Abstract
Objectives
The aims of the study were to determine the in vitro drug release of guar gum-coated capsules of ronidazole, and to evaluate the pharmacokinetics and efficacy of this formulation for the treatment of cats naturally infected with Tritrichomonas foetus.
Methods
The pharmacokinetics of ronidazole were evaluated in five healthy cats and five cats infected with T foetus. In a second step, the clinical efficacy of these capsules was evaluated by a controlled, randomised, double-blind clinical trial performed in 47 infected cats from French catteries. In this study, cats were randomly allocated to either the ronidazole treatment group (n = 25) or a placebo group (n = 22). Ronidazole (30 mg/kg) q24h for 14 days was administered to the treated cats. After 14 days of treatment, the presence of T foetus was tested by conventional PCR assay.
Results
In the pharmacokinetic study, a delayed peak plasma concentration was observed in healthy and infected cats, with no significant difference between these two groups (mean geometric mean of 9 h for time to maximum plasma concentration [Tmax], 21.6 µg/ml for time to maximum plasma concentration [Cmax] and 467.4 μg/h/ml for the area under the curve [AUC] in healthy cats; and 9.4 h for Tmax, 17.1 µg/ml for Cmax and 481 μg/h/ml for AUC in infected cats). In the clinical trial, T foetus was detected in 16% of cats from the treated group and 82% of cats from the placebo group at the end of the study (P <0.001). No clinical signs of adverse drug reactions were observed.
Conclusions and relevance
Oral administration of guar gum-coated capsules of ronidazole at a dose of 30 mg/kg once daily for 14 days delays the peak plasma concentration and eradicates infection in most cases.
Introduction
Tritrichomonas foetus is a flagellated protozoan parasite that is most widely known as a cause of reproductive disease in cattle. 1 Recently, the parasite has been identified as a pathogen in feline medicine, as it colonises the ileum, caecum and colon of cats, causing chronic large bowel diarrhoea.2,3 The faeces of infected cats are malodorous, semiformed to liquid in consistency, and sometimes contain blood and/or mucus. 2 Tritrichomonas foetus has been detected in North America, Europe, Asia and Oceania;2–15 consequently, this infection has been recognised as pandemic. The majority of infected cats come from shelters and breeding catteries in which the density of animals is high. Such conditions – especially environmental stress and immunological immaturity of young cats – increases exposure and susceptibility to the parasite. 2
Tritrichomonas foetus has been shown to be resistant to most traditionally used antiprotozoal drugs such as fenbendazole. Trichomonads lack mitochondria and derive their energy from organelles called hydrogenosomes. 16 This particular metabolism has been shown to make trichomonads susceptible to compounds from the 5-nitroimidazole family, such as metronidazole, tinidazole, nimorazole, dimetrazole and ronidazole.16,17 These molecules are able to diffuse into hydrogenosomes where they are reduced to cytotoxic nitro anions, which disrupt the parasites’ DNA. Several in vitro and in vivo studies have evaluated the efficacies of different 5-nitroimidazole compounds against T foetus.18–20 Ronidazole has been shown to kill T foetus in vitro, 18 and when 30 mg/kg was orally administered every 12 or 24 h for 14 days, it resolved diarrhoea and eradicated T foetus infection in cats.19,21,22 However, adverse effects, such as reversible neurological signs, were observed in cats treated with this drug. 23 The reported side effects include lethargy, trembling of extremities, ataxia, agitation, facial tremors and hyperesthesia. 23 These clinical signs could be linked to the rapid absorption, the high bioavailability and the slow elimination of ronidazole in some cats. 23
Pharmacokinetic studies have shown that coating ronidazole formulations in guar gum induces a delay in drug absorption consistent with colonic drug delivery. 24 Colonic delivery makes the drug available for local action and therefore it could be an effective treatment of intestinal infections caused by T foetus. This study had four aims: (1) to develop guar gum-coated capsules of ronidazole, (2) to determine the in vitro drug release of these capsules, (3) to evaluate the pharmacokinetics of ronidazole in this delayed-release formulation in healthy cats and cats infected with T foetus, and (4) to evaluate the efficacy of this targeted formulation for treatment of cats naturally infected with T foetus.
