Abstract
Objectives
The biologic variability of N-terminal pro-brain natriuretic peptide (NT-proBNP) and its impact on diagnostic utility is unknown in healthy cats and those with cardiac disease. The purpose of this study was to determine the biologic variation of NT-proBNP within-day and week-to-week in healthy adult cats.
Methods
Adult cats were prospectively evaluated by complete blood count (CBC), biochemistry, total thyroxine, echocardiography, electrocardiography and blood pressure, to exclude underlying systemic or cardiac disease. Adult healthy cats were enrolled and blood samples were obtained at 11 time points over a 6 week period (0, 2 h, 4 h, 6 h, 8 h, 10 h and at weeks 2, 3, 4, 5 and 6). The intra-individual (coefficient of variation [CVI]) biologic variation along with index of individuality and reference change values (RCVs) were calculated. Univariate models were analyzed and included comparison of the six different time points for both daily and weekly samples. This was followed by a Tukey’s post-hoc adjustment, with a P value of <0.05 being significant.
Results
The median daily and weekly CVI for the population were 13.1% (range 0–28.7%) and 21.2% (range 3.9–68.1%), respectively. The index of individuality was 0.99 and 1 for daily and weekly samples, respectively. The median daily and weekly RCVs for the population were 39.8% (range 17.0–80.5%) and 60.5% (range 20.1–187.8%), respectively.
Conclusions and relevance
This study demonstrates high individual variability for NT-proBNP concentrations in a population of adult healthy cats. Further research is warranted to evaluate NT-proBNP variability, particularly how serial measurements of NT-proBNP may be used in the diagnosis and management of cats with cardiac disease.
Introduction
Feline hypertrophic cardiomyopathy (HCM) is the most common cardiac disease in cats, frequently resulting in diastolic dysfunction, left atrial enlargement and congestive heart failure (CHF).1,2 The prevalence of heart murmurs in a suspected healthy population of cats has been reported as 21%, with only 53% of healthy cats with murmurs having echocardiographic evidence of underlying cardiac disease.3,4 Cardiomyopathy is challenging to diagnose as physical examination, electrocardiography (ECG) and thoracic radiography have limited sensitivity and specificity.5–7 Echocardiography is currently the clinical gold standard used to diagnose and differentiate cardiomyopathy in cats.
Use of serum or plasma biomarkers as a means of detecting cardiomyopathy is attractive because of its minimal invasiveness, ease of sample collection, widespread availability, including national laboratory and point-of-care testing, quantitative nature and cost-effectiveness when compared with echocardiography. Reference laboratory testing has a turnaround time that may reduce immediate usefulness for patient care; however, this is the test that was evaluated in this study as the point-of-care test (that provides results within 10 mins) is semi-quantitative. Brain natriuretic peptide (BNP) is secreted by atrial and ventricular myocytes in response to volume and pressure overload, and myocardial stretch.8–10 Brain natriuretic peptide is initially synthesized as a pro-hormone (proBNP), which is cleaved and released from myocytes as active BNP and inactive N-terminal proBNP (NT-proBNP). NT-proBNP correlates with plasma concentrations of active BNP, has a longer half-life than BNP and thus has been utilized as the more stable marker of cardiac disease.8–10 NT-proBNP concentrations may be increased in humans, dogs and cats with various cardiac disorders, though concentrations in cardiac patients often have a degree of overlap with clinically normal animals, particularly those with mild or subclinical heart disease.
