Abstract
This study aimed to investigate the efficacy and safety of transcutaneous electrical nerve stimulation (TENS) in postoperative acute pain control. PubMed, Scopus, and Cochrane Library were searched on 1–8 December 2022, for randomized controlled trials on the analgesic effects of TENS. The outcomes were pain intensity and opioid use (primary), and postoperative (PO) adverse events, blood pressure, and the duration of hospital stay (secondary); PROSPERO CRD42022333335. A total of 40 articles were included in the meta-analysis. Pain intensity at rest and during coughing for all types of surgeries combined was lower in the TENS group (standardized mean difference (SMD) = −0.51 [−0.61, −0.41], p < 0.00001, 29 studies, and −1.28 [−2.46, −0.09], p-value = 0.03, six studies, respectively). There was a statistically significant decrease in morphine requirements, as well as in the incidence of postoperative nausea and vomiting, dizziness, and pruritus. There was no difference between the groups in postoperative pain intensity during walking, in blood pressure, and only a borderline difference in the length of hospital stay. The subgroup analysis by surgery type did not show significant differences between the groups in pain severity at rest. Thus, TENS has a potential for pain control and postoperative recovery outcomes.
Keywords: acute pain, postoperative pain, transcutaneous electrical nerve stimulation, adverse events, hospital stay
1. Introduction
Acute pain is the most common symptom and patient complaint during the acute postoperative period. It can contribute to numerous unwanted physiological and pathological effects, such as cardiovascular activation, resulting in tachycardia, elevated blood pressure, and increased myocardial oxygen demand; it can also impair respiratory, endocrine, and other system impairments. For patients undergoing simple surgical procedures, pain is one of the most frequent reasons for overnight hospital stays [1,2]. Postoperative pain is also a major cause of prolonged hospitalization, leading to a potential increase in morbidity after surgery [1]. Improved pain management may enhance postoperative recovery and reduce morbidity [3].
Prescribing opioids for the management of moderate to severe pain often results in side effects, with the most common being nausea, vomiting, intestinal hypomotility, and respiratory depression. Interventional modalities of acute pain management, such as epidural analgesia, some newer plane blocks, including transversus abdominis plane block, erector spinae plane block, and quadratus lumborum block, can provide effective pain management but require experienced specialists and can result in complications, such as local anesthetic systemic toxicity [4,5,6,7,8,9,10,11]. Multimodal analgesia is usually recommended for pain reduction [3,12]. This approach may include opioids, regional analgesia, low-dose ketamine, and neurocognitive modalities [12]. Nonpharmacologic methods, including transcutaneous acupoint electrical stimulation (TENS) and “transcutaneous acupoint electrical stimulation” (TAES), have been studied to improve postoperative pain control and reduce analgesic drug requirements [13,14,15].
TENS is a physical method of controlled low-voltage electrical nerve stimulation used for the reduction of pain. The electricity is conducted by electrodes placed on the skin [16]. TENS is a non-invasive, portable, compact, easy-to-use, and safe method of pain control [16]. In the postoperative period, TENS is used as an add-on pain management modality to standard analgesics rather than a stand-alone modality. The sterile electrodes are applied parallel to the surgical incision, and additional electrodes are placed over the thoracic spinal nerves in the corresponding area [17]. The possible advantages of TENS in postoperative pain management include faster mobilization and improvement in deep respiratory function and coughing, which might also shorten the time to discharge from the hospital [17].
The original theory suggested that the activation of descending inhibitory pathways might be the mechanism of action of TENS. Previous studies also supported the mechanism of segmentally mediated inhibition. Therefore, TENS appears to activate both descending and segmental inhibition [18]. The analgesic effect of TENS is also produced by the pain gate control theory, which is characterized by an attenuation of the nociceptive stimulation of afferent fibers of large diameter in the dorsal horn [19]. Conventional TENS activates the A-α and A-β fibers, alleviating the pain. TENS also activates the endogenous opioid system. This activation is produced by high and low-frequency stimulations [20].
