Abstract
Overview:
Anaplasma species, Ehrlichia species and Rickettsia species are vector-borne pathogens infecting a wide variety of mammals, but causing disease in very few of them.
Infection in cats:
Anaplasma phagocytophilum is the most important feline pathogen among these rickettsial organisms, and coinfections are possible. Little information is available on the pathogenesis of these agents in cats. Clinical signs are usually reported soon after tick infestation. They are mostly non-specific, consisting of fever, anorexia and lethargy. Joint pain may occur.
Infection in humans:
Some rickettsial species (A phagocytophilum, Ehrlichia chaffeensis, Ehrlichia ewingii, Rickettsia conorii, Rickettsia rickettsii, Rickettsia felis, Rickettsia typhi and Candidatus Neoehrlichia mikurensis) are of zoonotic concern. Direct contact with cat saliva should be avoided because of potential contamination by R felis. Infected cats are ‘sentinels’ of the presence of rickettsial pathogens in ticks and fleas in a given geographical area, and they signal a risk for people exposed to vectors.
Agent properties and epidemiology
Obligate intracellular gram-negative coccoid organisms of the Anaplasma, Ehrlichia and Rickettsia genera are vector-borne members of the Rickettsiales order, infecting humans and a wide variety of domestic and wild animals worldwide. 1 They generally have a wide host specificity, evidenced by the fact that many mammalian species can be infected. Importantly, some hosts might serve as reservoirs of infection; however, susceptibility to disease is usually more restricted.
Anaplasma, Ehrlichia and Rickettsia species infections have been generally less studied in cats (Table 1) than in dogs. These organisms are difficult to culture in vitro. The advent of molecular genetics has, however, opened new avenues for the study of their infection biology. 1
Table 1.
Members of the Ehrlichia, Anaplasma and Rickettsia genera detected in cats in various countries
| Countries in which infection has been reported* | |
| Ehrlichia genus | |
| E canis | Canada, USA, Brazil, Portugal |
| E chaffeensis | USA, Brazil |
| E ewingii | USA |
| Ehrlichia species | Italy, USA, Kenya, France |
| Anaplasma genus | |
| A phagocytophilum | USA, Sweden, Finland, Poland, Switzerland, Germany, Italy, Spain |
| A platys | USA, Brazil |
| A platys-like | Italy |
| A bovis | Japan |
| Rickettsia genus | |
| R rickettsii | USA |
| R conorii | Spain |
| R massiliae | Spain |
| Rickettsia species | Italy |
Note that this list may not be exhaustive. It is possible that feline rickettsial infections are occurring in countries from which reports have not, to date, been published; particularly in areas where the competent tick vectors (see text) are abundant

Anaplasmataceae family
Anaplasma and Ehrlichia species are tick-borne pathogens of the Anaplasmataceae family and are pleomorphic intravacuolar organisms that replicate in haemopoietic cells. They give rise to a variety of cytoplasmic inclusions – small elementary bodies (0.2–0.4 μm diameter), larger reticulate bodies and morulae (up to 2–6 μm).
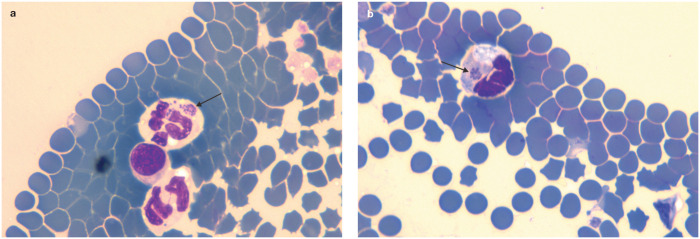
Anaplasma phagocytophilum replicates in myeloid cells (mostly in neutrophils; Figure 1) and is the agent of granulocytotropic anaplasmosis. It infects people and a wide range of animal species worldwide, especially in the northern hemisphere, and is the most important feline pathogen of the Anaplasma genus. Wild small mammals are the natural reservoirs. Antibody prevalence to A phagocytophilum in cats has been reported as 4.5–33.3% in Italy,3,4 2.0–8.0% in Spain,5,6 16.2% in Germany, 7 22.1% in Sweden, 8 and 1.8–38.0% in the USA.9–11 A phagocytophilum DNA was recently amplified in 0.4% of blood samples from cats admitted to veterinary clinics in southern Germany. 12
Figure 1.