Materials and methods
Drug formulation
Guar gum-coated capsules of ronidazole were prepared at Alfort Veterinary School (Paris, France), following the rules of the European pharmacopoeia. Ronidazole powder (Fagron) was weighed and mixed by geometric dilution with guar gum (Fagron), which was used as a specific drug carrier for ronidazole. Hard gelatin capsules of the appropriate size (Fagron) were then filled with the mixed powder, and caps were coated with three batches of acetophtalate cellulose solution (Fagron) to make them gastroresistant.
In vitro drug release
Guar gum-coated capsules of ronidazole (n = 6) were subjected to dissolution tests in simulated gastric and intestinal environments as previously described. 24 The simulated gastric fluid consisted of hydrochloric acid 0.1 N (400 ml, pH 2) in a beaker kept between 37°C and 40°C and stirred at 50 rpm with a stir bar for 3 h (average gastric emptying time in cat).25,26 The simulated intestinal environment test was similar, except that the HCl solution was replaced by phosphate buffer (pH 6.8). Drug release was monitored at various time intervals (10, 30, 45, 60, 120 and 180 mins in both the simulated gastric and intestinal environments) by withdrawing 5 ml of the sample and assessing drug concentration using a spectrophotometer (Jasco V 630 UV/VIS Spectrometer; Jasco) set at a wavelength of 313 nm. The ronidazole concentration in each of the samples was determined by comparing the absorbance of samples (5 ml) with calibration curves of absorbance plotted against ronidazole concentration. The calibration curves were generated from five different ronidazole dilutions (from 10 to 310 μg/ml and from 20 to 310 μg/ml) prepared in HCl and phosphate solutions, respectively. The calibration curves were linear with an R2 value ⩾0.99.
Ronidazole pharmacokinetics
Cats
The plasma concentrations of ronidazole were assessed in five healthy cats and five cats infected with T foetus. Infections were confirmed by PCR testing for the rRNA gene unit of T foetus. 27 The healthy cats included five intact male cats (4.7 kg ± 0.6 kg body weight) from the breeding group at the Animal Clinic Research Institute. The second group included five cats naturally infected with T foetus (four females, one male; 3.8 kg ± 1.8 kg body weight). These five infected cats came from a private French cattery. Cats were housed in the Veterinary School of Alfort (Paris, France) for the duration of the study. Authorisation of projects using animals for scientific use was granted by the ethics committee at the Ministry of Higher Education.
Ronidazole administration and sample collection
Fifteen hours prior to drug administration, a 22G catheter (BD Insyte-W; Becton Dickinson) was placed in the medial saphenous vein of each cat, to facilitate sample collection. To minimise the stress of catheter placement, cats were sedated with a combination of subcutaneous pethidine (Richter Pharma) and acepromazine (Calmivet; Vetoquinol). Food was withheld for 24 h prior to and 8 h after drug administration. Water was available ad libitum.
Cats received one guar gum-coated capsule of ronidazole, followed by tap water (5 ml) orally to ensure that the medication was swallowed. Blood samples (3 ml each) were collected via the intravenous catheter prior to and 1, 2, 4, 6, 8, 10, 12, 16, 24, 30 and 48 h after drug administration. Cumulative blood collection volumes were within published guidelines, to avoid adverse haemodynamic effects. 28 Blood was immediately transferred to tubes containing sodium citrate (BD Vacutainer; Becton Dickinson) and centrifuged to harvest plasma. Plasma was frozen and stored at −80°C until analysis.
Ronidazole assay
The quantitative determination of ronidazole in feline plasma samples was performed using high-performance liquid chromatography (HPLC) according to the procedure of Levine et al, 22 with modification. The HPLC system (LC-Net II/ADC; Jasco) consisted of a PU2089 JASCO pump, a LIChrospher 100RP18 encapped column (5 µM granule size, 250 × 4 mm; VWR International), an AS2051 sampler, a UV2075 detector (315 nm) and software (LACHROM; VWR International). A gradient elution system was used as follows: from 98% water (acidified with 0.2% H3PO4)/2% acetonitrile to 50% water/50% acetonitrile at 8 mins. Standard ronidazole was dissolved in acetonitrile to make a 1 mg/ml stock solution. Each plasma sample (0.5 ml) was extracted with acetonitrile (10 ml) and vortexed. The supernatant was collected in a clean glass tube and evaporated under a stream of compressed air at 40°C for 20 mins. Evaporated samples were reconstituted with mobile phase (200 μl) and vortexed. A 50 µl sample of each reconstituted solution was then injected into the HPLC system.
