Abstract
Objectives
The aim of this study was to evaluate and refine an ultrasound (US)-guided technique to block the brachial plexus (BP) at the level of the axillary space in live cats.
Methods
Eight adult experimental cats were enrolled into the study. The animals were sedated and positioned in dorsal recumbency with the limb to be blocked abducted 90º. The US transducer was placed in the axillary region and a non-traumatic peripheral nerve block needle was inserted in-plane with respect to the transducer, medial to the BP up to the level of the axillary artery. Lidocaine 1% (0.4 ml/kg) was injected as the needle was being progressively withdrawn in a caudal-to-cranial direction. The efficacy of the block was confirmed by evaluation of the motor and sensory functions of the blocked forelimb. Motor blockade was assessed observing the position of the blocked leg on standing and walking patterns. Sensory blockade was evaluated by the stimulation of mechanical nociceptors in the dermatomes supplied by the four major sensory nerves of the distal thoracic limb.
Results
The BP was successfully located by US in all cases. The achieved BP block was complete in six cats (75%) and partial in the remaining two cats (25%). All animals recovered uneventfully from the sedation and the BP blocks.
Conclusions and relevance
The US-guided block at the axillary space evaluated in this study is a feasible, reproducible and safe technique to block the BP plexus in experimental live cats.
Introduction
Pain management is essential in animals undergoing surgery. The current strategies to control pain are based on a balanced and multimodal approach, which includes the administration of a variety of analgesics with complementary effects, such as opioids, N-methyl-D-aspartate receptor antagonists, alpha(α)-2 agonists, non-steroidal anti-inflammatory drugs and local anaesthetics (LAs). 1 The use of an LA to block the transmission of the sensory input throughout a peripheral nerve or the spinal cord is highly effective in abolishing intraoperative nociception.2,3 Locoregional techniques are also useful to reduce the required doses of anaesthesia during surgery, and, later, the analgesic requirements during the postoperative phase.1,4–6
A number of methods have been described to perform peripheral nerve blocks (PNBs), which are mainly based on the different techniques employed to locate the target nerve(s). The insertion site of the needle and its trajectory in relation to the target nerves may be guided blindly by the use of simple anatomical landmarks, or may be guided more precisely by the use of nerve electrolocation and/or ultrasound (US)-guided techniques. 4,6 The success rate of a PNB depends on the precision of the LA injection. Moreover, when a solution of LA is accurately administered around the target nerves, lower doses can be employed, effectively reducing the potential for side effects. 7 US-guided techniques allow a direct visualisation of the spreading of LA around the target nerve(s), which may improve the onset time and the quality of the block, 8 thus reducing the volume of LA necessary to achieve the block in comparison to blind or electrolocation techniques. US-guided techniques may also reduce the risk for potential complications such as laceration of relevant vascular structures or nerve iatrogenic trauma. PNB-guided US techniques have been described extensively and are routinely employed in human anaesthesia.1,7
The brachial plexus (BP) block is a regional analgesic technique frequently used in human anaesthesia for surgical procedures carried out at the level of the hand, forearm and elbow as it provides complete analgesia distally from above the elbow joint. Similarly, its use has been reported in dogs and calves, to induce analgesia of the distal thoracic limb.1,4,9
Recently, an US-guided technique to block the BP at the level of the axillary space has been described in a cadaveric study carried out in cats. This study involved the comparison of two techniques to block the BP by the use of US in cadavers. The results of this study showed that the technique in which the animal was positioned in dorsal recumbency with the forelimb abducted 90º could be a safe and feasible procedure to block the BP in this species. 10 To our knowledge, there are no other descriptions of an US-guided technique to block the BP in the cat.
The purpose of the present study was to evaluate and refine this US-guided technique to block the BP at the level of the axillary space in experimental live cats.
