Abstract
Objectives
Currently, there are no published randomised, controlled veterinary trials evaluating the efficacy of antiepileptic medication in the treatment of myoclonic seizures. Myoclonic seizures are a hallmark of feline audiogenic seizures (FARS).
Methods
This prospective, randomised, open-label trial compared the efficacy and tolerability of levetiracetam (20–25 mg/kg q8h) with phenobarbital (3–5 mg/kg q12h) in cats with suspected FARS that experienced myoclonic seizures. Cats were included that had ⩾12 myoclonic seizure days during a prospective 12 week baseline period. This was followed by a 4 week titration phase (until a therapeutic serum concentration of phenobarbital was achieved) and a 12 week treatment phase.
Results
Fifty-seven cats completed the study: 28 in the levetiracetam group and 29 in the phenobarbital group. A reduction of ⩾50% in the number of myoclonic seizure days was seen in 100% of patients in the levetiracetam group and in 3% of patients in the phenobarbital group (P <0.001) during the treatment period. Levetiracetam-treated cats had higher freedom from myoclonic seizures (50.0% vs 0%; P <0.001) during the treatment period. The most common adverse events were lethargy, inappetence and ataxia, with no difference in incidence between levetiracetam and phenobarbital. Adverse events were mild and transient with levetiracetam but persistent with phenobarbital.
Conclusions and relevance
These results suggest that levetiracetam is an effective and well tolerated treatment for cats with myoclonic seizures and is more effective than phenobarbital. Whether it will prevent the occurrence of generalised tonic–clonic seizures and other forebrain signs if used early in the course of FARS is not yet clear.
Introduction
Feline audiogenic reflex seizures (FARS) represent a collection of seizure patterns, including myoclonic seizures, generalised tonic–clonic seizures (GTCSs) and absence seizures. The defining features include a geriatric onset (>10 years) of auditory-induced myoclonic seizures. 1 FARS occurs in pedigree and non-pedigree cats; among the pedigrees, the Birman breed is over-represented. 1 Avoiding certain sounds can reduce the seizures, although owners have reported that it is difficult to avoid noises, and the loudness of the sound also seems to increase the severity of seizures. 1 A pattern of audiogenic kindling has been observed in rats in which myoclonic seizures develop after numerous daily sound exposures and results in the spread of seizure discharges from brainstem to forebrain structures (ie, caudal colliculus, hippocampus, amygdala and neocortex).2,3 In the case of FARS, it is hypothesised that frequent sounds induce myoclonic seizures that progress to GTCSs. 1
Myoclonus can be classified in a number of ways but aetiologically it can be subdivided into physiological myoclonus (eg, hypnic jerks or ‘hiccups’), essential myoclonus (idiopathic or hereditary), epileptic myoclonus or symptomatic myoclonus (ie, myoclonus secondary to an underlying disorder). Epileptic and non-epileptic myoclonus often produce apparently similar clinical pictures indistinguishable from myoclonic seizures. When myoclonus occurs with GTCSs then epilepsy is inferred. 4 Myoclonus may only be one part of an epilepsy syndrome in people and domestic animals, and several problems regarding treatment exist. This is particularly true of FARS, where myoclonic seizures appear to be the most common type. Not all antimyoclonic drugs are antiepileptic, and only some antiepileptic drugs (AEDs) are antimyoclonic. 5 In addition, many of the myoclonic epilepsies reported in people are refractory to drug treatment. No study has investigated treatment response in cats with myoclonic seizures.
Levetiracetam is a novel AED that was approved at the turn of the 21st century for the treatment of partial epilepsies with or without secondary generalisation. It is structurally related to piracetam, which is commonly used in people to treat myoclonic seizures. Levetiracetam is efficacious in the treatment of myoclonus and progressive myoclonic epilepsies.6–12
In the face of the availability of newer AEDs such as levetiracetam, there is a need to reassess the role of first-generation AEDs, such as phenobarbital, in the treatment of myoclonic epilepsy. The majority of cats suffering from FARS suffer myoclonic and/or GTCSs as part of their syndrome. This provides a unique opportunity to assess the efficacy of antimyoclonic medication. The objective of this study was to explore whether levetiracetam or phenobarbital monotherapy are effective in the management of FARS.