Two decades ago, a meta-analysis comprising 21 studies examined the use of TENS in various surgeries and its effect on opioid consumption [21]. However, more studies have been published over the past twenty years; therefore, there is a need for additional analysis of the currently available data. Recently, meta-analyses have been conducted on the analgesic effects of TENS in orthopedic [22], pulmonary [23], inguinal hernia repair [24], gynecological [25], and cardiothoracic surgical interventions [26]. A recent large meta-analysis of 381 studies, comprising almost 25,000 patients, studied the analgesic effect of TENS in all-cause pain [27]. The authors state that aggregating pain intensity data regardless of the underlying medical condition is appropriate, as there is a lack of conclusive evidence establishing a connection between TENS outcomes and factors such as pathology, pain characteristics, medical diagnoses, or clinical context [27]. While this study comprises all types of pain, including chronic and non-surgical, it is important to also examine the effect of TENS on acute postoperative pain.
While these previous publications have examined the effect of TENS on postoperative pain, the evidence regarding the impact of TENS on pain and other postoperative outcomes is still inconclusive. Therefore, the goal of this work was to assess the efficacy and safety of TENS in acute postoperative pain management, as well as its influence on opioid consumption, ICU and hospital stay, and the rate of postoperative complication. We hypothesize that the use of TENS in various surgeries lowers pain scores and opioid consumption and might be associated with a reduction in postoperative adverse events and hospital length of stay.
2. Materials and Methods
2.1. Protocol
We used the PRISMA guidelines [28] and the Cochrane Handbook for Systematic Reviews of Interventions [29]. The protocol was registered in the PROSPERO database (CRD42022333335). We searched for suitable articles in PubMed, Scopus, and the Cochrane Library, published before December 2022. No gray literature was searched. We used the following search terms and combinations: (“transcutaneous electric nerve stimulation” OR (“transcutaneous” AND “electric” AND “nerve” AND “stimulation”) OR “transcutaneous electric nerve stimulation” OR (“transcutaneous” AND “electrical” AND “nerve” AND “stimulation”) OR “transcutaneous electrical nerve stimulation”) OR “transcutaneous acupoint electrical stimulation” AND (“pain, postoperative” OR (“pain” AND “postoperative”) OR “postoperative pain” OR (“postoperative” AND “pain”)). The filters used were “randomized controlled trials” and “English language”. No restrictions were used for the year or country of publication. Two authors independently worked on record screening and article searching. The search results retrieved from the mentioned databases were pooled in a spreadsheet, and duplicates were removed. Then, the two authors conducted the screening based on titles in accordance with the pre-specified inclusion criteria. The remaining articles were further screened based on the abstracts. Finally, the full texts were screened to identify those reporting the outcomes of interest. The final lists of articles were compared between the two authors. In case of disagreements, a third author was consulted to resolve the dispute.
2.2. Participants and Population
2.2.1. Inclusion Criteria
Patients: postoperative patients with no limitations on age, gender, or type of surgery.
Intervention: use of TENS.
Comparison: sham or no TENS (with standard postoperative pain management).
Outcomes: pain and other clinically important postoperative outcomes (please see below).
Study: randomized controlled trials (RCTs).
2.2.2. Exclusion Criteria
Types of studies: Study designs other than RCTs.
Outcomes: Studies not reporting the outcomes of interest.
2.3. Outcomes
The primary outcomes of our meta-analysis are postoperative (PO) pain (at rest, while walking, while coughing during a 72-h period postoperatively). The secondary outcomes were opioid consumption, postoperative adverse events, physiological parameters (blood pressure levels), and hospital stay duration (days).
2.4. Data Extraction and Statistical Methods
One author extracted and entered essential descriptive information (e.g., study goals and sample size). Another author extracted numeric data for meta-analysis from the studies. A third author further checked the descriptive and quantitative data, and disagreements were resolved by discussion. Data analysis was conducted using the software “Review Manager (RevMan) [Computer program]. Version 5.4. The Cochrane Collaboration, 2020”. The random-effects model was employed for the meta-analysis, in anticipation of heterogeneity resulting from the combination of various types of surgeries, patient populations, and the different scales used to report the outcomes of interest. All outcome variables were continuous. In some instances, we estimated statistics from the reported data using established statistical methods [30,31]. The effect size was reported as the standardized mean difference (SMD), mean difference (MD), or risk ratio (RR), with a 95% confidence interval. The utilization of SMD allowed for the standardized comparison of effect sizes across diverse outcome measures, such as pain scores and opioid use, accommodating the varied scales and units of measurement employed in the included studies. The risk ratio was used for analyzing dichotomous outcomes, such as adverse events, providing a measure of the relative risk between the TENS and control groups. Statistical significance was reported at p < 0.05. Heterogeneity was measured using the I2 statistic, which quantifies the proportion of total variation across studies due to heterogeneity. Additionally, the p-value of Cochran’s Q statistic was considered to evaluate the statistical significance of observed heterogeneity. A significance level of 0.1 was employed to determine whether the observed heterogeneity was statistically significant. Sensitivity analyses were performed for each outcome by running the model with the elimination of studies one by one and were reported only if the results were sensitive.