Multiple studies have investigated the ability of plasma NT-proBNP to distinguish echocardiographically normal cats from those with subclinical cardiomyopathy and to distinguish cardiogenic from non-cardiogenic causes of respiratory signs in cats.10-17 These studies have shown that NT-proBNP concentrations can distinguish healthy cats from those with subclinical disease (sensitivity 70.8–92.4%; specificity 93.9–100%), as well as distinguish cats with CHF from those with non-cardiac causes of dyspnea (sensitivity 90.2–93.9%; specificity 87.8–87.9%) with a reasonable degree of accuracy depending on the cut-off values used.10-17 Plasma NT-proBNP concentrations >100 pmol/l may suggest that morphologic cardiac changes are present and further cardiac evaluation is warranted to provide a definitive diagnosis and assess severity of disease.11-13 Plasma NT-proBNP concentrations >270 pmol/l in cats with respiratory signs support CHF as the cause of respiratory signs.14,15
Almost all current studies evaluating NT-proBNP for diagnosis, prognosis and management of feline cardiomyopathy involve sampling at a single time point. The intra-individual biologic variability of plasma NT-proBNP was evaluated in one study using both adult healthy cats and cats with cardiomyopathy diagnosed via echocardiogram. There was no difference between individual NT-proBNP values; however, this study only evaluated two time points: baseline and 7–10 days later. 18 This may not have been sufficient sampling to evaluate weekly variability and did not evaluate within-day variability. Within-day and week-to-week variability of NT-proBNP may affect the diagnostic utility of the test creating false-positive or false-negative results. False results could lead to increased cost associated with expensive diagnostics, such as an echocardiogram, that are unnecessary or a delay in the diagnosis of cardiac disease, which could result in increased morbidity and mortality. If significant variability exists, this may alter interpretation of results or sample timing for serial monitoring. The objective of this study was to determine the intra-individual biologic variation of NT-proBNP concentrations within-day and week-to-week in healthy adult cats.
Materials and methods
Animals
Apparently healthy client-owned cats were recruited for the study. Each cat was evaluated by complete blood count, serum biochemistry, total thyroxine (T4) concentration, urinalysis, complete echocardiography, ECG and blood pressure measurement prior to inclusion in the study, to exclude the presence of underlying systemic or cardiac disease. Cats with evidence of hyperthyroidism, hypertension, azotemia or systemic disease were excluded. For the purpose of this study, azotemia was defined as blood urea nitrogen (BUN) concentration >36 mg/dl and/or creatinine concentration >1.6 mg/dl. Hyperthyroidism was defined as total T4 concentration >3.0 µg/dl and/or the presence of a palpable thyroid nodule. Cats were considered normotensive if systolic blood pressure was <160 mmHg using a Doppler technique (Ultrasonic Doppler Flow Detector; PARKS Medical Electronics). The study was approved by the University of Florida Institutional Animal Care and Use Committee and written consent to participate in the study was obtained from owners.
Cardiac evaluation
A board-certified cardiologist (AHE) performed all echocardiographic studies using an ultrasound unit equipped with a 5.5–7.5 MHz phased array transducer with continuous monitoring of the ECG (GE Vivid 7 Dimension; GE). Echocardiographic examinations were performed without sedation on gently restrained cats in lateral recumbency to obtain short- and long-axis views. All standard two-dimensional and M-mode variables were measured according to recommendations set by the American Society of Echocardiography and published methodology in the veterinary literature. 19 Cats were considered free of cardiac disease if they did not have any color flow or spectral Doppler abnormalities, the left ventricular end diastolic posterior wall dimension and interventricular septal thicknesses at diastole were <6.0 mm, the left atrium: aorta ratio was <1.5 and there was no evidence of arrhythmias found on a six-lead ECG or auscultation.
Measurement of plasma NT-proBNP
In total, 3 ml blood was collected from each fasted (12 h) cat by jugular or medial saphenous venepuncture at 0, 2, 4, 6, 8 and 10 h, to assess within-day variability, and at weeks 1 (time 0 of within-day), 2, 3, 4, 5 and 6 at the same time of day (8–10 am), to assess week-to-week variability. Each blood sample was placed in an EDTA acid tube and centrifuged within 1 h of collection at −4°C for 10 mins at 12,352 x g. All samples were stored at −80°C until shipped in one batch to an external commercial laboratory for measurement of plasma NT-proBNP concentrations (IDEXX Laboratories). Plasma NT-proBNP concentration was measured using a commercially available horseradish peroxidase, colorimetric endpoint assay for quantitative determination of feline NT-proBNP (Cardiopet proBNP; IDEXX Laboratories). All assays for each cat were performed on the same plate to eliminate inter-assay variability. Additional tests performed on each sample included packed cell volume (PCV) and total protein (TP). BUN and creatinine were measured on every weekly sample.
