with or without anemia, 19 and in non-anemic conditions in which bone marrow is damaged, such as septicemia, endotoxic shock and hypoxia, as well as associated with certain drug administrations. 20
Figure 13.
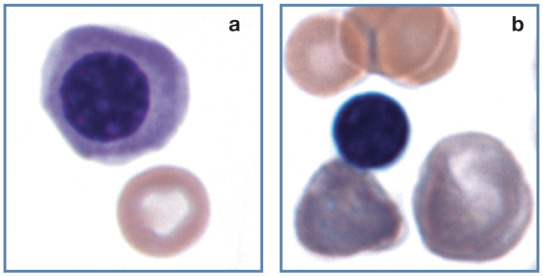
Nucleated erythrocytes. (a) Polychromatophilic rubricyte and discocyte in blood from a cat. (b) Metarubricyte with nuclear extrusion (left), a polychromatophilic erythrocyte (right) and discocytes (top) in blood from a 3-week-old kitten with a regenerative response to anemia. Wright-Giemsa stain
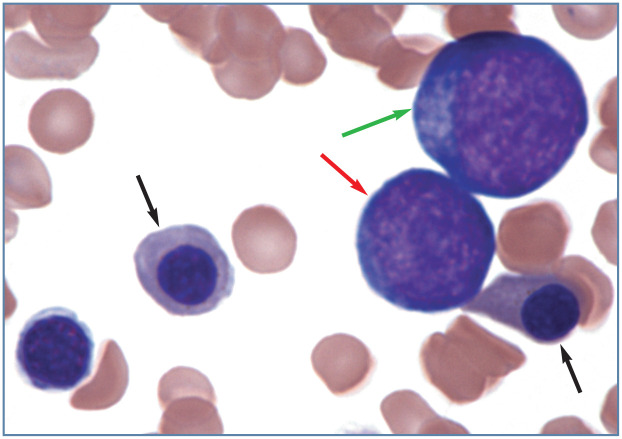
When nucleated erythrocyte precursors are frequent in the blood of an animal with nonregenerative anemia, conditions including myelodysplastic syndrome, erythroleukemia, infiltrative marrow disease and impaired splenic function should be considered. The presence of rubriblasts and prorubricytes in blood from an animal with non-regenerative anemia strongly suggests that a myeloid neoplasm is present (Figure 14). Nucleated erythrocytes may also be excessively large and erythrocyte nuclei may be lobulated or fragmented in cats with myeloid neoplasms. 1
Figure 14.
Two metarubricytes (black arrows), a prorubricyte (red arrow) and a rubriblast (green arrow) in blood from a cat with erythroleukemia (AML-M6). Wright-Giemsa stain
Erythrocyte inclusions
Howell-Jolly bodies
Howell-Jolly bodies (micronuclei) are small, spherical nuclear remnants that appear dark blue when stained with routine Romanowsky-type blood stains (Figure 15). They form in the bone marrow, following nuclear fragmentation or rupture of the nuclear membrane, with some nuclear material left behind when the metarubricyte nucleus is expelled. In contrast to dogs, Howell-Jolly bodies are often present in low numbers in normal cat blood. These inclusions are removed or ‘pitted’ by the spleen as erythrocytes squeeze through interendothelial slits of the splenic sinus. Their presence in cat blood results from a poor pitting function of the feline spleen. Howell-Jolly bodies are often increased in regenerative anemias. 1
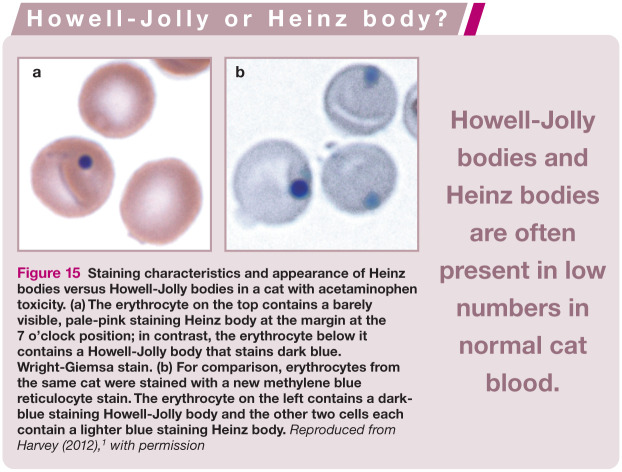
Figure 15.