Materials and methods
Study design
This prospective, multicentre, randomised, controlled, open-label study was conducted between February 2014 and April 2015, and coordinated at Davies Veterinary Specialists. Following a 12 week baseline period, cats were randomly allocated to receive levetiracetam or phenobarbital. Directions regarding the dosage (6–10 mg/kg PO for phenobarbital divided twice daily and 60–75 mg/kg PO for levetiracetam divided three times daily) were given via e-mail or telephone to the attending veterinarian. In the case of phenobarbital, a blood sample was collected 2 weeks after commencing medication, to assess the serum concentration of the drug. If the dose was subtherapeutic, the dosage was increased accordingly and a blood sample was collected 2 weeks later until a mid-range therapeutic concentration was achieved (20–35 µg/ml or 86.5–151 µmol/l). In both treatment groups, a titration period of 4 weeks was included, to allow the medication to reach steady-state concentrations. This period was extended in individual cats as required until therapeutic concentrations of phenobarbital were achieved. Following the titration period, a 12 week treatment period was observed.
Patients
Cats with a diagnosis of FARS were included. Cats were recruited from the pool of owners that had previously contacted the primary author (ML) regarding a questionnaire-led phenotypic study, 1 as well as new owners that had contacted the centre since completion of the original study. Diagnosis was achieved by video evidence of audiogenic myoclonic seizures. For inclusion, cats had to have experienced 12 or more days of myoclonic seizures during the prospective 12 week baseline period, have been on no previous antiepileptic medication and fulfil the criteria of the previously described phenotype for FARS. 1 Patient exclusion criteria included any concurrent disease that could represent a contraindication to the use of levetiracetam or phenobarbital, notably known pre-existing hepatic or renal dysfunction, previous or current treatment with antiepileptic medication, or signs suggestive of a progressive brain lesion (eg, circling, pacing, etc). All owners gave informed consent before participation in the study.
Assessments
Owners were requested to complete a seizure diary during the whole period of the study (comprising at least 26 weeks). They recorded the date, number and type of seizure (GTCSs, myoclonic or absence) on daily record cards. The primary investigator collated and confirmed this information with each owner and recorded it in an electronic spreadsheet. Owners were also instructed to include a record describing any signs of illness, change in activity or attitude. During the study, owners were requested to get on with daily life as normal and to make no attempts to produce the sounds responsible for eliciting their cats’ seizures.
The primary efficacy variable was the responder rate for myoclonic seizure days per week. Responders were defined as those experiencing a ⩾50% decrease in the mean number of myoclonic seizure days per week during the treatment period compared with baseline. Myoclonic seizure frequency was not selected as an efficacy variable as these seizures are frequently difficult to quantify owing to their repetitiveness. Secondary efficacy variables included mean percentage reduction from baseline in myoclonic seizure days/week; rates of seizure freedom from myoclonic seizures; and the total number of myoclonic seizure-free days.
A subjective measure was also recorded from each owner at the end of the study regarding their cat’s quality of life by asking whether it had improved, deteriorated or remained the same since starting the medication.
Adverse events were also recorded; their intensity and relationship to study medication were judged by the primary investigator (ML) in conjunction with the attending veterinarian.
Seizure classification
The definition of a GTCS is straightforward but includes variations beginning with a tonic, clonic or myoclonic phase. A GTCS usually commences with contraction of all skeletal muscles and loss of consciousness. The cat usually falls to the side with the legs stretched out and the head back. This is the tonic portion of the seizure. Sometimes a cat will vocalise or have facial twitching. Often the cat will salivate excessively or urinate. The tonic portion of the seizure is usually very brief and gives way to the clonic phase of the seizure. Once the clonic phase begins the cat will have rhythmic movements of the legs and chomping movements of the jaw.
A myoclonic seizure was defined as a sudden, brief, muscular jerk involving the limbs, neck or trunk (singly or in some combination) occurring as a single or irregularly recurrent event. An absence seizure was considered as the occurrence of an abrupt, transient apparent loss of consciousness with no motor activity. These definitions are in accordance with the International League Against Epilepsy classification of epileptic syndromes. 13
Statistical analysis
On the basis of a two-group continuity corrected χ2 test, a sample size of 72 cats (36 cats randomly assigned to each treatment group) was considered sufficient to attain a statistical power of 90% for detecting a treatment difference of 40% in responder rate, assuming responder rates of 70% and 30% in the levetiracetam and phenobarbital groups, respectively, and using a 5% two-sided significance level.