All calibration curves were linear with R² values of 0.99 or greater. The retention time of ronidazole, was 8.61 mins. The detection limit and quantification limit were 22.8 ng/ml and 47.2 ng/ml, respectively.
Pharmacokinetic calculations
The average of each cat’s normalised ronidazole concentration (cat’s ronidazole concentration at a given time point divided by the ratio of the cat’s dose to the mean dose) was calculated. Pharmacokinetic parameters were derived from the single-dose individual cat data using non-compartmental analysis as previously described. 24 Area under the curve (AUC), mean residence time (MRT), maximum plasma concentration (Cmax) and time to Cmax (Tmax) were evaluated. These parameters were calculated using PKSolver, an add-in used for pharmacokinetic and pharmacodynamic data analysis in Excel (Microsoft).29,30 These parameters were reported as the geometric mean and SD of the geometric mean. 24
Clinical trial
Identification of cases
Tritrichomonas foetus was evaluated using PCR according to protocol described by McMillen and Lew or using specific media (InPouch TF test; Biomed Diagnostics). 27 Media were immediately inoculated as already described. 31 The culture was incubated vertically at 25°C in the dark for 14 days and examined every day using optical microscopy at 160× magnification. A cat was considered as infected by T foetus if at least one motile trophozoite was observed in the media during these 14 days or if the PCR was positive.
Experimental design
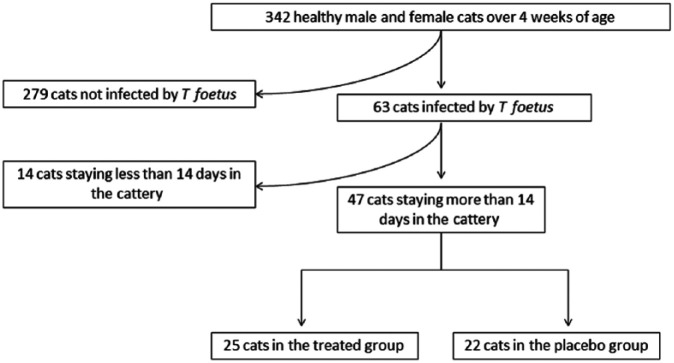
Between July 2010 and December 2011, a pre-inclusion screening phase was performed in 30 different French catteries. All healthy cooperative male and non-pregnant female cats of >4 weeks of age were included in this screening phase. A complete clinical examination was performed on the 342 eligible cats and a rectal swab was taken for the detection of T foetus by PCR or using the specific media (Figure 1). A total of 63/342 (18%) screened cat were infected by T foetus. Among these 63 positive cats, only cats staying in the cattery for the duration of the study were included in the clinical trial (n = 47). Before participation in the study, all breeders signed an informed consent document approved by the ethics committee of Alfort National Veterinary School, France.
Figure 1.
Flow chart description of cats that met the inclusion criteria for the study
Procedures
The clinical trial was a double-blind, randomised study comparing the efficacy of ronidazole (30 mg/kg of body weight once daily for 14 days) with placebo. Cats from the treated group (n = 25) received guar gum-coated capsules of ronidazole for a dose of approximately 30 mg/kg body weight of ronidazole (Table 1). The placebo group cats (n = 22) received capsules prepared by the same process but that lacked ronidazole. The randomisation scheme was based on a list of random numbers generated by computer. The breeder and veterinary investigator were blinded to the treatment.
Table 1.
Composition of the different capsules according to the weight of cats
| Ronidazole per capsule (mg) | Weight of cats (kg) receiving the capsule | Dose of ronidazole (mg/kg) | Number of cats in each weight range |
|---|---|---|---|
| 72 | 2–2.4 | 30–36 | 3 |
| 84 | 2.4–2.8 | 30–35 | 1 |
| 96 | 2.8–3.2 | 30–34.3 | 13 |
| 108 | 3.2–3.6 | 30–33.8 | 5 |
| 120 | 3.6–4 | 30–33.3 | 3 |
| 132 | 4–4.4 | 30–33 | 5 |
| 150 | 4.4–5 | 30–34.1 | 7 |
| 162 | 4.8–5.4 | 30–33.8 | 5 |
| 174 | 5.2–5.8 | 30–33.5 | 2 |
| 186 | >5.8 | 30–32.1 | 3 |
Evaluation of efficacy and safety
At the end of the 14 days of treatment, one rectal swab was collected from each cat and was stored at −80°C until testing by PCR according to protocol described by McMillen and Lew. 27 Cats were defined as still infected if this test was positive. A clinical examination was performed on each cat and the presence of adverse effects was recorded. The veterinary investigator who performed the clinical evaluation and rectal swabs was blinded to the treatment.