Presence of Anaplasma species morulae (arrows) in neutrophils from a 2-year-old male European shorthair cat (a) and a 6-year-old female European shorthair cat (b) in Poland. Reprinted with permission from Adaszek et al 2
Anaplasma platys replicates in mature platelets and is the agent of thrombocytotropic anaplasmosis, a disease well documented worldwide in dogs. 13 It has occasionally been detected in cats by PCR analysis.11,14,15
Anaplasma bovis has also been found by PCR, in feline blood samples from Japan. 16 Recently a novel, unclassified A platys-like strain from cats was characterised in Sardinia (Italy). This strain, despite its tropism for platelets, is closely related to others identified in ruminants. 17
Ehrlichia canis is the agent of canine monocytotropic ehrlichiosis, a disease described in tropical and temperate regions worldwide, with the exception of Australia. Ehrlichia chaffeensis is the agent of human monocytotropic ehrlichiosis, which has been reported mainly in the USA. The granulocytotropic pathogen Ehrlichia ewingii has been found in dogs and humans in midwestern and southern regions of the USA. In cats, E canis has been detected by PCR in blood samples.6,11,18–24 Less frequently, E chaffeensis and E ewingii have also been detected.11,20 Cats seropositive for E canis have been reported in Europe (6.0–18.0%),3–6,25,26 Brazil (5.5%) 20 and the USA (0.7%). 11
Another member of the Anaplasmataceae family is ‘Candidatus Neoehrlichia mikurensis’ (genus ‘Candidatus Neoehrlichia’), which leads to neoehrlichiosis, an emerging zoonosis. This tick-borne agent has been found mainly in immunocompromised patients, and in ticks and rodents, and recently also in two dogs,27,28 although so far not in cats. The infection may be underdiagnosed because diagnostic assays are not yet widely available.
Rickettsiaceae family
The Rickettsiaceae family includes the spotted fever group (SFG) and the typhus group agents. 1
There are more than 20 species within the SFG of the Rickettsia genus, some of which are important human pathogens. Historically, the most important zoonotic agents have been Rickettsia conorii (the cause of Mediterranean spotted fever in the Old World) and Rickettsia rickettsii (the agent of Rocky Mountain spotted fever in the Americas). However, recent molecular studies have increasingly focused on other rickettsial species that may be involved in human clinical disease. Rickettsia massiliae, for example, is now recognised as the most widely distributed rickettsial species that affects humans, being found worldwide. 29
R rickettsii and R conorii are both transmitted by ticks and infect dogs, sometimes causing clinical disease. Less information is available about the effect of these agents in cats. Feline infections involving R massiliae and R conorii have been confirmed (both by PCR and antibody testing) in endemic areas of Spain and Portugal,5,30–32 and cats seropositive for R rickettsii have been reported in the USA. 33
Rickettsia felis is emerging worldwide as a flea-borne SFG human pathogen; it is detected frequently in Ctenocephalides felis, less often in other flea species. 29 C felis is the vector and the recognised reservoir of R felis, which is vertically transmitted to successive generations of fleas. 34 Recent studies in parts of Europe have shown that Ctenocephalides infection rates range from 2.8% (in Albania) 35 to 54.2% (in Andalucia, Spain). 36 Cats are susceptible to R felis infection and seroconvert after exposure to infected fleas. However, they are probably not reservoir hosts as they experience short-term bacteraemia (blood PCR test results are usually negative in antibodypositive cats).4,32,37,38 In rare cases, R felis DNA can be amplified also from the skin or gingiva of cats with negative blood PCR results. 39 It is unknown whether R felis is present in other tissues of seropositive cats and whether it should be considered a feline pathogen.