Two-sample t-tests and Fisher’s exact tests were used to compare demographics and baseline seizure history between the treatment groups. Patients failing to complete the study were excluded from further analysis.
The treatment odds ratio and 95% confidence interval for the responder rate in myoclonic seizure days per week was calculated using a 2 × 2 contingency table and Fisher’s exact test. Seizure freedom rates were compared between treatment groups using Fisher’s exact test.
The secondary efficacy variables between treatment arms were tested with a Fisher’s exact test for categorical variables and the Wilcoxon rank sums test for continuous variables
A significance level of P <0.05 was established for all analyses.
Results
Patient disposition
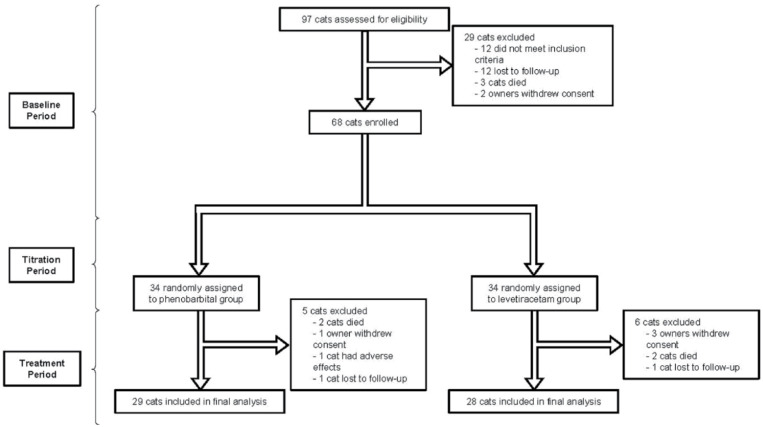
Ninety-seven cats underwent baseline assessment, of which 29 were found to be ineligible (12 did not meet the inclusion criteria, 12 cats were lost to follow-up, three cats died and two owners withdrew consent) and were not included (Figure 1). Therefore, 68 cats were randomised (34 to levetiracetam and 34 to phenobarbital). The baseline demographic characteristics of cats that were randomly assigned to each of the study groups are given in Table 1. There was no difference between treatment groups. All cats experienced myoclonic seizures during baseline, with 57/68 (84%) experiencing GTCSs in addition during the baseline period. Only five cats (7%) had a single reported absence seizure during baseline.
Figure 1.
Trial profile
Table 1.
Baseline characteristics of cats allocated to each treatment group
| Levetiracetam (n = 34) | Phenobarbital (n = 34) | P value | |
|---|---|---|---|
| Age (years) | 18 (12–23) | 19 (13–22) | 0.09 |
| Weight (kg) | 4 (2–8) | 4 (1–10) | 0.29 |
| Breed (n) | 0.97 | ||
| DSH | 17 | 19 | |
| DLH | 2 | 1 | |
| Birman | 8 | 9 | |
| Other | 7 | 5 | |
| Sex (n) | 0.70 | ||
| F | 17 | 15 | |
| FN | 13 | 9 | |
| M | 17 | 19 | |
| MN | 11 | 14 | |
| Age at onset of seizures (years) | 15 (10–19) | 16 (10–19) | 0.10 |
| Time from first seizure to study start point (years) | 3 (2–4) | 3 (2–4) | 1.00 |
Data are median (range) unless otherwise indicated
DLH = domestic longhair; DSH = domestic shorthair; F = female; M = male; N = neutered
The mean age of the cats at seizure onset was 15 years (median 15 years; range 10–19 years). Thirty-six cats were female (69%; 25/36 neutered) and 32 were male (88%; 28/32 neutered). Breeds comprised 36 domestic shorthairs, 17 Birmans, five Burmese, three domestic longhairs, two Bengals, and one of each of Maine Coon, British Shorthair, European Shorthair, Norwegian Forest Cat and Birman cross.