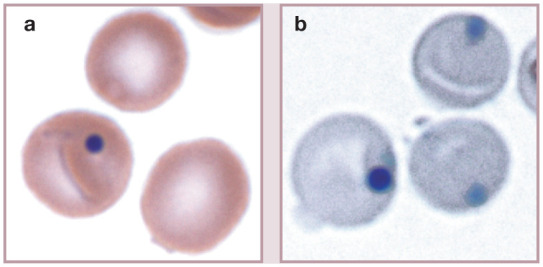
Staining characteristics and appearance of Heinz bodies versus Howell-Jolly bodies in a cat with acetaminophen toxicity. (a) The erythrocyte on the top contains a barely visible, pale-pink staining Heinz body at the margin at the 7 o’clock position; in contrast, the erythrocyte below it contains a Howell-Jolly body that stains dark blue. Wright-Giemsa stain. (b) For comparison, erythrocytes from the same cat were stained with a new methylene blue reticulocyte stain. The erythrocyte on the left contains a dark-blue staining Howell-Jolly body and the other two cells each contain a lighter blue staining Heinz body. Reproduced from Harvey (2012), 1 with permission
Heinz bodies
Heinz bodies are inclusions composed of oxidized, precipitated hemoglobin. They attach to the internal surfaces of erythrocyte membranes. Heinz bodies stain red to pale pink with Romanowsky-type stains, in contrast to Howell-Jolly bodies, which stain dark blue (Figure 15). Heinz bodies appear lighter blue than Howell-Jolly bodies when stained with reticulocyte stains. If they bind extensively to the inner surface of erythrocyte membranes, they may be recognized as small surface projections when the membrane binds around much of an inclusion (Figure 16). When intravascular hemolysis occurs, they may be visible as red to pale pink inclusions within erythrocyte ‘ghosts’ (Figure 17).
Figure 16.
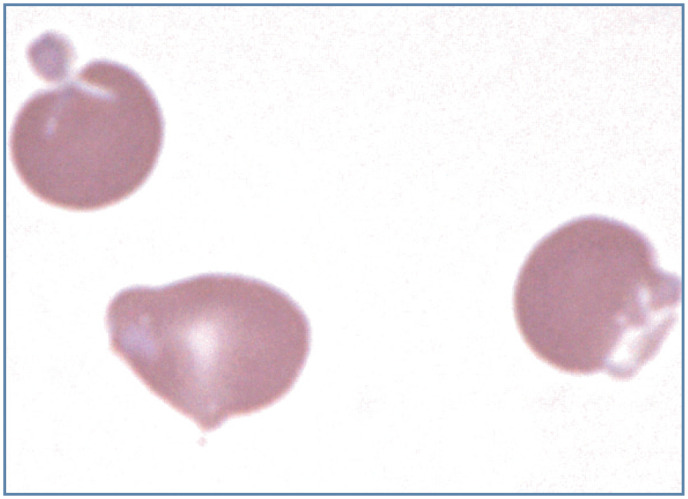
Heinz body hemolytic anemia in a cat with acetaminophen toxicity. The two erythrocytes on the left contain light-pink staining Heinz bodies, with prominent protrusion of the Heinz body in the top cell. The erythrocyte on the right is an eccentrocyte. Wright-Giemsa stain
Figure 17.