Materials and methods
Animals
This study was approved by the local animal care and ethics committee (code 101-2014). Eight adult experimental male cats with a mean weight of 4.3 kg (range 3.5–5.4 kg) were employed. The cats were declared healthy on the basis of the findings of a physical examination and haematological and biochemical analysis. The animals were fasted for 12 h before the trials but had free access to water. Cats were sedated by intramuscular (IM) administration of medetomidine (Domitor; Pfizer) 30 μg/kg and butorphanol (Turbogesic; Fort Dodge) 0.2 mg/kg. The animals were placed in dorsal recumbency with the limb to be blocked abducted 90º, as previously described. 10 The hair of the axillary area was clipped and the skin aseptically prepared. The animals were handled following the guidelines for humane care of experimental animals.
Methods
One forelimb was randomly selected to block the BP on each cat. Randomisation was performed by tossing a coin. Eight BP were located using a 4–13 MHz linear transducer (MyLab 70; Esaote) using the axillary approach to the BP previously described. 10 A surgical blade (11 Sovereign; Paramount Surgimed) was employed to perform a stab skin incision of a length of 2 mm, approximately 1 cm medially from the scapulohumeral joint. Then, a non-traumatic PNB-stimulating needle (PN) (Stimuplex D 0.71 × 50 mm 30°; B Braun Melsungen AG) was introduced and advanced in a cranial-to-caudal direction. The needle was inserted in-plane with respect to the US transducer (Figure 1a). The tip of the needle was positioned medial to the axillary artery, which was depicted using colour Doppler (Figure 1b). At this point, 0.4 ml/kg lidocaine 1% (Lidocaina 1%; B Braun Medical SA) was injected along the medial aspect of the entire BP as the needle was being progressively withdrawn in a caudal-to-cranial direction. 10 The location of the BP by an US-guided technique and the visualisation of the PN needle were always performed by the same investigator (AAG). The administration of LA was performed by a single operator (AAN). Negative pressure was applied before each injection, and injections were discontinued if an increasing resistance was detected during the injections. Sedation was antagonised with atipamezol (75 µg/kg, Antisedan; Orion Pharma) administered IM immediately after the blocks, to assess the success of the BP blockade. Cats were observed for a period of 72 h after the blocks, to determine the presence of potential complications.
Figure 1.
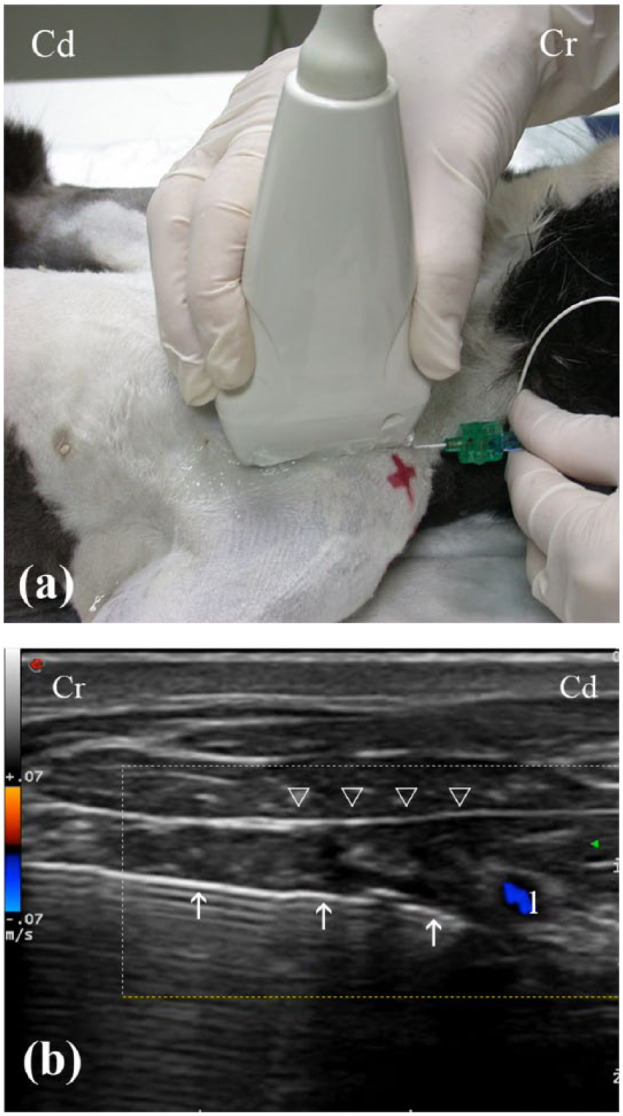
(a) Position of the ultrasound (US) transducer and needle for the axillary approach to the brachial plexus (BP) in the experimental cats. (b) Transverse US image of the BP. Note the visualisation of the needle (white arrows) in-plane with respect to the transducer along the medial aspect of the BP (white arrowheads). The tip of the needle was positioned medial to the axillary artery (1), which was depicted using colour Doppler. Cr = cranial; Cd = caudal
Blockade evaluation
Immediately after the administration of atipamezol (defined as T0), the motor and sensory functions of the blocked forelimb were qualitatively assessed as described below. Assessments were performed at 2 min intervals during the first 10 mins. Then, the animals were evaluated at 5 min intervals for another 90 mins and, finally, at 15 min intervals until the end of the procedure. The final assessment was performed 30 mins after a normal motor function was restored. All assessments were performed by the same investigator (AAN).