A total of 57 cats (84%) completed the study (Figure 1). Efficacy analysis therefore included a total of 57 cats (28 receiving levetiracetam; 29 receiving phenobarbital). Median daily phenobarbital dose (n = 29) was 6.250 mg/kg/day (range 3.34–15.00 mg/kg/day) with a mean phenobarbital serum concentration of 27.7 µg/ml (range 20.4–33.2 µg/ml). The median levetiracetam dose (n = 28) was 62.5 mg/kg/day (range 60.00–93.75 mg/kg/day).
Four cats, two in each of the levetiracetam and phenobarbital groups, died during the course of the treatment period and were not included in the efficacy analyses. Death was due to euthanasia in all cases, with three cats exhibiting progressive non-seizure forebrain signs and one cat in the levetiracetam group having sudden and severe dyspnoea. No post-mortem examinations were performed. Three owners of cats in the levetiracetam group withdrew consent and all cited the frequency with which medication was administered as their reason. One cat was lost to follow-up in the levetiracetam group. Three further cats were excluded from the phenobarbital group during the treatment period; one owner withdrew consent, one cat was lost to follow-up and one cat developed severe lethargy and was withdrawn.
Baseline parameters
Table 2 summarises the results for the comparisons of baseline myoclonic seizure frequency in both groups. There was no significant difference between the groups.
Table 2.
Frequency of myoclonic seizures at baseline in both treatment groups
| Levetiracetam group (n = 34) | Phenobarbital group (n = 34) | P value | |
|---|---|---|---|
| Myoclonic seizure frequency per day | 2.5 (0–18.0) | 2.3 (0–17.0) | 0.248 |
| Total myoclonic seizure-free days | 33.4 (22.0–53.0) | 35.0 (21.0–43.0) | 0.348 |
| Myoclonic seizures per week | 16.2 (0–57.0) | 17.5 (0–51.0) | 0.251 |
| Myoclonic seizure days per week | 4.2 (0–7.0) | 4.1 (0–7.0) | 0.348 |
Data are median (range)
Efficacy
Table 3 summarises the results for the myoclonic seizures in both groups.
Table 3.
Efficacy of levetiracetam and phenobarbital in the management of feline audiogenic reflex myoclonic seizures
| Levetiracetam group (n = 28) |
Phenobarbital group (n = 29) |
P value | |
|---|---|---|---|
| Number of cats achieving ⩾50% reduction from baseline in the number of myoclonic seizure days per week | 28 (100) | 1 (3) | <0.001 |
| Mean percentage reduction from baseline in the number of myoclonic seizure days per week | 98.8 ± 4.7 | 2.8 ± 23.3 | <0.001 |
| Number of cats achieving myoclonic seizure freedom | 14 (50) | 0 (0) | <0.001 |
| Mean percentage increase in myoclonic seizure-free days | 95.7 ± 8.8 | –57.0 ± 54.5 | <0.001 |
Data are n (%) or mean ± SD
Regarding GTCSs, these were infrequent with 57/68 cats having GTCSs during baseline (29/34 in the levetiracetam group and 30/34 in the phenobarbital group). The median number of GTCSs during baseline for all cats was 1 (range 0–3). During treatment, 44/68 cats experienced GTCSs (22 cats in each group) and the median number of GTCSs during treatment for all cats was 0 (range 0–1). In the levetiracetam-treated group, 23 had a decrease in the number of GTCSs on treatment and 11 were excluded owing either to lack of GTCSs at baseline or failure to complete the study. In the phenobarbital-treated group, 23 had a decrease in GTCSs on treatment, two remained with the same GTCSs frequency on treatment and nine were excluded owing to lack of GTCSs at baseline or failure to complete the study. No absence seizures were reported during the treatment period in any cat. Consequently, statistical analysis of GTCSs and absence seizures was not performed.