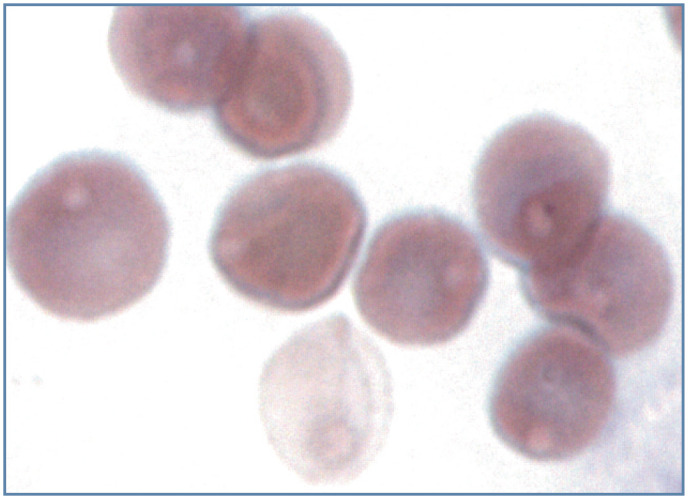
Heinz body hemolytic anemia in a cat with acetaminophen toxicity. The cell at the bottom has lysed and a pale-pink Heinz body is visible in the erythrocyte ‘ghost’. The remaining intact erythrocytes all contain pale-pink staining Heinz bodies. Wright-Giemsa stain
In contrast to other domestic animal species, normal cats may have up to 5% Heinz bodies within their erythrocytes. Not only is the cat spleen less efficient in the removal (pitting) of Heinz bodies from erythrocytes than are spleens of other species, but cat hemoglobin is more susceptible to denaturation by endogenous oxidants. 1
Increased numbers of Heinz bodies may occur:
In cats with spontaneous diseases such as diabetes mellitus (especially when ketoacidosis is present), hyperthyroidism and lymphoma; 21
In cats fed either commercial soft-moist diets containing propylene glycol or baby food containing onion powder;22,23
-
In cats undergoing repeated propofol anesthesia. 24
Although erythrocyte survival tends to be shortened, anemia is either mild or absent in the above conditions.
Acetaminophen toxicity is the most common cause of severe Heinz body hemolytic anemia in cats. Other reported causes of Heinz body hemolytic anemia in cats include methylene blue, benzocaine, phenazopyridine and methionine administration. 1
Basophilic stippling
Diffuse blue punctate inclusions in erythrocytes stained with Romanowsky-type blood stains are composed of aggregates of ribosomes and polyribosomes. These inclusions are similar in composition to precipitated ribosomes and polyribosomes produced using reticulocyte stains, but they form during the process of cell drying prior to staining, rather than during the staining process itself. Like other domestic mammals, diffuse basophilic stippling is often present, along with nucleated erythrocytes, in cats with lead poisoning. 19 Diffuse basophilic stippling commonly occurs in ruminants with regenerative anemia but it seldom occurs in cats with regenerative anemia. 1
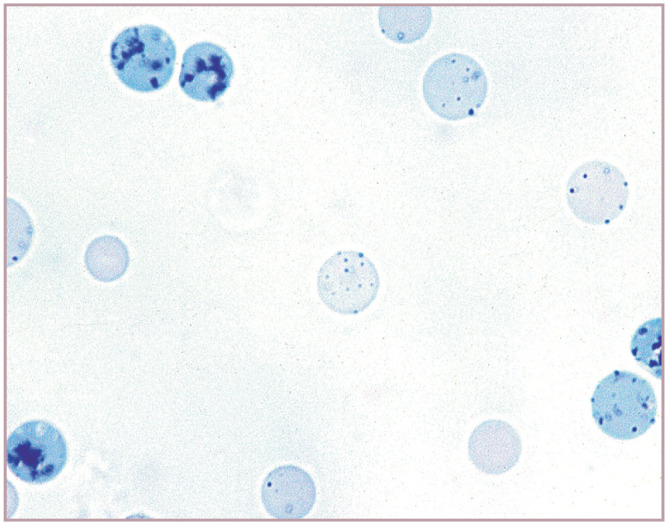
Focal basophilic stippling is typically positive for iron, as demonstrated with the Prussian blue or Perl’s iron stain. Iron-positive focal stippling has been reported in cats with dyserythropoiesis and myeloid neoplasms (Figure 18). 1
Figure 18.