Motor blockade
The evaluation of the motor block was assessed observing the position of the blocked leg on standing and walking patterns. A motor blockade was considered positive if motor or proprioceptive deficits were present and the cat was unable to bear weight with that forelimb (Figure 2). A three-point subjective rating scale was used: 1 1 = normal motor response (normal ability to walk or stand using the blocked limb); 2 = partial motor blockade (intermittent non-weightbearing, moderate lameness when walking); or 3 = complete motor blockade (complete non-weightbearing, weight-bearing on dorsal carpus >50% of the time, severe lameness when walking) (Table 1).
Figure 2.

Evaluation of the brachial plexus block using an axillary approach. A complete motor blockade can be observed as the cat is showing proprioceptive deficit and inability to bear weight after the administration of the local anaesthetic solution
Table 1.
Three-point scales employed to evaluate the degree and clinical signs of motor blockade and the degree of sensory blockade and clinical response to a mosquito clamp noxious stimulus
| Grade | Blockade | Clinical signs |
|---|---|---|
| Motor response | ||
| 1 | Normal | Normal ability to walk or stand using the blocked limb, no lameness |
| 2 | Partial blockade | Intermittent non-weightbearing, moderate lameness when walking |
| 3 | Complete blockade | Non-weightbearing, weightbearing on dorsal carpus >50% of the time, severe lameness when walking |
| Sensory response | ||
| 1 | Normal | Normal response with rapid withdrawal of the limb, weight shifting and/or head movement toward the testing site, and/or vocalisation |
| 2 | Partial blockade | Delayed or attenuated response to stimulus |
| 3 | Complete blockade | Absence of response to stimulus |
Sensory blockade
Sensory blockade was evaluated by the stimulation of mechanical nociceptors in the dermatomes supplied by the four major sensory nerves of the distal thoracic limb, as previously described. 1 The radial nerve was tested over the dorsal aspect of the antebrachiocarpal joint; the ulnar nerve testing site was 1 cm proximal to the accessory carpal pad; the musculocutaneous nerve blockade was assessed 1 cm distal to the medial epicondyle; the common dermatome for the median and ulnar nerves was located on the skin overlying the palmaromedial aspect of digit 2 (Table 2). Dermatomes were always evaluated in the same sequence: radial, musculocutaneous, ulnar and median/ulnar. A pair of mosquito clamps (modified by securing the tip with a cohesive bandage) was employed to elicit nociception. The clamps were progressively closed for a maximum time of 10 s until a positive response was elicited or until the first ratchet notch was locked, and the following three-point rating scale was employed: 1 1 = normal sensory response (withdrawal of the limb, weight shifting, orienting to testing site or vocalisation); 2 = partial sensory blockade (delayed or weak response); or 3 = complete sensory blockade (absence of response) (see Table 1).
Table 2.