Safety analysis
Safety analysis showed that 24% of cats (16/68) experienced apparent adverse events and that the majority were mild to moderate in nature. Treatment-related adverse events were reported by owners of five cats (5/34; 18%) in the levetiracetam group and included lethargy (4/5), mild inappetence (3/5), ataxia (2/5) and polydipsia (1/5). These signs resolved without any change in dosage after approximately 2 weeks. Adverse effects were reported in 11/34 cats (32%) receiving phenobarbital, including lethargy (8/11), ataxia (4/11), weakness (1/11) and behavioural changes (1/11). In one case the lethargy was severe enough to warrant withdrawal from the study. These reported signs were relatively persistent in this population, resolving in only 2/11 cats during the treatment period.
In an extension of this study, five patients switched to levetiracetam therapy after receiving phenobarbital because their owners desired improved seizure control. Of these patients, 3/5 had reported no further myoclonic seizures at the time of publication and 2/5 had just one myoclonic seizure per week.
Other information
Owners of cats in the phenobarbital group perceived no benefit from using the medication, with only adverse effects reported (see above). All owners with cats receiving levetiracetam commented on their cat appearing brighter and more responsive during the treatment period following initial transient effects of sedation and lethargy when reported. Fifteen of 28 owners (54%) had not realised their cat’s mentation had altered until observing the effects during treatment.
Discussion
FARS have provided a unique opportunity to compare the efficacy of phenobarbital and levetiracetam in the management of myoclonic seizures. Many owners and veterinarians alike have traditionally considered myoclonus to be an age-related finding or potentially a condition associated with concurrent renal or cardiac disease. In making this assumption it has provided a pool of AED-naive patients on which to base the grounds of our study. We have therefore been able to evaluate the antimyoclonic efficacy of phenobarbital and levetiracetam in the management of FARS. It has long been suggested that medical management for myoclonic seizures in people contrasts with that for GTCSs. 5 This study provides the first veterinary evidence that levetiracetam is superior to phenobarbital in the management of myoclonic seizures.
The pharmacokinetics of levetiracetam in 10 healthy cats were evaluated following the disposition of a single dose of the drug via oral and intravenous routes. 14 Although limited information on the pharmacokinetics has been published, this study supported the use of levetiracetam at 20–25 mg/kg q8h. The only efficacy study of levetiracetam in cats reported its use as an adjunct to phenobarbital in 10 epileptic cats with GTCSs. 15 The study reported a reduction of more than 50% in seizure frequency in 7/10 cats following administration and the medication appeared to be well tolerated. Our results support the tolerability but appear to show a more dramatic response to levetiracetam when used as an antimyoclonic medication.
Levetiracetam is indicated in people as a monotherapy in the treatment of partial-onset seizures and as adjunctive treatment for myoclonic seizures and GTCSs. In a recent double-blind, placebo-controlled trial, levetiracetam (3000 mg/day) was shown to be effective as adjunctive therapy in 120 idiopathic generalised epilepsy patients aged 12–65 years with uncontrolled myoclonic seizures. Just over half (58.3%) of these patients achieved a >50% reduction in myoclonic seizure days per week, compared with 23.3% in the placebo group. 16 Another double-blind, placebo-controlled trial has shown adjunctive levetiracetam to be effective in controlling GTCSs, myoclonic seizures and all seizure types in patients with idiopathic generalised epilepsy compared with placebo. 17 The median percentage reduction in seizure days per week between the prospective baseline period and treatment period was 62.8% for levetiracetam and 24.7% for placebo. The results of these two double-blind, placebo-controlled studies are in line with the findings of open-label studies in humans,18–21 confirming the usefulness of levetiracetam in idiopathic generalised epilepsies with myoclonic seizures.
It is still not clear how levetiracetam exerts its antiepileptic effect. It does not, like many other AEDs, bind to the gamma (γ)-aminobutyric acid A–benzodiazepine receptor complex, does not inhibit voltage-gated sodium channels and does not inhibit low-voltage-activated Ca2+ channels. 22 It has been found to bind to synaptic vesicle glycoprotein 2A (SV2A), which is one of three isoforms of the SV2 protein, and the isoform most widely distributed in the brain. 22 SV2A is thought to inhibit presynaptic Ca2+ channels, so reducing neurotransmitter release. 23 There is a strong correlation between affinity of levetiracetam for the SV2A binding site and the seizure protection given to audiogenic mouse models of epilepsy. 22 Thus, although no molecular mechanisms of action are described for levetiracetam, it is possible that its antimyoclonic actions are mediated via the SV2A protein.