The quantitative NT-proBNP assay has been previously validated in cats with an intra-assay coefficient of variation (CV) of 6.2%, 6.3%, 4.1%, 1.6%, 2.7% and 2.9% from lowest to highest concentrations, and an inter-assay CV of 8.8%, 8.2%, 7.7%, 4.3%, 4.4% and 6.0% from lowest to highest concentrations (47–871 pmol/l), respectively. The limit of detection is 10 pmol/l with a limit of quantitation of 24 pmol/l. 20
Statistical and data analysis
The data were tested for the presence of outliers with Reed’s criterion test, and outliers were excluded from further statistical analysis. The coefficient of variation is defined as SD divided by the mean. The total CV (CVT) was calculated for each cat’s daily (0, 2, 4, 6, 8 and 10 h) and weekly (week 1, 2, 3, 4, 5 and 6) NT-proBNP concentrations. The analytical CV (CVA) for the NT-proBNP assay was assessed in a validation study using 324 samples and was 6.2%. 20 Between-cat CV (CVG) was calculated for both daily and weekly NT-proBNP concentrations. Intra-individual CV (CVI) was calculated for each cat’s daily and weekly NT-proBNP concentrations using the following formula:
Indices of individuality were calculated from the CVs using the following formula:
To estimate what constituted a significant change in NT-proBNP concentrations beyond individual and assay variation, reference change values (RCV) were calculated with a 95% confidence interval for each cat’s daily and weekly NT-proBNP concentrations using the following formula:21,22
A value of 1.96 was used for zp, indicating a 5% probability of type I error.
The percentage change was calculated between a single baseline compared with each of the other time points. Each time point acted as a baseline for comparison for each of the different time points for daily and weekly samples. Variables analyzed included PCV and TP with additional variables analyzed only at the weekly NT-proBNP samples including creatinine, BUN, blood pressure, body condition score, weight (kg), pulse, respiratory rate and temperature.
Percentage change data were analyzed using a repeated measure mixed model nominating subject and intercept as random effects and nesting replication within subject with a compound symmetry covariance structure. The models analyzed a comparison of the six different time points followed by a Tukey’s post-hoc adjustment. All analyses were modeled in univariate where time acted as the sole predictor. All analyses were considered significant with an alpha of 0.05 and carried out using SAS 9.3.
Results
Study population
Twenty-one adult cats were initially screened for inclusion in the study but only 13 cats met the inclusion criteria. One cat had an elevated total T4 concentration and two cats were found to be azotemic with isothenuria. The remaining five cats were excluded owing to personality characteristics making them difficult to restrain for multiple venepunctures. One cat was an outlier based on Reed’s criterion test and was excluded from analysis, thus resulting in a study population of 12 adult cats.
The study population of 12 adult healthy cats consisted of seven male neutered and five female spayed cats. There were 11 domestic shorthairs and 1 Maine Coon. Ages ranged from 3.5 to 9.0 years (median 5.0 years). Weights ranged from 3.9 to 6.3 kg (median 5.1 kg). The median NT-proBNP concentrations were calculated for the daily and weekly time points (Table 1).
Table 1.
N-terminal pro-brain natriuretic peptide (NT-proBNP) concentrations for all cats at each daily and weekly time point
| Daily time points | Daily NT-proBNP concentrations (pmol/l) | Weekly time points | Weekly NT-proBNP concentrations (pmol/l) |
|---|---|---|---|
| 0 h | 23 (9–35) | Week 1 | 23 (9–35) |
| 2 h | 21.5 (10–43) | Week 2 | 21.5 (9–54) |
| 4 h | 21.5 (10–41) | Week 3 | 23 (10–49) |
| 6 h | 23 (10–45) | Week 4 | 23.5 (12–62) |
| 8 h | 23 (16–46) | Week 5 | 24.5 (9–46) |
| 10 h | 27 (17–53) | Week 6 | 34 (19–90) |
Data are median (range)
Individual variability
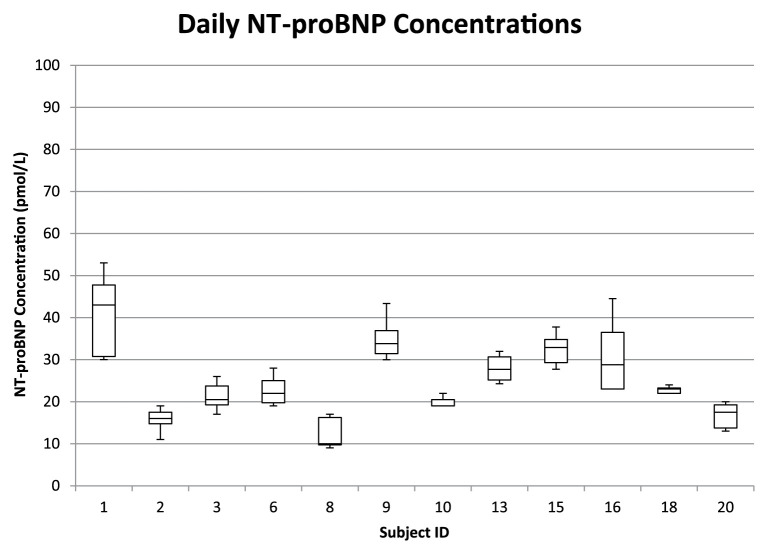
The median daily and weekly NT-proBNP concentrations are shown in Figures 1–4, respectively. All samples were within the manufacturers’ reported reference interval (RI) of <100 pmol/l (Cardiopet proBNP; IDEXX Laboratories). The median daily NT-proBNP CVI was 13.1% (range 0–28.7%) and the median daily RCV was 39.8% (range 17.0–80.5%). The median weekly CVI was 21.2% (range 3.9–68.1%) and the median weekly RCV was 60.5% (range 20.1–187.8%).