Localisation of dermatomes employed for sensory blockade evaluation
| Nerve | Dermatome |
|---|---|
| Radial | Dorsal aspect of the antebrachiocarpal joint |
| Musculocutaneous | 1 cm distal to the medial epicondyle |
| Ulnar | 1 cm proximal to the accessory carpal pad |
| Ulnar/median | Palmaromedial aspect of digit 2 |
The following times (mins) were also recorded in this study and defined as follows: time to onset of motor blockade = time from the BP block to the time in which signs of partial or complete motor blockade were noted; duration of motor blockade = time interval in which a partial or complete motor blockade was observed; time to onset of sensory blockade = time from BP block to the time at which signs of partial or complete sensory blockade were evidenced; duration of sensory blockade = time interval in which a partial or complete sensory blockade was observed. The BP block was considered to be clinically effective when complete motor and sensory blockades (score of 3) were observed.
Results
The BP was successfully located by US in all the experimental cats. It appeared as a cluster of small, round hypoechoic structures surrounded by a thin hyperechoic rim. No resistance was noted to the injections of LA and a negative blood aspiration was obtained in all cases. Sedation was quickly antagonised in all cats within a range of 4–10 mins.
The injected LA solution was observed by US spreading along the medial aspect of the BP in 6/8 cases (Figure 3a,b). In one animal, the spreading of the LA solution could not be observed (cat 4). Most of the solution was found to be spreading around an area medial and caudal to the axillary artery in another case (cat 8).
Figure 3.
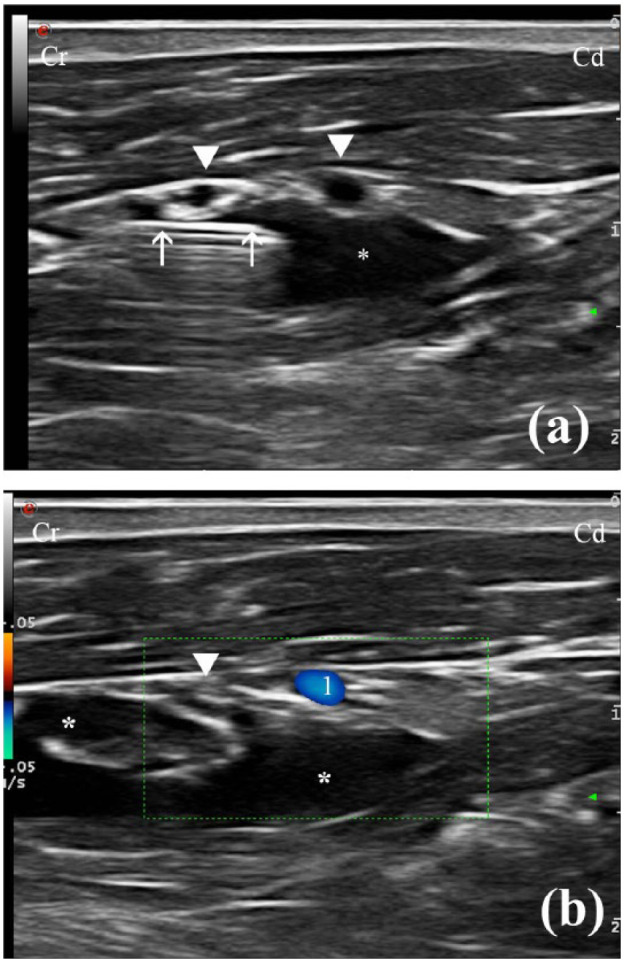
(a) Transverse ultrasound (US) image showing the administration of the local anaesthetic solution (*) along the medial aspect of the brachial plexus (BP) (white arrowheads) as the needle (white arrows) is being progressively withdrawn in a caudal-to-cranial direction. (b) Transverse US image using colour Doppler showing the distribution of the local anaesthetic solution (*) along the BP (white arrowhead). Cr = cranial; Cd = caudal; 1 = axillary artery
Motor blockade
A partial or a complete motor blockade was observed in all cats. In 6/8 cats (75%) the blockade was complete (score 3), while two cats (25%) (cats 4 and 8) showed a partial motor blockade (score 2) (Table 3). The onset time and duration of the motor blockade are summarised in Table 4. The mean onset time to observe motor blockade was 12 mins. The fastest onset time was 8 mins in 2/8 cats. In one case (1/8), the onset time was delayed to 20 mins. The mean duration of the motor blockade was 94 mins. The shorter duration of the motor block was 40 mins in one cat (1/8), and the longest was 120 mins in another case (1/8).