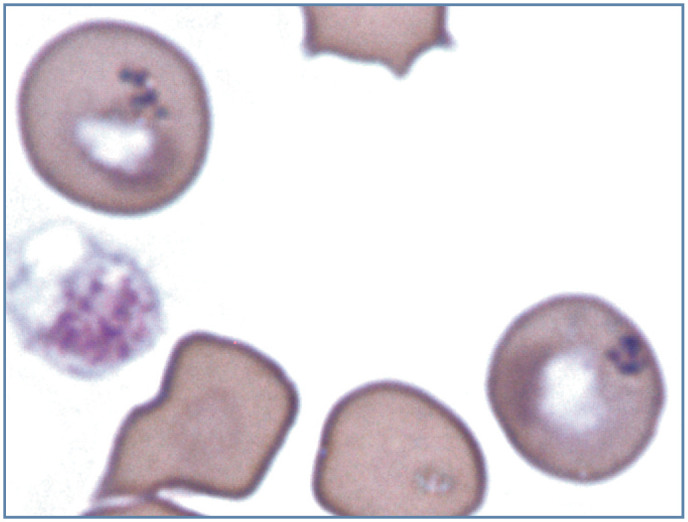
Focal basophilic stippling in two erythrocytes from a cat with dyserythropoiesis and persistent mild anemia. These are called siderotic inclusions because they stained positive for iron using the Perl’s iron stain. In the human literature, siderotic inclusions have been referred to as Pappenheimer bodies. A platelet (left) is also present. Modified Wright’s stain. Photograph of a stained blood film from a 2012 ASVCP slide review submitted by P Cazzini, M Camus, J Messick, B Garner and K Latimer
Infectious agents of erythrocytes
A number of infectious agents are recognized to occur on or in the erythrocytes of cats. These include epicellular hemotrophic mycoplasmas (formerly Haemobartonella species) and intracellular protozoal parasites (Babesia felis [not in the United States] and Cytauxzoon species). Protozoa have a nucleus within their cytoplasm. Hemotrophic mycoplasmas are bacteria and, therefore, lack nuclei. These infectious agents of erythrocytes generally cause hemolytic anemia, which may be mild to severe depending on the pathogenicity of the organism and the susceptibility of the host.
Infectious agents must be differentiated from staining artefacts, as well as from noninfectious inclusions.
Hemotrophic mycoplasmas
Three distinctly different hemoplasmas have been identified in cats based on 16S rRNA gene sequences:
Mycoplasma haemofelis (formerly Haemobartonella felis) appears as small, blue staining cocci, rings or rods on the surface of erythrocytes (Figures 19 and 20a). 25 Typically these organisms are observed on the periphery of erythrocytes in thin areas of the blood film (Figure 20b). These bacteria are smaller than Howell-Jolly bodies and Cytauxzoon felis organisms. M haemofelis occurs in cyclic parasitemias; consequently, it is not always identifiable in blood even during acute infections. In contrast to dogs infected with Mycoplasma haemocanis, splenectomy or splenic pathology is not required for M haemofelis to be visualized in stained blood films and cause anemia in cats. 25
Mycoplasma haemominutum is rarely observed in stained blood films from infected cats. When seen, M haemominutum organisms appear to stain less densely and are about half the size of M haemofelis organisms. M haemofelis is an important pathogen in cats, but M haemominutum appears to be minimally pathogenic.
Candidatus Mycoplasma turicensis has been documented in blood from many cats using PCR-based assays, but it has not yet been identified in stained blood films from infected cats, presumably because of low numbers of organisms present.
Figure 19.