Figure 1.
Box and whisker plots of the within-day median and interquartile plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) concentrations in 12 healthy adult cats. Whiskers denote range. The box represents the 25th–75th percentile. The horizontal bar represents the median. The reported reference interval for healthy adult cats is <100 pmol/l
Figure 2.
Box and whisker plots of the weekly median and interquartile plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) concentrations in 12 healthy adult cats. See Figure 1 for key
Figure 3.
Box and whisker plots of the daily median and interquartile plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) concentrations in 12 healthy adult cats and the outlier cat that was excluded. See Figure 1 for key
Figure 4.
Box and whisker plots of the weekly median and interquartile plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) concentrations in 12 healthy adult cats and the outlier cat that was excluded. See Figure 1 for key
The calculated CVs were used to determine the indices of individuality for both daily and weekly NT-proBNP concentrations. The index of individuality was 0.99 and 1 for the daily and weekly samples, respectively. These values are within the gray zone, suggesting that a population-based RI should be used with caution. Analytical variation of the assay was less than half individual variation (CVA <½CVI) for NT-proBNP in both daily and weekly samples indicating adequate precision for this analyte.
Discussion
The use of NT-proBNP in the assessment and management of cardiac disease is increasing, based on communication with IDEXX Laboratories (CA Mainville, 2015, personal communication). In view of the growing interest in its use for identifying heart disease and guiding medical therapy response, the issue of variability is important. This study demonstrated a high individual variability for NT-proBNP concentrations in a population of adult healthy cats. In order to determine if a population- or subject-based RI would be more appropriate an index of individuality was calculated. An index of individuality of <0.6 indicates that subject-based reference values are more appropriate to use and when the index of individuality is >1.4 a population-based RI is more appropriate.22,23 When the index of individuality is between 0.6 and 1.4, as occurred in this study, a population-based range should be used with caution and a subject-based RI should be considered. Furthermore, when the CVI is less than the CVG, as also occurred in this study, a subject-based RI may be more sensitive than a population-based RI for detection of disease. A subject-based RI may able to identify subjects with significant changes in the concentration of a biochemical marker that would still fall within a population-based RI. Thus, a subject-based RI could be more sensitive in detecting changes in the health of an individual.20,24,25
When a subject-based RI is being considered, a reference change value, which is the difference between consecutive test results in an individual that is statistically significant, may be more sensitive in detecting significant change within a cat. This is based on intra-individual biologic variation and analytical error. Analytical error includes both imprecision (variation) and inaccuracy (bias). Analytic error for the NT-proBNP quantitative assay used in this study was previously determined during a method validation study. 20 The current study found large reference change values of 39.8% and 60.5% for daily and weekly NT-proBNP concentrations in healthy adult cats, respectively. In essence, a serial measurement of NT-proBNP would have to increase or decrease by up to 60% from the previous measurement to be considered a significant change.