Table 3.
Results obtained for each cat for the motor and sensory blockades obtained with an ultrasound-guided technique to block the brachial plexus at the level of the axillary space in cats
| Cat | Motor blockade | Sensory blockade |
|---|---|---|
| 1 | 3 | 3 |
| 2 | 3 | 3 |
| 3 | 3 | 3 |
| 4 | 2 | 2 |
| 5 | 3 | 3 |
| 6 | 3 | 3 |
| 7 | 3 | 3 |
| 8 | 2 | 2 |
1 = normal; 2 = partial blockade; 3 = complete blockade
Table 4.
Onset times and duration of the motor blockade obtained with an ultrasound-guided technique to block the brachial plexus at the level of the axillary space in cats
| Cat | Onset time (mins) | Duration of block (mins) |
|---|---|---|
| 1 | 10 | 120 |
| 2 | 15 | 105 |
| 3 | 8 | 97 |
| 4 | 15 | 40 |
| 5 | 20 | 105 |
| 6 | 10 | 105 |
| 7 | 8 | 75 |
| 8 | 10 | 105 |
| Mean (range) | 12 (8–20) | 94 (40–120) |
Sensory blockade
Sensory blockade was difficult to evaluate owing the temperament of the cats. The only dermatome that could be consistently evaluated in all cases was the common dermatome of the median and ulnar nerves. Assessments of the other dermatomes were considered unreliable and excluded from the study. The sensory blockade evaluated at that dermatome was found to be complete in 6/8 cats (75%) (score 3). Again, cats 4 and 8 showed a partial sensory blockade (25%) (score 2) (Table 3). The time to onset and duration of the sensory blockade are summarised in Table 5. The mean time to onset for a sensory blockade was 5 mins (range 2–10 mins). In 2/8 cats it was detected at 2 mins and in another two cats at 10 mins. The mean duration of this blockade was of 56 mins. It ranged from 25 mins (1/8 cats) to 75 mins (3/8 cats). The time to onset and duration of the motor blockade were longer in all cases than that for sensory blockade.
Table 5.
Time to onset and duration of the sensory blockade obtained with an ultrasound-guided technique to block the brachial plexus at the level of the axillary space in cats
| Cat | Onset time (mins) | Duration time of block (mins) |
|---|---|---|
| 1 | 2 | 75 |
| 2 | 10 | 55 |
| 3 | 4 | 46 |
| 4 | 2 | 23 |
| 5 | 10 | 75 |
| 6 | 4 | 75 |
| 7 | 6 | 45 |
| 8 | 4 | 55 |
| Mean (range) | 5 (2–10) | 56 (23–75) |
Complications
Recovery from sedation and BP blocks was uneventful in all the cases and no complications were found.
Discussion
The aim of this study was to evaluate and refine an US-guided technique to block the BP at the level of the axillary space in live cats. The results of this study show that the BP was successfully located by US in all cases, and that the BP block was clinically effective in 6/8 cats (75%). In the remaining two cats (25%), despite the suboptimal spreading of LA, a partial BP blockade (sensory and motor) was present.
In human medicine, US guidance is the standard of care for administration of locoregional analgesia.11–13 Ultrasonographic guidance offers an important advantage over electrolocation or blind techniques: direct visualisation of the target nerves and accurate localisation of the vascular structures by the use of Doppler may reduce the need for multiple needle passes, thus avoiding the risk of nerve and vascular laceration.14,15 In the current veterinary clinical setting, techniques based on anatomic landmarks or nerve electrolocation are widely employed to conduct these blocks.1,9,13 There is still scarce information regarding the use of US-guided techniques to carry out these blocks in small animals, more particularly in felines. To our knowledge, this is the first study to investigate the use of US guidance to block the BP in live cats.