Rickettsia typhi is a worldwide flea-borne rickettsial pathogen of the typhus group. It is the agent of murine or endemic typhus, transmitted to humans, cats or wild animal reservoirs by infected rat (Xenopsylla species) or cat (C felis) fleas.
Just as in humans and dogs, coinfections with multiple vector-borne pathogens occur in cats. The consequences of such mixed infections are unknown.11,15,23,40
Pathogenesis and clinical signs
Little information is available on the pathogenesis of rickettsial agents in cats.
A limited number of studies involving experimental infections or exposure to A phagocytophilum or R felis in cats exist. In one such study, intraperitoneal inoculation of A phagocytophilum-infected blood in a small number of cats resulted in mild disease, with only transient fever and no associated changes in appetite or general appearance. However, mild reductions in total leukocyte, neutrophil and lymphocyte counts, significant reductions in packed cell volume, and a transient increase in alanine transaminase and aspartate aminotransferase values were detected. 46 Antinuclear antibodies and increased expression of IFNγ mRNA were also found in infected cats, but they showed normal antibody responses to feline herpesvirus and feline leukaemia virus vaccination 2 weeks postinfection. When experimental infection with A phagocytophilum was performed in feline immunodeficiency virus-infected cats, upregulation of IL-10 expression was observed instead of IFNγ, but the clinical course of anaplasmosis was similar. 46
Recent experimental exposure of four cats to wild-caught adult Ixodes scapularis ticks induced a largely subclinical dual infection with A phagocytophilum and Borrelia burgdorferi. 40 In fact, a transient lymphopenia was the only abnormality detected during monitoring of the cats’ general appearance, appetite, body temperature and complete blood count for 13 weeks after tick infestation. 40
In naturally exposed cats, clinical signs of feline granulocytotropic anaplasmosis are usually reported soon after tick infestation. They are mostly non-specific, consisting of fever, anorexia, lethargy and dehydration. Clinicopathological abnormalities include mild or moderate thrombocytopenia, anaemia and lymphopenia.2,47–51 Lameness and swollen joints, epistaxis or pain on abdominal palpation have been less frequently reported.2,47,52 The clinical course is usually short and not severe; abnormalities resolve quickly, particularly when antibiotic treatment is given.
Natural infection with A platys in a cat in Brazil was associated with anorexia, lethargy, urinary tract infection and thrombocytopenia. 14 In another cat, persistent A platys infection was found concurrently with Bartonella henselae, Bartonella koehlerae and ‘Candidatus Mycoplasma haemominutum’ infections; the cat was also diagnosed with multiple myeloma. 15 In both cases, the pathogenic role of A platys was not clearly established. In the cat with multiple myeloma, the associated immune system dysfunction could have been responsible for increased susceptibility to the coinfections. 53
Experimental subcutaneous inoculation of cats with a canine strain of E canis was not successful in inducing infection, and the pathogenesis of feline monocytotropic ehrlichiosis in cats is not known. 54 Natural disease has been confirmed by molecular testing in only a few cases and mainly manifests as nonspecific signs such as fever, anorexia and lethargy; more rarely, hyperaesthesia, joint pain, pale mucous membranes, lymph node and spleen enlargement, and haemorrhagic diathesis (petechiae, vitreous haemorrhage) have also been reported. 54 Haematology can reveal non-regenerative anaemia, thrombocytopenia, pancytopenia and increased or decreased white cell counts. 54 Bone marrow hypoplasia is found in cats with pancytopenia or anaemia and thrombocytopenia on haematology. 18 The most consistent biochemical abnormality seen with feline monocytotropic ehrlichiosis has been hyperproteinaemia and polyclonal or monoclonal gammopathy, which is also typical of the chronic course of canine monocytotropic ehrlichiosis. 54 Antinuclear antibodies have been found in some cats and neutrophilic polyarthritis was diagnosed in a cat with joint signs. 18
In a study evaluating clinically ill cats for evidence of rickettsial infection, no association was found between antibody positivity and fever, and no febrile cat was found to be PCR positive for R felis or R rickettsii. 55 Another study investigated possible links between Ehrlichia species or A phagocytophilum infections and anaemia, but no significant associations were detected. 56
Diagnosis
The main indication for undertaking diagnostic investigations for rickettsial disease is a febrile syndrome affecting cats exposed to ticks in endemic areas; in particular, strays or outdoor pet cats not protected by the regular use of appropriate ectoparasiticides.