Statistical analysis
Statistical analysis for ronidazole pharmacokinetic analysis
Normality and equality of variances of the different pharmacokinetic parameters were evaluated in the two groups of cats infected or not by T foetus, using the Shapiro–Wilk test and Fisher’s test, respectively. For pharmacokinetic parameters with unequal variances (AUC and MRT) a Student’s t-test for unequal variances was used to compare these parameters in the two groups of cats. For the other pharmacokinetic parameters with equal variances (Cmax, Tmax) a Student’s t-test was used. Type one error was set at alpha = 0.05. Data were analysed using free software (Tanagra).
Statistical analysis for the field trial
The efficacy of ronidazole (vs placebo) was evaluated by comparing the two groups, using χ2 statistical tests. The χ2 test was performed to evaluate the efficacy of ronidazole treatment, in which the proportion of infected cats in treated and placebo groups were compared. Medians were compared using the non-parametrical Wilcoxon statistical test. Type one error was set at alpha = 0.05. Data were analysed using free software (Epi Info; US Centers for Disease Control and Prevention).
Results
In vitro drug release
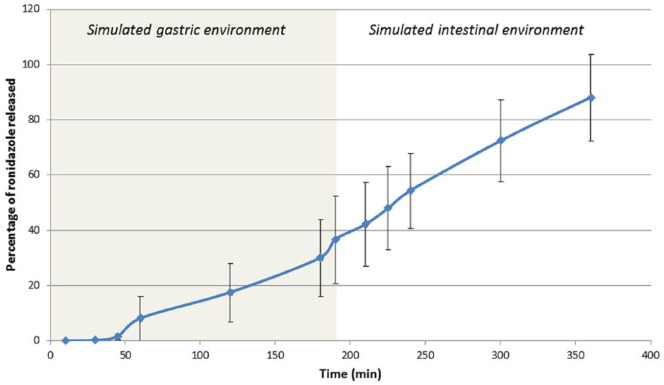
In vitro drug release studies showed that 30 ± 13.9% (mean ± SD) of ronidazole was released from capsules after 3 h in a simulated gastric environment. 32 The release of ronidazole from capsules continued after moving these capsules to the simulated intestinal fluids, and release reached 54.3 ± 13.5% 1 h later and 87.9 ± 15.7% 3 h later (Figure 2).
Figure 2.
Percentage of ronidazole released (mean and SD) vs time in a simulated gastric environment (time 0–180 mins) followed by a simulated intestinal environment (time 180–360 mins)
Ronidazole pharmacokinetics
Plasma ronidazole concentrations over time in the two groups of cats (healthy cats and cats infected by T foetus) are summarised in Table 2. In both groups, the plasma concentration of ronidazole was less than the lower limit of quantification at time zero for all cats. Ronidazole pharmacokinetic values were not significantly different between the two groups of cats (Table 3). Ronidazole was detected in the plasma of all 10 cats 1 h after administration, with a mean Tmax of 9 ± 2.3 h for healthy cats and 9.4 ± 4 h in cats infected by T foetus (P = 0.708). The mean Cmax was 21.6 ± 4 µg/ml and 17.1 ± 9.2 µg/ml in healthy cats and cats infected by T foetus, respectively (P = 0.499; Table 2). No adverse neurological events associated with ronidazole administration were observed in any cats.
Table 2.
Concentration vs time for ronidazole in cat plasma (normalised for dose) following oral administration of a single oral dose (30 mg/kg) of guar gum ronidazole capsules
| Time (h) | Ronidazole concentrations in cats not infected by T foetus (µg/ml) | Ronidazole concentrations in cats infected by T foetus (µg/ml) |
|---|---|---|
| 0 | 0 ± 0 | 0 ± 0 |
| 1 | 3.5 ± 3.4 | 5.7 ± 2.9 |
| 2 | 7.1 ± 3.2 | 7.8 ± 2.6 |
| 4 | 11.0 ± 4.0 | 13.8 ± 5.8 |
| 6 | 14.8 ± 2.8 | 16.4 ± 9.7 |
| 8 | 18.7 ± 5.1 | 17.2 ± 11.8 |
| 10 | 18.6 ± 5.2 | 16.3 ± 10.4 |
| 12 | 17.3 ± 4.0 | 14.8 ± 9.5 |
| 16 | 15.1 ± 3.6 | 12.9 ± 5.0 |
| 24 | 9.2 ± 2.6 | 11.8 ± 9.7 |
| 30 | 7.2 ± 2.0 | – |
| 48 | 2.2 ± 0.8 | 4.3 ± 2.0 |
Data are mean ± SD
T foetus = Tritrichomonas foetus
Table 3.