It has been described in human anaesthesia that US-guided techniques are more likely to be successful and also to have a faster onset and a longer duration than blind or electrolocation techniques. 16
In a previous study carried out in experimental cats, the onset time and duration of a BP blockade using blind techniques was reported. 17 This study was conducted to compare the effects of bupivacaine 0.25% (at a volume of 0.4 ml/kg) and 0.75% (at a volume of 1.3 ml/kg). In our study, a volume of lidocaine of 0.4 ml/kg was also employed. The mean onset time of the motor and sensory blockade was 5.5 mins and 12.5 mins, respectively, in the group in which bupivacaine 0.25% was used. 17 The results from our study showed similar onset times for the motor and sensory blockade (5 mins and 12 mins, respectively). These times were within the normal range of 5–15 mins classically described for lidocaine onset time.8,18 The mean duration of the motor and sensory blockades were 124.16 and 181.66 mins, respectively, when bupivacaine 0.25% was injected. 17 These times were longer than the times found in our study, 56 and 94 mins, respectively. It is well documented that bupivacaine has a longer duration of action than lidocaine.18–20
In our study, we decided to use lidocaine to perform the BP blocks as it has a safer profile than bupivacaine.20,21 Bupivacaine is four times more potent than lidocaine for causing myocardial depression and about 16 times more potent as an arrythmogenic.20,21 Finally, lidocaine induces sensory and motor blockades of short duration compared with bupivacaine, allowing us to perform the procedures in a shorter time, which was important for the comfort of the cats.
LAs are known to cause systemic toxicity when high doses are administered. A dangerously high plasma concentration can also be reached following an accidental intravascular injection or by an increased absorption from the injection site.22,23 Blind approaches to the BP and large volumes of LA (1 ml/kg) are commonly used in veterinary medicine. The relatively large volume of LA necessary to achieve an effective block when blind techniques are used can lead to discomfort and an increased risk of side effects or overdose. 24 This fact is particularly important in cats as they are more susceptible than dogs to secondary toxicity to LAs.25,26 A recent study compared the efficacy of different volumes of LA using electrolocation or a blind guided-technique to block the BP in canine cadavers. 23 It was concluded that under electrolocation the administration of a lower volume of injectate (0.2 ml/kg) had a similar success rate than the blinded technique using a higher volume of LA (1 ml/kg). As was mentioned above, with the use of US-guided techniques volumes as low as 0.15 ml/kg can be successfully employed in dogs. 27 Mosing et al reported in cats undergoing orthopaedic surgery the use of a mixture of lidocaine–bupivacaine (at a volume of 0.6 ml/kg) to block the BP at the level of the thoracic limb using the electrolocation technique. 28 These authors concluded that the BP block reduced intraoperative isoflurane requirements and pain early in the postoperative period compared with procedures carried out without BP blockade. 28 Recently, a cadaveric study carried out in cats determined the minimum volume of methylene blue necessary to produce a complete staining of the BP nerves using a blind approach to this plexus. Results from this study found that a potentially successful BP block could be achieved with a total volume of injectate of 5–6 ml. 29 These results show the need to administer a high volume of LA when blind approaches are employed to block the BP in cats, similarly to other studies conducted in dogs. 29 In our study, the success rate of the BP block was of 75%, despite using a moderate volume of 0.4 ml/kg of lidocaine 1%, which was equivalent, in accordance to the cat’s weight, to final volumes of injectate ranging from 1.40–2.16 ml. A partial sensory and motor blockade was observed in two cats. In both cases the distribution of LA was considered suboptimal. In one cat, spreading of LA could not be observed by US, while in the other the injectate travelled medially and caudally to the axillary artery. These findings may also explain the brief duration of the motor blockade observed in those cats. It is possible that the administration of a larger volume of LA could have produced a better distribution of the injectate, improving the intensity of the BP block in those two cats.10,29
The results of the present study indicate that a moderate volume of 0.4 ml/kg could be employed to block the BP by US guidance in cats. However, the aim of this study was not to establish the optimal dose/volume for an US-guided BP block in cats, and further clinical research is still needed to determine it.