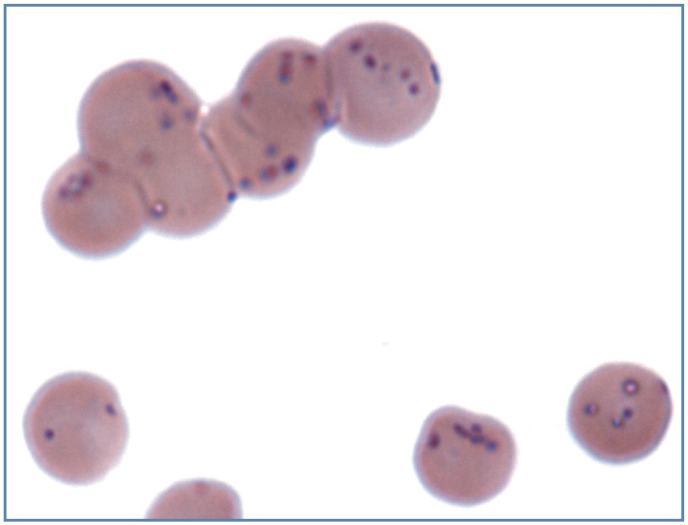
Large numbers of small blue/purple-staining Mycoplasma haemofelis bacterial organism on the surface of erythrocytes from a cat with a natural infection. Wright-Giemsa stain
Figure 20.
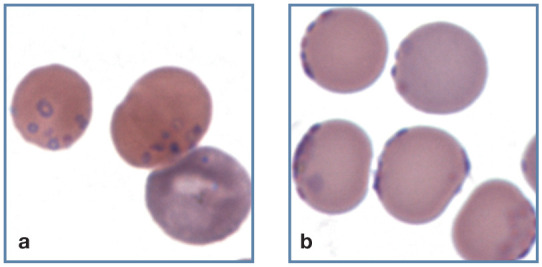
Large numbers of small blue/purple-staining Mycoplasma haemofelis organisms on the surface of erythrocytes from two cats with a natural infection. (a) Ring forms of the organism can be seen on the surface of an erythrocyte. A polychromatophilic erythrocyte (aggregate reticulocyte) without organisms is also present (bottom right). (b) Organisms appear on the periphery of erythrocytes in a thin area of the blood film. Wright-Giemsa stain
Cytauxzoon felis
C felis is a protozoal organism that infects feline erythrocytes and results in high mortality in the United States (Figure 21a). 26 it is morphologically similar to small Babesia organisms. The nuclei of the merozoites in erythrocytes stain magenta or blue and their cytoplasm appears light blue. C felis has both a tissue phase and an erythrocyte phase. The schizonts of Cytauxzoon develop in macrophages that are adherent to many vessels within the body. These schizonts are generally not seen in blood films (see Part 2, Figure 18), but can easily be identified by exfoliative cytology or histopathology of tissues from infected cats.
Figure 21.
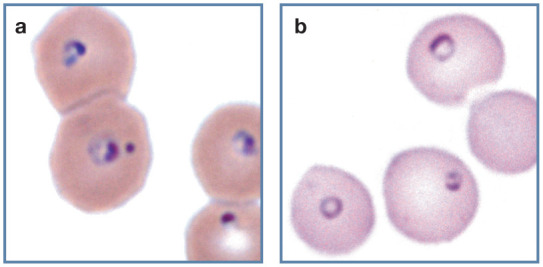
Protozoal parasites in feline erythrocytes. (a) Erythrocytes containing Cytauxzoon felis merozoites in blood from a domestic cat with a natural infection. Organisms have a magenta staining nucleus and pale-blue staining cytoplasm. Wright-Giemsa stain. (b) Erythrocytes containing Babesia felis in blood from a cat from South Africa. Wright stain. Reproduced from Harvey (2012), 1 with permission
Different Cytauxzoon species have been reported in domestic and wild cats outside of the Americas. These infections appear to be subclinical with persistent erythroparasitemia. 27
Babesia species
In contrast to Cytauxzoon, Babesia protozoa proliferate only in erythrocytes. Both large and small Babesia species have been reported in wild and domestic cats. Diseases associated with these organisms have not been well characterized, with the exception of Babesia felis (Figure 21b). This small Babesia species can cause a life-threatening hemolytic anemia in cats in South Africa. 28
Key Points
There are important hematology findings that can only be determined by microscopic examination of stained blood films.