All NT-proBNP concentrations in this study were within the RI, with most of the values in the lower half of this range. Based on the RCV calculated, a significant change in NT-proBNP could occur, but the concentration may remain within the RI. Thus, a subject-based RI for NT-proBNP concentrations may be more sensitive at detecting changes over time. However, further studies are needed to determine if these changes correlate with development of cardiac disease prior to the NT-proBNP concentration exceeding the RI.
Many factors contribute to total individual variability, including pre-analytic factors, analytic variability and biologic variability. Pre-analytic factors include patient preparation and sample collection and handling such as vial type, collection system, transportation, centrifugation and storage. The methods used in this study to minimize the impact of pre-analytic factors through careful cat preparation and blood collection protocol should result in a more accurate estimate of intrinsic biologic variability. Routine use of those methods may not be practical in clinical settings and thus the overall variation may be higher in this environment. Artefactual increases in biologic variation can occur when the analytic variation is high, indicating imprecision of the assay. When CVA approaches CVI it is hard to determine whether any variation is intra-subject or due to limitations of the equipment. Previously described tolerable imprecision is defined as a CVA < 0.5 CVI. 22 This was achieved in this study and thus is unlikely to effect the biologic variation.
The daily NT-proBNP concentrations were found to increase significantly throughout the day in this study, indicating a possible circadian rhythm. However, samples were not collected for the full 24 h, so this could not be fully evaluated. It is possible that stress from repeated blood draws during the day and week-to-week may have had an increased effect on NT-proBNP. By obtaining samples at the same time of day for the weekly samples we controlled for potential circadian variability as a possible contributor to the weekly biologic variation. 26
There was significant variation in NT-proBNP concentrations over time in both the daily and weekly samples. The cause for the biologic variability in this study is not clear and may be influenced by physical exertion, fluid intake, subtle changes in intra-ventricular pressure/volume status or alterations in peptide clearance mechanisms. Mature BNP is removed from circulation by the clearance receptors C-type natriuretic peptide receptor (NPR-C). N-terminal fragments of proBNP are removed more slowly from circulation than their C-terminal counterparts because clearance of these peptides is more dependent on renal excretion. 27
We excluded one cat from statistical analysis as an outlier. This cat had increased NT-proBNP concentrations that were above the RI for all of the daily samples (range 91–264 pmol/l) and weeks 1 and 2 (range 91–115 pmol/l) before returning to within the RI. Outliers can have a profound effect on summary statistics, especially variances. 23 No reason was found for such dramatic variations to occur in this one cat across multiple samples both within day and across multiple weeks. This cat may have had underlying systemic or cardiac disease that we were unable to identify or may represent a false-positive. A second echocardiogram performed at the end of the study did not reveal evidence of cardiac disease. Other non-cardiac factors may have resulted in an increased NT-proBNP but were not identified. Long-term follow-up would be needed to determine if cardiac disease develops in this cat in the future.
One of the limitations of this study was individual variation in degree of exertion and water intake, which may result in variability in NT-proBNP concentrations. Circadian rhythm is another factor that may affect individual variation. All weekly samples were taken at the same time at each visit to try and minimize this as a possible factor. However, this may not be representative of daily practice and the effective variability may be larger. Based on these results, it may be best to sample cats in a fasted state and at the same time of day for serial measurements.
There is growing interest in using serial measurements of NT-proBNP concentrations in monitoring the success of drug therapy in cats with cardiac disease. In human studies, inpatient monitoring of decompensated CHF suggests that serial NT-proBNP measurements are useful for detecting short-term cardiac improvement with therapy and improved survival outcomes.28-32 Similar data are not currently available in cats. It is important to note that the RCVs established in healthy cats during this study may be inappropriate for use in monitoring sick patients as biologic variability and RCV in diseased patients may be higher or lower than healthy individuals. 33 Thus, further studies in cats with stable cardiac disease are needed to determine the biologic variation and RCV in this population.
Conclusions
This study demonstrates that high individual variability of NT–proBNP exists in a population of adult healthy cats. Further research is warranted to understand this variability and determine how serial measurements of NT-proBNP may be used to identify and guide management in cats with underlying cardiac disease.
Acknowledgments
We are grateful to the technicians and veterinarians who assisted in data collection and care of the cats in this study. We are also grateful to the college faculty, staff and student cat owners who volunteered for the study.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was funded by IDEXX Laboratories, Westbrook, ME, USA and an intramural resident research grant at the University of Florida, Gainesville, FL.