The incidence of complications following a BP block is unknown in dogs and cats. Possible complications include haematoma due to vessel laceration or puncture, pneumothorax, diaphragmatic hemiparesis (secondary to phrenic nerve block), intrathoracic injection and nerve damage.7,23 In a recent study, two different positions for an US-guided axillary BP blockade were compared in feline cadavers. An axillary approach with the cat in dorsal recumbency and with the forelimb to be blocked abducted 90° was found to be the safest technique, as no complications were reported. On the contrary, the approach carried out with the thoracic limbs flexed and orientated caudally was associated with complications such as intrathoracic injection, staining of the phrenic nerve and haematoma. 10 Because of these descriptions, in the present study, the axillary approach with the animals in dorsal recumbency and the forelimb to be blocked abducted 90° was selected. No complications related to the BP blockade technique employed here were observed in any cat.
The main limitations of this study include the low number of animals enrolled in the experience, and the use of experimental cats which exhibited at times an un-cooperative behaviour, thus limiting the final number of dermatomes that could be reliably assessed to evaluate the efficacy of the sensitive block.
Conclusions
The US-guided technique evaluated here was found to be a feasible, reproducible and safe method to block the BP plexus in experimental live cats. Further studies, using more animals and, ideally, performed in a clinical setting are still necessary to establish the optimal dose/volume of LA to increase the clinical success of this technique.
Acknowledgments
We would like to thanks Hill’s Pet Nutrition (Spain) for the scholarship granted to Agustina Anson supporting her postgraduate training.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Accepted: 30 October 2015
References
- 1. Trumpatori BJ, Carter JE, Hash J, et al. Evaluation of a midhumeral block of the radial, ulnar, musculocutaneous and median (RUMM block) nerves for analgesia of the distal aspect of the thoracic limb in dogs. Vet Surg 2010; 39: 785–796. [DOI] [PubMed] [Google Scholar]
- 2. Lemke KA, Dawson SD. Local and regional anesthesia. Vet Clin North Am Small Anim Pract 2000; 30: 839–857. [DOI] [PubMed] [Google Scholar]
- 3. Mathews KA. Neuropathic pain in dogs and cats: if only they could tell us if they hurt. Vet Clin North Am Small Anim Pract 2008; 38: 1365–1414. [DOI] [PubMed] [Google Scholar]
- 4. Rioja E, Sinclair M, Chalmers H, et al. Comparison of three techniques for paravertebral brachial plexus blockade in dogs. Vet Anaesth Analg 2012; 39: 190–200. [DOI] [PubMed] [Google Scholar]
- 5. Hofmeister EH, Kent M, Read MR. Paravertebral block for forelimb anesthesia in the dog- an anatomic study. Vet Anaesth Analg 2007; 34: 139–142. [DOI] [PubMed] [Google Scholar]
- 6. De Marzo C, Crovace A, De Monte V, et al. Comparison of intra-operative analgesia provided by intravenous regional anesthesia or brachial plexus block for pancarpal arthrodesis in dogs. Res Vet Sci 2012; 93: 1493–1497. [DOI] [PubMed] [Google Scholar]
- 7. Mahler SP, Adogwa AO. Anatomical and experimental studies of brachial plexus, sciatic, and femoral nerve location using peripheral nerve stimulation in the dog. Vet Anaesth Analg 2008; 35: 80–89. [DOI] [PubMed] [Google Scholar]
- 8. Liu SS, Ngeow J, John RS. Evidence basis for ultrasound-guided block characteristics: onset, quality, and duration. Reg Anesth Pain Med 2010; 35 Suppl 2: S26–S35. [DOI] [PubMed] [Google Scholar]
- 9. Campoy L, Bezuidenhout AJ, Gleed RD, et al. Ultrasound-guided approach for axillary brachial plexus, femoral nerve, and sciatic nerve blocks in dogs. Vet Anaesth Analg 2010; 37: 144–153. [DOI] [PubMed] [Google Scholar]