Blood films should be prepared as soon as possible after blood sample collection to minimize artefactual changes that can distort blood cell morphology.
When using Romanowsky-type stains, the appearance of stained blood films depends on the quality of the fixative and stains, the staining and rinse times, and the pH of the stains and rinse water.
Stained blood films should first be scanned using a low-power objective to look for erythrocyte autoagglutination, leukocyte aggregates, platelet aggregates, microfilaria and abnormal cells that might be missed during the differential leukocyte count.
As a quality control measure, the number of leukocytes and platelets present should be estimated to assure that the number present on the slide is consistent with the total leukocyte and platelet counts measured by the analyzer.
Erythrocytes may exhibit rouleaux, agglutination and aggregate reticulocytes, and include Howell-Jolly bodies, Heinz bodies and basophilic stippling; nucleated red blood cells may also be present.
Feline erythrocytes are anucleated bioconcave discs, but there may also be abnormally shaped erythrocytes present (poikilocytosis). These include echinocytes, acanthocytes, keratocytes, schistocytes, spherocytes, elliptocytes, dacryocytes, stomatocytes, eccentrocytes and erythrocytes with hemoglobin crystals.
A number of infectious agents occur on or in the erythrocytes of cats. These include epiceullar hemotrophic mycoplasmas and intracellular Cytauxzoon and Babesia protozoal parasites.
Footnotes
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Harvey JW. Veterinary hematology. A diagnostic guide and color atlas. St Louis, Mo: Elsevier Saunders, 2012. [Google Scholar]
- 2. Mitzner BT. Why automated differentials fall short. J Am Anim Hosp Assoc 2001; 37: 117–118. [DOI] [PubMed] [Google Scholar]
- 3. Bain BJ. Diagnosis from the blood smear. N Engl J Med 2005; 353: 498–507. [DOI] [PubMed] [Google Scholar]
- 4. Welles EG, Hall AS, Carpenter DM. Canine complete blood counts: a comparison of four in-office instruments with the ADVIA 120 and manual differential counts. Vet Clin Pathol 2009; 38: 20–29. [DOI] [PubMed] [Google Scholar]
- 5. Fujino Y, Nakamura Y, Matsumoto H, et al. Development and evaluation of a novel in-clinic automated hematology analyzer, ProCyte Dx, for canine erythrocyte indices, leukogram, platelet counts and reticulocyte counts. J Vet Med Sci 2013; 75: 1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lilliehook I, Tvedten HW. Errors in basophil enumeration with 3 veterinary hematology systems and observations on occurrence of basophils in dogs. Vet Clin Pathol 2011; 40: 450–458. [DOI] [PubMed] [Google Scholar]
- 7. Granat F, Geffre A, Bourges-Abella N, et al. Feline reference intervals for the Sysmex XT-2000iV and the ProCyte DX haematology analysers in EDTA and CTAD blood specimens. J Feline Med Surg 2014; 16: 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Houwen B. Blood film preparation and staining procedures. Lab Hematol 2000; 6: 1–7. [DOI] [PubMed] [Google Scholar]
- 9. Allison RW, Velguth KE. Appearance of granulated cells in blood films stained by automated aqueous versus methanolic Romanowsky methods. Vet Clin Pathol 2010; 39: 99–104. [DOI] [PubMed] [Google Scholar]
- 10. Weiss DJ. Uniform evaluation and semiquantitative reporting of hematologic data in veterinary laboratories. Vet Clin Pathol 1984; 13: 27–31. [DOI] [PubMed] [Google Scholar]