Two studies have provided evidence that levetiracetam, unlike other AEDs, may have modulatory effects on activity-dependent plasticity and its behavioural consequences. Löscher et al demonstrated that administration of levetiracetam during induction of kindling resulted in a persistent reduction in after-discharge duration, even after discontinuation of treatment in rats. 24 A second study investigated a strain of rats that developed spontaneous seizures in adulthood. 25 They were given long-term levetiracetam before these seizures developed. Even though these seizures continued to develop, a significant decrease in the frequency and duration of both tonic and absence seizures was noted compared with untreated animals. These data suggest that levetiracetam has a different spectrum of action to other AEDs, which may relate to the novel mechanism of action via SV2A. The observation in our study of cats appearing brighter and more responsive provides tentative evidence to suggest levetiracetam influences behavioural consequences of FARS. However, it cannot be excluded that this change in demeanour may simply be the result of freedom from the myoclonic seizures and hence lack of postictal signs.
Rarely, cats have only myoclonic seizures as a manifestation of FARS; more frequently, the myoclonus may predominate, and GTCSs may be infrequent. 1 The predominant seizure type in the cats of our study was myoclonic, while >80% also had GTCSs and <10% had absence seizures. Whether a build up of myoclonic jerks eventually leads to a GTCS is not entirely proven. Many cats are reported to be indifferent to myoclonic jerks, with owners frequently electing to monitor their frequency. 1 In some, however, these constitute a concern when owners observe a train of myoclonic jerks culminating in a single GTCS. This observation, combined with the available data suggesting FARS is a progressive disorder, 1 implies that early medical intervention is an advantage. When levetiracetam is prescribed, owners perceive their cats to be brighter as a result. It is also of note that electroencephalography has not been performed in cats with FARS and so it is uncertain whether myoclonus in this syndrome is of forebrain or brainstem origin.
Two unanswered questions from these results, in our view, are: (1) will levetiracetam prevent GTCSs in the same way it prevents myoclonus? (2) will levetiracetam prevent progression to GTCSs if used as an early interventional therapy? Previous work suggests that both may be a possibility.24,25
Conclusions
When myoclonus is frequent in cats with FARS, and when agreement exists between the owner and the veterinarian that medical treatment is justified, treatment with levetiracetam is likely to be effective. Whether it will prevent the occurrence of GTCSs if used early in the course of the disease is not clear.
Acknowledgments
The authors thank all the owners and primary veterinarians for their hard work and dedication in this study.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Accepted: 24 November 2015
References
- 1. Lowrie M, Bessant C, Harvey RJ, et al. Audiogenic reflex seizures in cats. J Feline Med Surg 2016; 18: 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kesner RP. Subcortical mechanisms of audiogenic seizures. Exp Neurol 1966; 15: 192–205. [DOI] [PubMed] [Google Scholar]
- 3. Garcia-Cairasco N. A critical review on the participation of inferior colliculus in acoustic-motor and acoustic-limbic networks involved in the expression of acute and kindled audiogenic seizures. Hear Res 2002; 168: 208–222. [DOI] [PubMed] [Google Scholar]
- 4. Faught E. Clinical presentations and phenomenology of myoclonus. Epilepsia 2003; 11: 7–12. [DOI] [PubMed] [Google Scholar]
- 5. Pranzatelli MR, Nadi NS. Mechanism of action of antiepileptic and antimyoclonic drugs. Adv Neurol 1995; 67: 329–360. [PubMed] [Google Scholar]