2.5. Assessment of the Methodological Quality and the Publication Bias
Two authors independently assessed the methodological quality. The Cochrane Risk of Bias tool 2.0 [32] was utilized to evaluate the methodological quality of the studies. The risk of bias was categorized as “high”, “low”, or “medium/some concerns”, based on the provided description of randomization and blinding procedures, as well as the reporting of results. Furthermore, we assessed the certainty of evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) [33]. Five outcomes (pain at rest at 24 h, morphine consumption at 24 h, PONV, pruritus, and hospital length of stay) were evaluated for upgrading or downgrading based on the risk of bias, imprecision, inconsistency, and indirectness. Each of these outcomes received a certainty of evidence grading ranging from “very low” to “high”. Additionally, we conducted a comprehensive assessment of publication bias utilizing both funnel plots and Egger’s regression test.
3. Results
3.1. Article Search Results
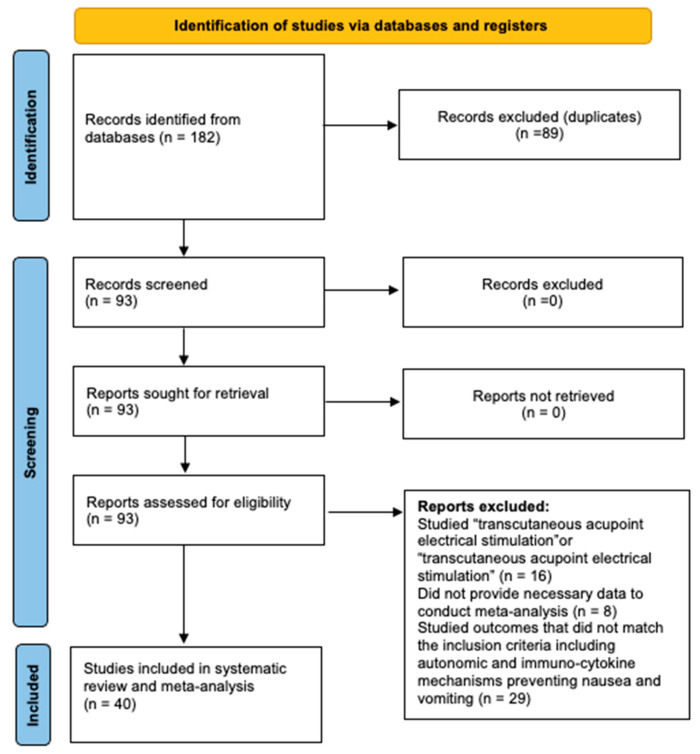
In total, 182 articles were initially identified (Figure 1). Of them, 89 duplicates were removed. Subsequently, 93 RCTs were screened. After screening the titles and abstracts, 53 articles were excluded. Finally, a total of 40 articles [2,13,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71] with 2265 (TENS—1137, control—1128) patients were included in the meta-analysis (Table 1).
Figure 1.
PRISMA diagram.
Table 1.
Study characteristics. Abbreviations: RoM, range of motion; TKA, total knee arthroplasty; VAS, visual analog scale; LAS, linear analog scale; N, number; PONV, postoperative vomiting and nausea; TENS, transcutaneous electrical nerve stimulation; QoR, quality of recovery; NRS, numeric rating scale; TEAS, transcutaneous acupoint stimulation; QoL, quality of life [2,13,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71].