Ronidazole pharmacokinetic values in 10 cats (five infected by Tritrichomonas foetus and five uninfected) after a single dose of guar gum ronidazole capsules
| Parameters | Units | Cats not infected by T foetus |
Cats infected by T foetus |
P value | ||||
|---|---|---|---|---|---|---|---|---|
| Geometric mean | Range of each parameter | SD | Geometric mean | Range of each parameter | SD | |||
| AUC | μg/h/ml | 467.3 | 415.4–528.3 | 47.0 | 481.0 | 357.0–900.4 | 230.3 | 0.687 |
| Cmax | μg/ml | 21.6 | 18.5–28.5 | 4.0 | 17.1 | 10.7–32.8 | 9.2 | 0.499 |
| MRT | h | 21.3 | 16.4–23.2 | 2.9 | 30.8 | 15.6–47.2 | 13.1 | 0.125 |
| Tmax | h | 9.0 | 6.0–12.0 | 2.3 | 9.4 | 6.0–16.0 | 4.0 | 0.708 |
AUC = area under the curve from time zero to infinity; Cmax = maximum plasma concentration; MRT = mean residence time; Tmax = time to maximum plasma concentration
Clinical trial
Cat characteristics
A total of 47 cats from 14 catteries were included in the study and randomised into ronidazole treatment (n = 25) or placebo (n = 22) groups. Cats had a median age of 17 months (range 3 months–5 years); most were females (55 %; Table 4). The median weight of cats the included cats was 3.7 kg (range 2.2–7.7 kg).
Table 4.
Baseline characteristics of the study sample of the 47 cats
| Variables | Total (n = 47) | Placebo (n = 22) | RDZ (n = 25) | P value |
|---|---|---|---|---|
| Females, % (n) | 26 (55) | 50 (11) | 60 (15) | 0.49 |
| Median (range) age (months) | 17 (3–64) | 18 (5–64) | 17 (3–47) | 0.98 |
| Median (range) weight (kg) | 3.7 (2.2–7.6) | 3.4 (2.8–4.7) | 4 (2.8–7.6) | 0.07 |
| Breed, % (n) | ||||
| Bengal | 17 (8) | 14 (3) | 20 (5) | 0.56 |
| Norwegian Forest Cat | 36 (17) | 45 (10) | 28 (7) | 0.21 |
| Maine Coon | 23 (11) | 14 (3) | 32 (8) | 0.14 |
| Burmilla | 6 (3) | 0 (0) | 12 (3) | 0.09 |
| Sphynx | 2 (1) | 5 (1) | 0 (0) | 0.28 |
| Birman | 4 (2) | 9 (2) | 0 (0) | 0.12 |
| Persian | 4 (2) | 9 (2) | 0 (0) | 0.21 |
| Siberian | 4 (2) | 5 (1) | 4 (1) | 1.00 |
| European Shorthair | 2 (1) | 0 (0) | 4 (1) | 1.00 |
| Median (range) size of breeds (in number of cats) | 17 (3–48) | 17 (3–48) | 17 (3–48) | 0.56 |
RDZ = ronidazole treatment
Treatment efficacy and safety
After 14 days of treatment, 47% (22/47) of cats tested positively (PCR) for infection. Tritrichomonas foetus was not detected in 84% (21/25) of the cats who received ronidazole nor in 18% (4/22) of the cats in the placebo group (P <0.001). No clinical signs of adverse drug reactions were reported by the breeder or observed after clinical examination by the veterinary investigator.
Discussion
In an in vitro study, ronidazole tablets without a guar gum coating released all drug within 10 mins in a simulated gastric environment. 24 In our study, only 30% of ronidazole was released from our guar gum-coated capsules after 3 h in gastric conditions; this increased to 88% after a further 3 h in simulated intestinal conditions. The results of this in vitro study shows that this medication will undergo intestinal release. This result was confirmed by the in vivo study. Orally administered immediate-release ronidazole capsules had a Tmax of 1 h. 23 However, guar gum-coated ronidazole tablets with colonic release had a Tmax 14.5 h. 24 In our study, the maximal plasma concentration was reached approximately 9 h after ingestion of the guar gum-coated capsules of ronidazole, and this intermediate Tmax confirms that ronidazole was released from our guar gum-coated capsules in the intestine.