Accepted: 30 November 2015
References
- 1. Paige CF, Abbott JA, Elvinger F, et al. Prevalence of cardiomyopathy in apparently healthy cats. J Am Vet Med Assoc 2009; 234: 1398–1403. [DOI] [PubMed] [Google Scholar]
- 2. Riesen SC, Kovacevic A, Lombard CW, et al. Prevalence of heart disease in symptomatic cats: an overview from 1998 to 2005. Schweiz Arch Tierheilkd 2007; 149: 65–71. [DOI] [PubMed] [Google Scholar]
- 3. Cote E, Manning AM, Emerson D, et al. Assessment of the prevalence of heart murmurs in overtly healthy cats. J Am Vet Med Assoc 2004; 225: 384–388. [DOI] [PubMed] [Google Scholar]
- 4. Nakamura RK, Rishniw M, King MK, et al. Prevalence of echocardiographic evidence of cardiac disease in apparently healthy cats with murmurs. J Feline Med Surg 2011; 13: 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schober KE, Maerz I, Ludewig E, et al. Diagnostic accuracy of electrocardiography and thoracic radiography in the assessment of left atrial size in cats: comparison with transthoracic 2 dimensional echocardiography. J Vet Intern Med 2007; 21: 709–718. [DOI] [PubMed] [Google Scholar]
- 6. Cote E, Manning AM, Emerson D, et al. Assessment of the prevalence of heart murmurs in overtly healthy cats. J Am Vet Med Assoc 2004; 225: 384–388. [DOI] [PubMed] [Google Scholar]
- 7. Wood MJ, Picard MH. Utility of echocardiography in the evaluation of individuals with cardiomyopathy. Heart 2004; 90: 707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biondo AW, Ehrhart EJ, Sisson D, et al. Immunohistochemistry of atrial and brain natriuretic peptides in control cats and cats with hypertrophic cardiomyopathy. Vet Pathol 2003; 40: 501–506. [DOI] [PubMed] [Google Scholar]
- 9. Vanderheyden M, Goethals M, Verstreken S, et al. Wall stress modulates brain natriuretic peptide production in pressure overload cardiomyopathy. J Am Coll Cardiol 2004; 44: 2349–2354. [DOI] [PubMed] [Google Scholar]
- 10. Connolly DJ, Soares Magalhaes RJ, Syme HM, et al. Circulating natriuretic peptides in cats with heart disease. J Vet Intern Med 2008; 22: 96–105. [DOI] [PubMed] [Google Scholar]
- 11. Wess G, Daisenberger P, Mahling M, et al. Utility of measuring plasma N-terminal pro-brain natriuretic peptide in detecting hypertrophic cardiomyopathy and differentiating grades of severity in cats. Vet Clin Pathol 2011; 40: 237–244. [DOI] [PubMed] [Google Scholar]
- 12. Fox PPR, Rush JE, Reyonolds CA, et al. Multicenter evaluation of plasma N-terminal probrain natriuretic peptide (NT-proBNP) as a biochemical screening test for asymptomatic (occult) cardiomyopathy in cats. J Vet Intern Med 2011; 25: 1010–1016. [DOI] [PubMed] [Google Scholar]
- 13. Tominaga Y, Miyagawa Y, Toda N, et al. The diagnostic significance of plasma N-terminal pro-B-type natriuretic peptide concentration in asymptomatic cats with cardiac enlargement. J Vet Med Sci 2011; 73: 971–975. [DOI] [PubMed] [Google Scholar]
- 14. Connolly DJ, Soares Magalhaes RJ, Fuentes VL, et al. Assessment of the diagnostic accuracy of circulating natriuretic peptide concentrations to distinguish between cats with cardiac and non-cardiac causes of respiratory distress. J Vet Cardiol 2009; 11 Suppl 1: S41–S50. [DOI] [PubMed] [Google Scholar]
- 15. Fox PR, Oyama MA, Reynolds C, et al. Utility of plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) to distinguish between congestive heart failure and non-cardiac causes of acute dyspnea in cats. J Vet Cardiol 2009; 11 Suppl 1: S51–S61. [DOI] [PubMed] [Google Scholar]