| Author, Year, Country | Study Goals | Age | N of Patients: Total (TENS/Control); % Male | % Male (TENS/Control) | Groups | Diagnosis | Comorbidities | Type of Surgery | The Timing of TENS | Method of Pain Measurement | Study Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Beckwee, 2017, Belgium [41] | Pain, knee RoM, analgesic use | 71.8 (7.3) 72.9 (7.6) |
53 (25/28) | 32%/39.3% | TENS Sham |
- | - | TKA | 40 min | 100 mm VAS before and after, daily | No effects on pain |
| Forogh, 2017, Iran [56] | Pain, IKDC, RoM | 26 (4.1) 26.31 (4.33) |
70 (35/35) | 100% | TENS No TENS, both groups exercise |
Injury to the ACL | - | Post-anterior cruciate ligament reconstruction | 20 sessions, 4 weeks, 35 min/day | 100 mm VAS | No effect on knee function and pain |
| Asgari, 2018, Iran [37] | Pain, fentanyl use, PONV | 31.35 (4.89) 31.15 (6.28) |
80 (40/40) | 0% | TENS No TENS, 50 mg fentanyl |
Ectopic pregnancy, infertility, ovarian cysts, ovarian torsion | - | Laparoscopic Gynecologic Surgery | 20 min for patients who complained of pain | 10-cm VAS before, and 5, 10, 20, 30 min after treatment | TENS is not superior to fentanyl for pain relief |
| Bjersa, 2014, Sweden [62] | Pain, QoR-40, extra analgesia use, EDA infusion rate, total TENS use time | 69.1 65.5 |
20 (9/11) | 56%/73% | TENS Sham |
- | - | Pancreatic resection: Ad Modum Whipple pancreaticoduodenectomy |
30 min sessions; for 24 h post-op | Pain-O-Meter, estimation on 100-mm scale | Supports use of high-frequency TENS |
| Bjersa, 2015, Sweden [38] | Pain, QoR-40, total analgesia use, time of TENS use |
67.9 (11.6) 74.1 (10.3) |
28 (15/13) | 53%/86% | TENS Sham |
Colon diseases and malignancies, unknown | - | Open colon resection | No time limits; each session—30 min; for 24 h post-op | Pain-O-Meter, estimation on 100-mm scale | Benefits of TENS |
| Cuschieri, 1985, UK [63] | Pain, morphine use, ABG | 51 57 |
106 (53/53) | 43%/40% | TENS Sham |
- | - | Abdominal surgery | 3 days post-op | LAS, before + after twice daily for 3 days | Results do not support TENS use |
| Galli, 2014, Brazil [57] | Pain | 44.32 (9.98) 44.22 (8.21) |
74 (37/37) | 57%/38% | TENS Sham |
Healthy kidney donors | HTN, asthma, gastritis, hypothyroidism | Open nephrectomy | For 1 h during first post-op day | NRS before and after | TENS decreases pain and increases max expiratory pressure |
| Hamza, 1999, USA [67] | PCA demands and doses, sedation, fatigue, discomfort, pain, nausea, side effects |
43 (11) 44 (11) 45 (10) 43 (9) |
100 (25/25/25/25) | 0% | PCA + sham PCA + low-frequency TENS PCA + high-frequency TENS PCA + mixed-frequency TENS |
- | - | Major gynecological procedures | Every 2 h during the day | 100 mm VAS at baseline, 24, 48 h | TENS decreases post-op opioid analgesic use and opioid-related side effects |
| Laitinen, 1991, Finland [68] | Pain, BP, HR, RR, side effects | 63.4 (7.8) 50.2 (8.6) 56.6 (11.5) 61.4 (8.4) 52.2 (8.4) 40.6 (11.4) 49.6 (16.9) 46.9 (14) |
60 (10/10/20/20) | 20%/0%/0%/0%/3% | Control Indomethacin Low-frequency TENS + indomethacin High-frequency TENS + indomethacin |
Cholecystitis | - | Cholecystectomy | 16 h | No/mild/moderate/severe every 4 h | Neither indomethacin nor TENS reduce the postoperative opiate requirement. |
| Rakel, 2003, USA [60] | Pain, walking function, vital capacity | 20–77 40 (15) |
33 | 48% | Pharmacologic analgesia + TENS Pharmacologic + sham TENS Pharmacologic only |
- | End-stage renal disease, diabetes | - | 15 min, 2–4 h between the sessions | NRS 0–20 | Reduces pain and increases walking function post-op |