The presence of colonic trichomonads is associated with mild-to-moderate lymphoplasmacytic and neutrophilic colitis in cats. 33 However, colonic inflammation does not seem to affect ronidazole absorption, as no significant difference in pharmacokinetic parameters was observed between healthy and infected cats.
Tritrichomonas foetus and Giardia species are two enteropathogens frequently observed in cats with reported prevalences of 14–82% and 0.6–80.0%, respectively.7,9,12,34–37 Their prevalence in cats depends on the test population (age, clinical status and origin of cats) and detection method employed. Significant co-occurrence of T foetus with Giardia species has been observed in cats, with co-infection prevalence rates ranging from 4.3% to 54.0%.9,15,36,37 Ronidazole has also been shown to have a highly antiprotozoal effect against these two parasites. 38 The intestinal liberation of ronidazole from guar gum-coated capsules of ronidazole could be useful for the treatment of cats co-infected by these two protozoa.
In our study, administration of the guar gum-coated capsules of ronidazole once a day for 14 days, at a dose of 30 mg/kg body weight eliminated T foetus infection in 84% of cats. The efficacy of ronidazole has already been described in in vitro and in vivo studies;18,19 however, despite its efficacy in T foetus elimination, ronidazole has been reported to cause adverse effects, such as reversible neurological signs.15,39 The pharmacokinetic profile of ronidazole after administration of immediate-release capsules indicates that the full drug absorption occurs rapidly after oral administration. 23 This leads to a high plasma concentration of ronidazole, which could be the cause of neurointoxication in cats. In our study, the cats treated with the guar gum-coated capsules of ronidazole had mean plasma concentrations of 21.6 and 17.1 µg/ml in healthy cats and infected cats, respectively. These plasma concentrations are lower than those described in cats treated with oral immediate-release capsules (35 µg/ml). 23 In this study, no adverse neurological events associated with ronidazole administration were observed in any cats, suggesting that the guar gum-coated capsules of ronidazole could decrease the maximal plasma concentration and so limit the development of neurological signs.
Sixteen percent of the treated cats were still infected at the end of the study. This could be explained by a recontamination of treated cats. Indeed, all cats enrolled were recruited from catteries and consequently lived in dense housing conditions. Moreover, ronidazole-treated cats were housed with placebo-treated cats excreting the parasite during the study. High housing density (low number of square feet of facility area per cat) was identified as a likely risk factor of T foetus infection in cats. 36 Consequently, the density of animals in catteries could promote environmental contamination and recontamination. Tritrichomonas foetus does not form true cysts, but during unfavourable environmental conditions the trophozoites can display a pseudocyst stage, probably allowing a prolonged survival of the parasite.40,41 This treatment failure could also be attributed to inappropriate administration of capsules or a resistance to ronidazole. In vivo and in vitro resistance of feline T foetus isolates to ronidazole has already been documented in two intact cats. 20 Eighteen percent of cats in the placebo group presented a spontaneous elimination of the parasite. This result is in accordance with a previous study in which 46% of cats spontaneously eliminated T foetus. 42 The importance of latent infections to spread T foetus or cause later development of secondary sequelae is unknown.