- 16. Singletary GE, Rush JE, Fox PR, et al. Effects of NT-proBNP assay on accuracy and confidence of general practitioners in diagnosing heart failure or respiratory disease in cats with respiratory signs. J Vet Intern Med 2012; 26: 542–546. [DOI] [PubMed] [Google Scholar]
- 17. Kellihan HB, Mackie BA, Stepien RL. NT-proBNP, NT-proANP and cTnI concentrations in dogs with pre-capillary pulmonary hypertension. J Vet Cardiol 2011; 13: 171–182. [DOI] [PubMed] [Google Scholar]
- 18. Reynolds CA, Kellihan HB, Alivernini A, et al. Weekly variability of plasma NT-proBNP measurements in cats with and without heart disease [abstract]. J Vet Intern Med 2010; 24: 730. [Google Scholar]
- 19. Petric AD, Rishniw M, Thomas WP. Two-dimensionally-guided M-mode and pulsed wave Doppler echocardiographic evaluation of the ventricles of apparently healthy cats. J Vet Cardiol 2012; 14: 423–430. [DOI] [PubMed] [Google Scholar]
- 20. Mainville CA, Clark GH, Esty KJ, et al. Validation of immunoassay for the quantification of N-Terminal Pro-B-Type natriuretic peptide in feline blood. J Vet Diagn Invest 2015; 27: 414–421. [DOI] [PubMed] [Google Scholar]
- 21. Walton RM. Subject-based reference values: biological variation, individuality, and reference change values. Vet Clin Pathol 2012; 41: 175–181. [DOI] [PubMed] [Google Scholar]
- 22. Cotlove E, Harris EK, Williams GZ. Biological and analytic components of variation in long-term studies of serum constituents in normal subjects. III. Physiological and medical implications. Clin Chem 1970; 16: 1028–1032. [PubMed] [Google Scholar]
- 23. Fraser CG, Harris EK. Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci 1989; 27: 409. [DOI] [PubMed] [Google Scholar]
- 24. Solberg HE. Subject-based reference values. Scand J Clin Lab Invest Suppl 1995; 222: 7–10. [DOI] [PubMed] [Google Scholar]
- 25. Fraser CG. Inherent biological variation and reference values. Clin Chem Lab Med 2004; 42: 758–764. [DOI] [PubMed] [Google Scholar]
- 26. Guo YF, Stein PK. Circadian rhythm in the cardiovascular system: chronocardiology. Am Heart J 2003; 145: 779–786. [DOI] [PubMed] [Google Scholar]
- 27. Sisson DD. Neuroendocrine evaluation of cardiac disease. Vet Clin Small Anim 2004; 34: 1105–1126. [DOI] [PubMed] [Google Scholar]
- 28. Kazanegra R, Chen V, Garcia, et al. A rapid test for B-type natriuretic peptide correlates with falling wedge pressures in patients treated for decompensated heart failure: a pilot study. J Card Fail 2001; 7: 21–29. [DOI] [PubMed] [Google Scholar]
- 29. Johnson W, Omland T, Hall C, et al. Neurohormonal activation rapidly decreases after intravenous therapy with diuretics and vasodilators for class IV heart failure. J Am Coll Cardiol 2002; 39: 1623–1629. [DOI] [PubMed] [Google Scholar]
- 30. Teerlink JR, Massie BM, Calucci WS, et al. Rapid and sustained reductions of plasma B-type natriuretic peptide concentrations in patients with acutely decompensated heart failure treated with levosimendan: The REVIVE II study. J Am Coll Cardiol 2006; 47: 86A. [Google Scholar]
- 31. Gackowski A, Isnard R, Golmard JL, et al. Comparison of echocardiography and plasma B-type natriuretic for monitoring the response to treatment in acute heart failure. Eur Heart J 2004; 25: 1788–1796. [DOI] [PubMed] [Google Scholar]
- 32. Dhaliwal A, Deswal A, Pritchett AM, et al. How much reduction is needed in BNP levels to reduce future clinical events with treatment during hospitalization for heart failure. J Am Coll Cardiol 2006; 79: 72A. [Google Scholar]
- 33. Ricos C, Iglesias N, Garcia-Lario JV, et al. Within-subject biological variation in disease: collated data and clinical consequences. Ann Clin Biochem 2007; 44: 343–352. [DOI] [PubMed] [Google Scholar]