Blood or buffy-coat smear evaluation may be useful for cytological diagnosis of Ehrlichia and Anaplasma species infections. In general, intracytoplasmic inclusion bodies are more frequently found in granulocytotropic anaplasmosis than in monocytotropic ehrlichiosis. A phagocytophilum inclusion bodies are found in 1–24% of circulating neutrophils in cats with natural granulocytotropic anaplasmosis. In experimentally infected cats they appear 7–9 days postinfection, 46 or over the first 10 weeks after tick infestation. 40 Following antibiotic therapy they are no longer visible.40,47,50 With A platys, bacteraemia is cyclical, recurring at 1–2 week intervals, with a higher percentage of circulating infected platelets during the early cycles. 57
Antibodies to rickettsial infections can be detected by immunofluorescence and ELISA techniques. Cross-reaction is possible between A phagocytophilum and A platys but not E canis, although E canis can cross-react with other Ehrlichia species. Antibodies against A phagocytophilum were detected in an experimental study within 14 days of infection, and seroconversion occurs in natural infections even in antibiotic-treated cats. 46 In cats experimentally exposed to infected ticks, antibodies against A phagocytophilum appeared 2–6 weeks after infestation. 40 In the case of a positive immunofluorescence test at first sampling, a four-fold increase in the titre over 4 weeks may confirm the acute course of the infection.46–48,50 Some cats testing positive to E canis by PCR were found to be antibody negative despite the chronic course of their disease. 18 This suggests that a negative antibody test does not rule out a diagnosis of ehrlichiosis in cats with compatible clinical signs.
Blood PCR analysis is a sensitive and specific method for confirming diagnosis at the onset of acute clinical signs when antibody testing is usually still negative.40,46 Because of overlapping clinical signs, the use of genus-inclusive primers for Ehrlichia–Anaplasma and Rickettsia species genera in PCRs has been suggested, followed by sequencing of any resulting PCR products to determine the infecting species. 1 However, a recent study has demonstrated that some genus-specific PCRs also detect Pseudomonas sequences and may lead to false-positive results that may only be recognised after sequencing analysis. 28 Alternatively, species-specific real-time TaqMan assays may be faster and more sensitive options for the molecular detection of rickettsaemia.
Samples for microscopic detection or PCR should be collected prior to the initiation of antibiotic treatment.
Treatment
Doxycycline is the antibiotic of choice for treating rickettsial infections in cats, although currently there are only retrospective case reports supporting this recommendation [EBM grade III].2,47,48,50,51 It is administered at 10 mg/kg PO q24h for 28 days. Clinical improvement is seen in the first 24–48 h, unless coinfections not susceptible to doxycycline (eg, protozoal vector-borne agents) are present, or other complications develop such as severe bleeding. Animals generally respond well to treatment but may remain persistently infected.

In cases testing negative by microscopy, PCR or serology, or while awaiting results – and where there is a strong clinical suspicion of rickettsial infection – treatment should be initiated to prevent the potential rapid progression of clinical disease.
Prevention
As vectors are the main routes of transmission of rickettsial infections, regular application of appropriate ectoparasiticide spot-on treatments or collars may protect cats from becoming infected, as is well recognised in dogs.