- 6. Genton P, Gélisse P. Antimyoclonic effect of levetiracetam. Epileptic Disord 2000; 2: 209–212. [PubMed] [Google Scholar]
- 7. Frucht SJ, Louis ED, Chuang C, et al. A pilot tolerability and efficacy study of levetiracetam in patients with chronic myoclonus. Neurology 2001; 57: 1112–1114. [DOI] [PubMed] [Google Scholar]
- 8. Krauss GL, Bergin A, Kramer RE, et al. Suppression of post-hypoxic and post-encephalitic myoclonus with levetiracetam. Neurology 2001; 56: 411–412. [DOI] [PubMed] [Google Scholar]
- 9. Kinrions P, Ibrahim N, Murphy K, et al. Efficacy of levetiracetam in a patient with Unverricht-Lundborg progressive myoclonic epilepsy. Neurology 2003; 60: 1394–1395. [DOI] [PubMed] [Google Scholar]
- 10. Crest C, Dupont S, Leguern E, et al. Levetiracetam in progressive myoclonic epilepsy: an exploratory study in 9 patients. Neurology 2004; 62: 640–643. [DOI] [PubMed] [Google Scholar]
- 11. Magaudda A, Gelisse P, Genton P. Antimyoclonic effect of levetiracetam in 13 patients with Unverricht-Lundborg disease: clinical observations. Epilepsia 2004; 45: 678–681. [DOI] [PubMed] [Google Scholar]
- 12. Sharpe DV, Patel AD, Abou-Khalil B, et al. Levetiracetam monotherapy in juvenile myoclonic epilepsy. Seizure 2008; 17: 64–68. [DOI] [PubMed] [Google Scholar]
- 13. International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia 1989; 30: 389–399. [DOI] [PubMed] [Google Scholar]
- 14. Carnes MB, Axlund TW, Boothe DM. Pharmacokinetics of levetiracetam after oral and intravenous administration of a single dose to clinically normal cats. Am J Vet Res 2011; 72: 1247–1252. [DOI] [PubMed] [Google Scholar]
- 15. Bailey KS, Dewey CW, Boothe DM, et al. Levetiracetam as an adjunct to phenobarbital treatment in cats with suspected idiopathic epilepsy. J Am Vet Med Assoc 2008; 15: 867–872. [DOI] [PubMed] [Google Scholar]
- 16. Noachtar S, Andermann E, Meyvisch P, et al. Levetiracetam for the treatment of idiopathic generalized epilepsy with myoclonic seizures. Neurology 2008; 70: 607–616. [DOI] [PubMed] [Google Scholar]
- 17. Berkovic SF, Knowlton RC, Leroy RF, et al. Placebo-controlled study of levetiracetam in idiopathic generalized epilepsy. Neurology 2007; 69: 1751–1760. [DOI] [PubMed] [Google Scholar]
- 18. Grünewald R. Levetiracetam in the treatment of idiopathic generalized epilepsies. Epilepsia 2005; 46; 154–160. [DOI] [PubMed] [Google Scholar]
- 19. Labate A, Colosimo E, Gambardella A, et al. Levetiracetam in patients with generalised epilepsy and myoclonic seizures: an open label study. Seizure 2006; 15: 214–218. [DOI] [PubMed] [Google Scholar]
- 20. Specchio LM, Gambardella A, Giallonardo AT, et al. Open label, long-term, pragmatic study on levetiracetam in the treatment of juvenile myoclonic epilepsy. Epilepsy Res 2006; 71: 32–39. [DOI] [PubMed] [Google Scholar]
- 21. Specchio N, Boero G, Michelucci R, et al. Effects of levetiracetam on EEG abnormalities in juvenile myoclonic epilepsy. Epilepsia 2008; 49: 663–669. [DOI] [PubMed] [Google Scholar]
- 22. Lynch BA, Lambeng N, Nocka K, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci U S A 2004; 29: 9861–9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vogl C, Mochida S, Wolff C, et al. The synaptic vesicleglycoprotein 2A ligand levetiracetam inhibits presynaptic Ca2+ channels through an intracellular pathway. Mol Pharmacol 2012; 82: 199–208. [DOI] [PubMed] [Google Scholar]
- 24. Löscher W, Hönack D, Rundfeldt C. Antiepileptogenic effects of the novel anticonvulsant levetiracetam (ucb L059) in the kindling model of temporal lobe epilepsy. J Pharmacol Exp Ther 1998; 284: 474–479. [PubMed] [Google Scholar]
- 25. Ji-qun C, Ishihara K, Nagayama T, et al. Long-lasting antiepileptic effects of levetiracetam against epileptic seizures in the spontaneously epileptic rat (SER): differentiation of levetiracetam from conventional antiepileptic drugs. Epilepsia 2005; 46: 1362–1370. [DOI] [PubMed] [Google Scholar]