- 10. Anson A, Laredo FG, Gil F, et al. Comparison of two techniques for ultrasound-guided axillary brachial plexus blockade in cats. J Feline Med Surg 2015; 17: 476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marhofer P, Chan VW. Ultrasound-guided regional anesthesia: current concepts and future trends. Anesth Analg 2007; 104: 1265–1269. [DOI] [PubMed] [Google Scholar]
- 12. Kapral S, Janrasits O, Schaberning C, et al. Lateral infraclavicular plexus block vs. axillary block for hand and forearm surgery. Acta Anaesthesiol Scand 1999; 43: 1047–1052. [DOI] [PubMed] [Google Scholar]
- 13. Bagshaw HS, Larenza MP, Seiler GS. A technique for ultrasound-guided paravertebral brachial plexus injections in dogs. Vet Radiol Ultrasound 2009; 50: 649–654. [DOI] [PubMed] [Google Scholar]
- 14. Sites BD, Brull R. Ultrasound guidance in peripheral regional anesthesia: philosophy, evidence-based medicine, and techniques. Curr Opin Anaesthesiol 2006; 19: 630–639. [DOI] [PubMed] [Google Scholar]
- 15. Gray AT. Ultrasound-guided regional anesthesia: current state of the art. Anesthesiology 2006; 104: 368–373. [DOI] [PubMed] [Google Scholar]
- 16. Abrahams MS, Aziz MF, Fu RF, et al. Ultrasound guidance compared with electrical neurostimulation for peripheral nerve block: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth 2009; 102: 408–417. [DOI] [PubMed] [Google Scholar]
- 17. Freitas PM, Lima PCA, Mota FCD, et al. Comparison between the use of 0.25% and 0.75% bupivacaine solution for the brachial plexus blockade in cats. Ars Vet 2002; 18: 218–222. [Google Scholar]
- 18. Skarda RD. Local and regional anesthesia in ruminants and swine. Vet Clin North Am 1996; 12: 579–626. [DOI] [PubMed] [Google Scholar]
- 19. Lawal FM, Adetunji A. A comparison of epidural anaesthesia with lignocaine, bupivacaine and a lignocaine-bupivacaine mixture in cats. J S Afr Vet Assoc 2009; 80: 243–246. [DOI] [PubMed] [Google Scholar]
- 20. Movafegh A, Razazian M, Hajimaohamadi F, et al. Dexamethasone added to lidocaine prolongs axillary brachial plexus blockade. Anesth Analg 2006; 102: 263–267. [DOI] [PubMed] [Google Scholar]
- 21. Chadwick HS. Toxicity and resuscitation in lidocaine- or bupivacaine-infused cats. Anesthesiology 1985; 63: 385–390. [DOI] [PubMed] [Google Scholar]
- 22. Stoelting R, Hillier SC. Pharmacology and physiology of anesthetic practice. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2006. [Google Scholar]
- 23. Ricco Ac, Shih A, Killos M, et al. Different volumes of injectate using electrostimulator and blinded techniques for brachial plexus block in dogs. Vet Rec 2013; 173: 608–611. [DOI] [PubMed] [Google Scholar]
- 24. Kimura Y, Kamada Y, Kimura A, et al. Ropivacaine-induced toxicity with overdose suspected after axillary brachial plexus block. J Anesth 2007; 21: 413–416. [DOI] [PubMed] [Google Scholar]
- 25. Court MH, Grenblatt DJ. Molecular basis for deficient acetaminophen glucuronidation in cats. Biochem Pharmacol 1997; 53: 1041–1047. [DOI] [PubMed] [Google Scholar]
- 26. Robertson SA, Taylor PM. Pain management in cats – past, present and future. Part 2. Treatment of pain – clinical pharmacology. J Feline Med Surg 2004; 6: 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Campoy L, Martin-Flores M, Looney AL, et al. Distribution of a lidocaine-methylene blue solution staining in brachial plexus, lumbar plexus and sciatic nerve blocks in the dog. Vet Anaesth Analg 2008; 35: 348–354. [DOI] [PubMed] [Google Scholar]
- 28. Mosing M, Reich H, Moens Y. Clinical evaluation of the anaesthetic sparing of brachial plexus block in cats. Vet Anaesth Analg 2010; 37: 154–161. [DOI] [PubMed] [Google Scholar]
- 29. Mencalha R, Fernandes N, dos Santos Sousa CA, et al. A cadaveric study to determine the minimum volume of methylene blue to completely color the nerves of brachial plexus in cats. An update in forelimb and shoulder surgeries. Acta Cir Bras 2014; 29: 382–388. [DOI] [PubMed] [Google Scholar]