In endemic areas, cats should be tested for rickettsial blood-borne infections to confirm they are negative before being used as blood donors. 58
Key Points
Anaplasma species, Ehrlichia species and Rickettsia species are vector-borne pathogens, the most important of which in cats is Anaplasma phagocytophilum.
The geographical distribution of these rickettsial organisms overlaps with that of their competent vectors (ticks or fleas).
Blood or buffy-coat smear evaluation may be useful for cytological diagnosis of Ehrlichia and Anaplasma species infections.
Antibodies to rickettsial infections can be detected by immunofluorescence and ELISA techniques, but cross-reactions may occur between organisms of the same genus.
Blood PCR analysis is a sensitive and specific method for confirming diagnosis at the onset of acute clinical signs and before starting therapy, when antibody testing is usually still negative.
Doxycycline is the first-choice antibiotic for treating rickettsial infections.
Regular application of appropriate ectoparasiticide spot-on treatments or collars protects cats from infection.
In endemic areas, blood donors should be tested for rickettsial blood-borne infections.

Acknowledgments
The ABCD is deeply indebted to Marian C Horzinek, the ‘spiritus rector’ and past chairman of ABCD for many years, who sadly passed away during the finalisation of these guidelines.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article. The ABCD is supported by Merial, but is a scientifically independent body and its members receive no stipends from Merial.
References
- 1. Allison RW, Little SE. Diagnosis of rickettsial diseases in dogs and cats. Vet Clin Pathol 2013; 42: 127–144. [DOI] [PubMed] [Google Scholar]
- 2. Adaszek L, Górna M, Skrzypczak M, et al. Three clinical cases of Anaplasma phagocytophilum infection in cats in Poland. J Feline Med Surg 2013; 15: 333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ebani VV, Bertelloni F. Serological evidence of exposure to Ehrlichia canis and Anaplasma phagocytophilum in Central Italian healthy domestic cats. Ticks Tick Borne Dis 2014; 5: 668–671. [DOI] [PubMed] [Google Scholar]
- 4. Persichetti MF, Solano-Gallego L, Serrano L, et al. Detection of vector-borne pathogens in cats and their ectoparasites in southern Italy. Parasit Vectors 2016; 9: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Solano-Gallego L, Hegarty B, Espada Y, et al. Serological and molecular evidence of exposure to arthropod-borne organisms in cats from northeastern Spain. Vet Microbiol 2006; 118: 274–277. [DOI] [PubMed] [Google Scholar]
- 6. Ayllón T, Diniz PP, Breitschwerdt EB, et al. Vector borne diseases in client-owned and stray cats from Madrid, Spain. Vector Borne Zoonotic Dis 2012; 12: 143–149. [DOI] [PubMed] [Google Scholar]
- 7. Hamel D, Bondarenko A, Silaghi C, et al. Seroprevalence and bacteremia of Anaplasma phagocytophilum in cats from Bavaria and Lower Saxony (Germany). Berl Munch Tierarztl Wochenschr 2012; 125: 163–167. [PubMed] [Google Scholar]
- 8. Elfving K, Malmsten J, Dalin AM, et al. Serological and molecular prevalence of Rickettsia helvetica and Anaplasma phagocytophilum in wild cervids and domestic mammals in the central parts of Sweden. Vector Borne Zoonotic Dis 2015; 15: 529–534. [DOI] [PubMed] [Google Scholar]
- 9. Magnarelli LA, Bushmich SL, Ijdo JW, et al. Seroprevalence of antibodies against Borrelia burgdorferi and Anaplasma phagocytophilum in cats. Am J Vet Res 2005; 66: 1895–1899. [DOI] [PubMed] [Google Scholar]
- 10. Billeter SA, Spencer JA, Griffin B, et al. Prevalence of Anaplasma phagocytophilum in domestic felines in the United States. Vet Parasitol 2007; 147: 194–198. [DOI] [PubMed] [Google Scholar]