Conclusions
Peak plasma concentration of ronidazole can be delayed and T foetus infection can be eradicated by a 14 day course of once-daily oral administration of guar gum-coated capsules of ronidazole at a dose of 30 mg/kg.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Accepted: 16 November 2015
References
- 1. Rae DO, Chenoweth PJ, Genho PC, et al. Prevalence of Tritrichomonas fetus in a bull population and effect on production in a large cow-calf enterprise. J Am Vet Med Assoc 1999; 214: 1051–1055. [PubMed] [Google Scholar]
- 2. Gookin JL, Breitschwerdt EB, Levy MG, et al. Diarrhea associated with trichomonosis in cats. J Am Vet Med Assoc 1999; 215: 1450–1454. [PubMed] [Google Scholar]
- 3. Xenoulis PG, Lopinski DJ, Read SA, et al. Intestinal Tritrichomonas foetus infection in cats: a retrospective study of 104 cases. J Feline Med Surg 2013; 15: 1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pham D. Chronic intermittent diarrhea in a 14-month-old Abyssinian cat. Can Vet J 2009; 50: 85–87. [PMC free article] [PubMed] [Google Scholar]
- 5. Burgener I, Frey C, Kook P, Gottstein B. Tritrichomonas fetus: a new intestinal parasite in Swiss cats [article in German]. Schweiz Arch Tierheilkd 2009; 151: 383–389. [DOI] [PubMed] [Google Scholar]
- 6. Frey CF, Schild M, Hemphill A, et al. Intestinal Tritrichomonas foetus infection in cats in Switzerland detected by in vitro cultivation and PCR. Parasitol Res 2009; 104: 783–788. [DOI] [PubMed] [Google Scholar]
- 7. Gunn-Moore DA, McCann TM, Reed N, et al. Prevalence of Tritrichomonas foetus infection in cats with diarrhoea in the UK. J Feline Med Surg 2007; 9: 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holliday M, Deni D, Gunn-Moore DA. Tritrichomonas foetus infection in cats with diarrhoea in a rescue colony in Italy. J Feline Med Surg 2009; 11: 131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuehner KA, Marks SL, Kass PH, et al. Tritrichomonas foetus infection in purebred cats in Germany: prevalence of clinical signs and the role of co-infection with other enteroparasites. J Feline Med Surg 2011; 13: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miro G, Hernandez L, Montoya A, et al. First description of naturally acquired Tritrichomonas foetus infection in a Persian cattery in Spain. Parasitol Res 2011; 109: 1151–1154. [DOI] [PubMed] [Google Scholar]
- 11. Profizi C, Cian A, Meloni D, et al. Prevalence of Tritrichomonas foetus infections in French catteries. Vet Parasitol 2013; 196: 50–55. [DOI] [PubMed] [Google Scholar]
- 12. Doi J, Hirota J, Morita A, et al. Intestinal Tritrichomonas suis (=T. foetus) infection in Japanese cats. J Vet Med Sci 2012; 74: 413–417. [DOI] [PubMed] [Google Scholar]
- 13. Lim S, Park SI, Ahn KS, et al. First report of feline intestinal trichomoniasis caused by Tritrichomonas foetus in Korea. Korean J Parasitol 2010; 48: 247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kingsbury DD, Marks SL, Cave NJ, et al. Identification of Tritrichomonas foetus and Giardia spp. infection in pedigree show cats in New Zealand. N Z Vet J 2010; 58: 6–10. [DOI] [PubMed] [Google Scholar]
- 15. Bell ET, Gowan RA, Lingard AE, et al. Naturally occurring Tritrichomonas foetus infections in Australian cats: 38 cases. J Feline Med Surg 2010; 12: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kulda J. Trichomonads, hydrogenosomes and drug resistance. Int J Parasitol 1999; 29: 199–212. [DOI] [PubMed] [Google Scholar]
- 17. Moreno SN, Mason RP, Docampo R. Distinct reduction of nitrofurans and metronidazole to free radical metabolites by Tritrichomonas foetus hydrogenosomal and cytosolic enzymes. J Biol Chem 1984; 259: 8252–8259. [PubMed] [Google Scholar]
- 18. Kather EJ, Marks SL, Kass PH. Determination of the in vitro susceptibility of feline Tritrichomonas foetus to 5 antimicrobial agents. J Vet Intern Med 2007; 21: 966–970. [DOI] [PubMed] [Google Scholar]
- 19. Gookin JL, Copple CN, Papich MG, et al. Efficacy of ronidazole for treatment of feline Tritrichomonas foetus infection. J Vet Intern Med 2006; 20: 536–543. [DOI] [PubMed] [Google Scholar]
- 20. Gookin JL, Stauffer SH, Dybas D, et al. Documentation of in vivo and in vitro aerobic resistance of feline Tritrichomonas foetus isolates to ronidazole. J Vet Intern Med 2010; 24: 1003–1007. [DOI] [PubMed] [Google Scholar]