- 11. Alsaker RD, Laber J, Stevens JB, et al. A comparison of polychromasia and reticulocyte counts in assessing erythrocyte regenerative response in the cat. J Am Vet Med Assoc 1977; 170: 39–41. [PubMed] [Google Scholar]
- 12. Christopher MM, Lee SE. Red cell morphologic alterations in cats with hepatic disease. Vet Clin Pathol 1994; 23: 7–12. [DOI] [PubMed] [Google Scholar]
- 13. O’Keefe DA, Schaeffer DJ. Hematologic toxicosis associated with doxorubicin administration in cats. J Vet Intern Med 1992; 6: 276–283. [DOI] [PubMed] [Google Scholar]
- 14. Peterson JL, Couto CG, Wellman ML. Hemostatic disorders in cats: a retrospective study and review of the literature. J Vet Intern Med 1995; 9: 298–303. [DOI] [PubMed] [Google Scholar]
- 15. Skopichev VG, Smirnova OO. Echinocytosis and changes of medium weight molecules content in endo- and exogenous intoxications [article in Russian]. Morfologiia 2010; 137: 31–35. [PubMed] [Google Scholar]
- 16. Brazzell JL, Borjesson DL. Evaluation of plasma antithrombin activity and D-dimer concentration in populations of healthy cats, clinically ill cats, and cats with cardiomyopathy. Vet Clin Pathol 2007; 36: 79–84. [DOI] [PubMed] [Google Scholar]
- 17. Scavelli TD, Hornbuckle WE, Roth L, et al. Portosystemic shunts in cats: seven cases (1976-1984). J Am Vet Med Assoc 1986; 189: 317–325. [PubMed] [Google Scholar]
- 18. Tholen I, Weingart C, Kohn B. Concentration of D-dimers in healthy cats and sick cats with and without disseminated intravascular coagulation (DIC). J Feline Med Surg 2009; 11: 842–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knight TE, Kumar MS. Lead toxicosis in cats - a review. J Feline Med Surg 2003; 5: 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ben-oz J, Segev G, Bilu G, et al. Peripheral nucleated red blood cells in cats and their association with age, laboratory findings, diseases, morbidity and mortality - a retrospective case-control study. Israel J Vet Med 2014; 69: 182–191. [Google Scholar]
- 21. Christopher MM. Relation of endogenous Heinz bodies to disease and anemia in cats: 120 cases (1978-1987). J Am Vet Med Assoc 1989; 194: 1089–1095. [PubMed] [Google Scholar]
- 22. Christopher MM, Perman V, Eaton JW. Contribution of propylene glycol-induced Heinz body formation to anemia in cats. J Am Vet Med Assoc 1989; 194: 1045–1056. [PubMed] [Google Scholar]
- 23. Robertson JE, Christopher MM, Rogers QR. Heinz body formation in cats fed baby food containing onion powder. J Am Vet Med Assoc 1998; 212: 1260–1266. [PubMed] [Google Scholar]
- 24. Matthews NS, Brown RM, Barling KS, et al. Repetitive propofol administration in dogs and cats. J Am Anim Hosp Assoc 2004; 40: 255–260. [DOI] [PubMed] [Google Scholar]
- 25. Messick JB, Harvey JW. Hemotrophic mycoplasmosis (hemobartonellosis). In: Greene CE. (ed). Infectious diseases of the dog and cat. St Louis, Mo: Elsevier Saunders, 2012, pp 310–319. [Google Scholar]
- 26. Sherrill MK, Cohn LA. Cytauxzoonosis: diagnosis and treatment of an emerging disease. J Feline Med Surg 2015; 17: 940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carli E, Trotta M, Chinelli R, et al. Cytauxzoon sp. infection in the first endemic focus described in domestic cats in Europe. Vet Parasitol 2012; 183: 343–352. [DOI] [PubMed] [Google Scholar]
- 28. Ayoob AL, Prittie J, Hackner SG. Feline babesiosis. J Vet Emerg Crit Care (San Antonio) 2010; 20: 90–97. [DOI] [PubMed] [Google Scholar]