- 11. Hegarty BC, Qurollo BA, Thomas B, et al. Serological and molecular analysis of feline vector-borne anaplasmosis and ehrlichiosis using species-specific peptides and PCR. Parasit Vectors 2015; 8: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bergmann M, Englert T, Stuetzer B, et al. Prevalence of selected rickettsial infections in cats in Southern Germany. Comp Immunol Microbiol Infect Dis 2015; 42: 33–36. [DOI] [PubMed] [Google Scholar]
- 13. Sainz A, Roura X, Miró G, et al. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit Vectors 2015; 8: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lima MLF, Soares PT, Ramos CAN, et al. Molecular detection of Anaplasma platys in a naturally-infected cat in Brazil. Braz J Microbiol 2010; 41: 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qurollo BA, Balakrishnan N, Cannon CZ, et al. Co-infection with Anaplasma platys, Bartonella henselae, Bartonella koehlerae and ‘Candidatus Mycoplasma haemominutum’ in a cat diagnosed with splenic plasmacytosis and multiple myeloma. J Feline Med Surg 2014; 16: 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sasaki H, Ichikawa Y, Sakata Y, et al. Molecular survey of Rickettsia, Ehrlichia, and Anaplasma infection of domestic cats in Japan. Ticks Tick Borne Dis 2012; 3: 307–310. [DOI] [PubMed] [Google Scholar]
- 17. Zobba R, Anfossi AG, Visco S, et al. Cell tropism and molecular epidemiology of Anaplasma platys-like strains in cats. Ticks Tick Borne Dis 2015; 6: 272–280. [DOI] [PubMed] [Google Scholar]
- 18. Breitschwerdt EB, Abrams-Ogg AC, Lappin MR, et al. Molecular evidence supporting Ehrlichia canis-like infection in cats. J Vet Intern Med 2002; 16: 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oliveira LS, Mourão LC, Oliveira KA, et al. Molecular detection of Ehrlichia canis in cats in Brazil. Clin Microbiol Infect 2009; 15: 53–54. [DOI] [PubMed] [Google Scholar]
- 20. Braga M, André MR, Freschi CR, et al. Molecular and serological detection of Ehrlichia spp. in cats on São Luís island, Maranhaão, Brazil. Rev Bras Parasitol Vet 2012; 21: 37–41. [DOI] [PubMed] [Google Scholar]
- 21. Braga IA, Santos LG, Melo AL, et al. Hematological values associated to the serological and molecular diagnostic in cats suspected of Ehrlichia canis infection. Rev Bras Parasitol Vet 2013; 22: 470–474. [DOI] [PubMed] [Google Scholar]
- 22. Braga IA, Santos LG, Ramos DG, et al. Detection of Ehrlichia canis in domestic cats in the central-western region of Brazil. Braz J Microbiol 2014; 45: 641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maia C, Ramos C, Coimbra M, et al. Bacterial and protozoal agents of feline vector-borne diseases in domestic and stray cats from southern Portugal. Parasit Vectors 2014; 7: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. André MR, Herrera HM, Fernandes Sde J, et al. Tick-borne agents in domesticated and stray cats from the city of Campo Grande, state of Mato Grosso do Sul, Midwestern Brazil. Ticks Tick Borne Dis 2015; 6: 779–786. [DOI] [PubMed] [Google Scholar]
- 25. Aguirre E, Tesouro MA, Amusatequi I, et al. Assessment of feline ehrlichiosis in central Spain using serology and a polymerase chain reaction technique. Ann N Y Acad Sci 2004; 1026: 103–105. [DOI] [PubMed] [Google Scholar]
- 26. Ortuño A, Gauss CB, Garaa F, et al. Serological evidence of Ehrlichia spp. exposure in cats from northeastern Spain. J Vet B Infect Dis Vet Public Health 2005; 52: 246–248. [DOI] [PubMed] [Google Scholar]