- 21. Lim S, Park SI, Ahn KS, et al. Efficacy of ronidazole for treatment of cats experimentally infected with a Korean isolate of Tritrichomonas foetus. Korean J Parasitol 2012; 50: 161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. LeVine DN, Gookin JL, Papich MG. Twice-daily dosing of RDZ no longer recommended for treatment of intestinal Tritrichomonas foetus infection. J Feline Med Surg 2014; 16: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. LeVine DN, Papich MG, Gookin JL, et al. Ronidazole pharmacokinetics after intravenous and oral immediate-release capsule administration in healthy cats. J Feline Med Surg 2011; 13: 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Papich MG, Levine DN, Gookin JL, et al. Ronidazole pharmacokinetics in cats following delivery of a delayed-release guar gum formulation. J Vet Pharmacol Ther 2013; 36: 399–407. [DOI] [PubMed] [Google Scholar]
- 25. Goggin JM, Hoskinson JJ, Kirk CA, et al. Comparison of gastric emptying times in healthy cats simultaneously evaluated with radiopaque markers and nuclear scintigraphy. Vet Radiol Ultrasound 1999; 40: 89–95. [DOI] [PubMed] [Google Scholar]
- 26. Wyse CA, McLellan J, Dickie AM, et al. A review of methods for assessment of the rate of gastric emptying in the dog and cat: 1898–2002. J Vet Intern Med 2003; 17: 609–621. [DOI] [PubMed] [Google Scholar]
- 27. McMillen L, Lew AE. Improved detection of Tritrichomonas foetus in bovine diagnostic specimens using a novel probe-based real time PCR assay. Vet Parasitol 2006; 141: 204–215. [DOI] [PubMed] [Google Scholar]
- 28. Diehl KH, Hull R, Morton D, et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol 2001; 21: 15–23. [DOI] [PubMed] [Google Scholar]
- 29. Dansirikul C, Choi M, Duffull SB. Estimation of pharmacokinetic parameters from non-compartmental variables using Microsoft Excel. Comput Biol Med 2005; 35: 389–403. [DOI] [PubMed] [Google Scholar]
- 30. Zhang Y, Huo M, Zhou J. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed 2010; 99: 306–314. [DOI] [PubMed] [Google Scholar]
- 31. Gookin JL, Foster DM, Poore MF, et al. Use of a commercially available culture system for diagnosis of Tritrichomonas foetus infection in cats. J Am Vet Med Assoc 2003; 222: 1376–1379. [DOI] [PubMed] [Google Scholar]
- 32. Goggin JM, Hoskinson JJ, Butine MD, et al. Scintigraphic assessment of gastric emptying of canned and dry diets in healthy cats. Am J Vet Res 1998; 59: 388–392. [PubMed] [Google Scholar]
- 33. Yaeger MJ, Gookin JL. Histologic features associated with Tritrichomonas foetus-induced colitis in domestic cats. Vet Pathol 2005; 42: 797–804. [DOI] [PubMed] [Google Scholar]
- 34. Epe C, Rehkter G, Schnieder T, et al. Giardia in symptomatic dogs and cats in Europe – results of a European study. Vet Parasitol 2010; 173: 32–38. [DOI] [PubMed] [Google Scholar]
- 35. De Santis-Kerr AC, Raghavan M, Glickman NW, et al. Prevalence and risk factors for Giardia and coccidia species of pet cats in 2003–2004. J Feline Med Surg 2006; 8: 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gookin JL, Stebbins ME, Hunt E, et al. Prevalence of and risk factors for feline Tritrichomonas foetus and Giardia infection. J Clin Microbiol 2004; 42: 2707–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paris JK, Wills S, Balzer HJ, et al. Enteropathogen co-infection in UK cats with diarrhoea. BMC Vet Res 2014; 10: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fiechter R, Deplazes P, Schnyder M. Control of giardia infections with ronidazole and intensive hygiene management in a dog kennel. Vet Parasitol 2012; 187: 93–98. [DOI] [PubMed] [Google Scholar]
- 39. Rosado TW, Specht A, Marks SL. Neurotoxicosis in 4 cats receiving ronidazole. J Vet Intern Med 2007; 21: 328–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mattern CF, Honigberg BM, Daniel WA. Fine-structural changes associated with pseudocyst formation in Trichomitus batrachorum. J Protozool 1973; 20: 222–229. [DOI] [PubMed] [Google Scholar]
- 41. Pereira-Neves A, Campero CM, Martinez A, Benchimol M. Identification of Tritrichomonas foetus pseudocysts in fresh preputial secretion samples from bulls. Vet Parasitol 2011; 175: 1–8. [DOI] [PubMed] [Google Scholar]
- 42. Foster DM, Gookin JL, Poore MF, et al. Outcome of cats with diarrhea and Tritrichomonas foetus infection. J Am Vet Med Assoc 2004; 225: 888–892. [DOI] [PubMed] [Google Scholar]