- 27. Diniz PP, Schulz BS, Hartmann K, et al. “Candidatus Neoehrlichia mikurensis” infection in a dog from Germany. J Clin Microbiol 2011; 49: 2059–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hofmann-Lehmann R, Wagmann N, Meli ML, et al. Detection of ‘Candidatus Neoehrlichia mikurensis' and other Anaplasmataceae and Rickettsiaceae in Canidae in Switzerland and Mediterranean countries. Schweiz Arch Tierheilkd 2016; 158: 691–700. [DOI] [PubMed] [Google Scholar]
- 29. Brouqui P, Parola P, Fournier PE, et al. Spotted fever rickettsioses in southern and eastern Europe. FEMS Immunol Med Microbiol 2007; 49: 2–12. [DOI] [PubMed] [Google Scholar]
- 30. Alves AS, Milhano N, Santos-Silva M, et al. Evidence of Bartonella spp., Rickettsia spp. and Anaplasma phagocytophilum in domestic, shelter and stray cat blood and fleas, Portugal. Clin Microbiol Infect 2009; 15: 1–3. [DOI] [PubMed] [Google Scholar]
- 31. Vilhena H, Martinez-Díaz V, Cardoso L, et al. Feline vector-borne pathogens in the north and centre of Portugal. Parasit Vectors 2013; 6: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Segura F, Pons I, Miret J, et al. The role of cats in the eco-epidemiology of spotted fever group diseases. Parasit Vectors 2014; 7: 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Case JB, Chomel B, Nicholson W, et al. Serological survey of vector-borne zoonotic pathogens in pet cats and cats from animal shelters and feral colonies. J Feline Med Surg 2006; 8: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wedincamp J, Jr, Foil LD. Vertical transmission of Rickettsia felis in the cat flea (Ctenocephalides felis Bouché). J Vector Ecol 2002; 27: 96–101. [PubMed] [Google Scholar]
- 35. Silaghi C, Knaus M, Rapti D, et al. Survey of Toxoplasma gondii and Neospora caninum, haemotropic mycoplasmas and other arthropod-borne pathogens in cats from Albania. Parasit Vectors 2012; 7: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Márquez FJ, Muniain MA, Rodríquez-Liebana JJ, et al. Incidence and distribution pattern of Rickettsia felis in peridomestic fleas from Andalusia, Southeast Spain. Ann N Y Acad Sci 2006; 1078: 344–346. [DOI] [PubMed] [Google Scholar]
- 37. Wedincamp J, Jr, Foil LD. Infection and seroconversion of cats exposed to cat fleas (Ctenocephalides felis Bouché) infected with Rickettsia felis. J Vector Ecol 2000; 25: 123–126. [PubMed] [Google Scholar]
- 38. Hawley JR, Shaw SE, Lappin MR. Prevalence of Rickettsia felis DNA in the blood of cats and their fleas in the United States. J Feline Med Surg 2007; 9: 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lappin MR, Hawley J. Presence of Bartonella species and Rickettsia species DNA in the blood, oral cavity, skin and claw beds of cats in the United States. Vet Dermatol 2009; 20: 509–514. [DOI] [PubMed] [Google Scholar]
- 40. Lappin MR, Chandrashekar R, Stillman B, et al. Evidence of Anaplasma phagocytophilum and Borrelia burgdorferi infection in cats after exposure to wild-caught adult Ixodes scapularis. J Vet Diagn Invest 2015; 27: 522–525. [DOI] [PubMed] [Google Scholar]
- 41. Jameson LJ, Medlock JM. Tick surveillance in Great Britain. Vector Borne Zoonotic Dis 2011; 11: 403–412. [DOI] [PubMed] [Google Scholar]
- 42. Claerebout E, Losson B, Cochez C, et al. Ticks and associated pathogens collected from dogs and cats in Belgium. Parasit Vectors 2013; 6: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pennisi MG, Persichetti MF, Serrano L, et al. Ticks and associated pathogens collected from cats in Sicily and Calabria (Italy). Parasit Vectors 2015; 8: 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. ECDC. Tick species – distribution maps. European Centre for Disease Prevention and Control. http://ecdc.europa.eu/en/healthtopics/vectors/vector-maps/Pages/VBORNET-maps-tick-species.aspx (accessed October 1, 2106).
- 45. Spada E, Proverbio D, Galluzzo P, et al. Molecular study on selected vector-borne infections in urban stray colony cats in northern Italy. J Feline Med Surg 2014; 16: 684–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Foley JE, Leutenegger CM, Dumler JS, et al. Evidence for modulated immune response to Anaplasma phagocytophila sensu lato in cats with FIV-induced immunosuppression. Comp Immunol Microbiol Infect Dis 2003; 26: 103–113. [DOI] [PubMed] [Google Scholar]
- 47. Bjöersdorff A, Svendenius L, Owens JH, et al. Feline granulocytic ehrlichiosis – a report of a new clinical entity and characterization of the infectious agent. J Small Anim Pract 1999; 40: 20–24. [DOI] [PubMed] [Google Scholar]
- 48. Lappin MR, Breitschwerdt EB, Jensen WA, et al. Molecular and serological evidence of Anaplasma phagocytophilum infection in cats in North America. J Am Vet Med Assoc 2004; 225: 893–896. [DOI] [PubMed] [Google Scholar]
- 49. Schaarschmidt-Kiener D, Graf F, von Loewenich FD, et al. Anaplasma phagocytophilum infection in a cat in Switzerland. Schweiz Arch Tierheilkd 2009; 151: 336–341. [DOI] [PubMed] [Google Scholar]
- 50. Heikkilä HM, Bondarenko A, Mihalkov A, et al. Anaplasma phagocytophilum infection in a domestic cat in Finland: case report. Acta Vet Scand 2010; 52: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Savidge C, Ewing P, Andrews J, et al. Anaplasma phagocytophilum infection of domestic cats: 16 cases from northeastern USA. J Feline Med Surg 2016; 18: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tarello W. Microscopic and clinical evidence for Anaplasma (Ehrlichia) phagocytophilum infection in Italian cats. Vet Rec 2005; 156: 772–774. [DOI] [PubMed] [Google Scholar]
- 53. Pratt G, Goodyear O, Moss P. Immunodeficiency and immunotherapy in multiple myeloma. Br J Haematol 2007; 138: 563–579. [DOI] [PubMed] [Google Scholar]
- 54. Lappin MR, Breitschwerdt ER. Ehrlichia spp. infection (feline monocytotropic ehrlichiosis). In Greene CE. (ed). Infectious diseases of the dog and cat. St Louis, MO: Elsevier, 2012, pp 238–241. [Google Scholar]
- 55. Bayliss D, Morris AK, Horta MC, et al. Prevalence of Rickettsia species antibodies and Rickettsia species DNA in the blood of cats with and without fever. J Feline Med Surg 2009; 11: 266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ishak AM, Radecki S, Lappin MR. Prevalence of Mycoplasma haemofelis, “Candidatus Mycoplasma haemominutum”, Bartonella species, Ehrlichia species, and Anaplasma phagocytophilum DNA in the blood of cats with anemia. J Feline Med Surg 2006; 9: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Harvey JW, Simpson CF, Gaskin JM. Cyclic thrombocytopenia induced by a Rickettsia-like agent in dogs. J Infect Dis 1978; 137: 182–188. [DOI] [PubMed] [Google Scholar]
- 58. Pennisi MG, Hartmann K, Addie DD, et al. Blood transfusion in cats. ABCD guidelines for minimising risks of infectious iatrogenic complications. J Feline Med Surg 2015; 17: 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]