| Silva, 2012, Brazil [13] | Pain, PONV | 52 (14) 44 (16) |
42 (21/21) | 7% | TENS Sham TENS |
Cholecystitis | - | Laparoscopic cholecystectomy | 30 min during 24h post-op | 11-point VNS, VAS (0–10) | Decreases pain and PONV |
| Yu, 2020, China [52] | QoR, pain at rest, MMSE, PONV, medication use | 48.5 (16.2) 45.9 (17.5) |
60 (30/30) | 0% | TEAS Sham TEAS |
- | - | Gynecological laparoscopic surgery | 30 min before anesthesia | 100 mm VAS | Improves QoR, MMSE; reduces pain, PONV |
| Zhang, 2017, China [53] | Pain, bladder spasm episodes | 64.5 (54–79) | 66 (30/36) | 100% | TENS No TENS |
BPH, bladder disease | - | Bladder or prostate surgery | 3 days post-op, each session for 60 min | VAS 0–10 | Relieves post-op bladder spasms |
| Zhang, 2018, China [54] | Pain, time to first: defecation, flatulence, diet; LOS, HRV | 68 (1.4) 64 (2.6) |
42 (21/21) | 86%/71% | TEAS Sham TEAS |
GI cancers | - | Open abdominal surgery for cancers | 1 h, twice daily, 3 d | VAS 0–10 | Improves major post-op symptoms |
| Chiu, 1999, Taiwan [42] | Pain, total PCA morphine use, N of nurse calls for analgesia | 53.1 (2.7) 56.0 (3.1) |
60: (30/30) | 75% | TENS on acupoints TENS on sham acupoints |
Symptomatic hemorrhoids | - | Hemorrhoidectomy | Postoperative, 2 times a day | 0–10 | Complications—hemopericardium, better pain relief |
| Elboim-Gabyzon, 2019, Israel [43] | Pain, FAC, physical performance | 78.06 (8.45), 80.26 (9.83) |
41: (18/23) | 13%/33% | TENS Sham TENS |
Intertrochanteric or sub-trochanteric fracture | Yes | Hip fracture surgery | Postoperative | NRS 0–10 | Pain relief |
| Benedetti, 1997, Italy [66] | Time to analgesia, total medication use, pain | - | 103 106 112 |
Not given | TENS Sham TENS No TENS |
Empyema, myasthenia gravis | - | Posterolateral thoracotomy, muscle-sparing thoracotomy, costotomy, sternotomy, and video-assisted thoracoscopy |
1 h post-op, 1 h rest interval, 1h more | NRS 0–10 | Useful for mild to moderate pain; ineffective for severe pain |
| Engen, 2015, USA [44] | Pain, analgesia use, patient satisfaction | 61.5 (11.21), 61.8 (13.13) |
56: (28/28) | 30%/55% | TENS + opioids Opioids only |
- | - | Thoracoscopic surgery | 48 h post-operatively | VAS 0–10 | No effect on pain or morphine use |
| Erden, 2016, Turkey [36] | Pain, analgesic use | 54.9 (13.3), 50.0 (12.7) |
40: (20/20) | 70%/80% | TENS No TENS |
Lung cancer | Chronic disease | Posterolateral thoracotomy | Postoperative 30 min | VAS | Reduces pain |
| Erdogan, 2005, Turkey [46] | Pain, FEV1, FVC, PaO2, PaCO2, doses of analgesia, sedation, side effects | 55.6 (11.9) 52.93 (11.48) |
116 (60/56) | 63%/57% | TENS No TENS |
Lung cancer | - | Posterolateral thoracotomy | For 20 min at 3-h intervals for 3 days | VAS 0–10 | Routine use recommended |
| Ferreira, 2011, Brazil [40] | Pain | 49 (14), 55.0 (14.9) |
30: (15/15) | 67%/53% | TENS Sham TENS |
Lung cancer | - | Thoracotomy | Second post-op day | VAS 10 cm | Reduces pain severity |
| Fiorelli, 2011, Italy [2] | Cytokines, pain, respiratory function, medication usage | 64 (1), 64 (4.1) |
50: (25/25) | 74%/61% | TENS Sham TENS |
Lung cancer | - | Standard posterolateral thoracotomy | 48 h post-op, 30 min | VAS 0–10 | Reduces pain |
| Gregorini, 2010, Brazil [58] | Pain, respiratory function | 59.9 (10.3) | 25: (13/12) | 72% | TENS Sham TENS |
- | - | Elective cardiac surgery | Third post-op day | VAS | Reduces pain |
| Jahangirifard, 2018, Iran [61] | Pain, respiratory function, narcotics use, drain secretions, ICU LoS, N requests for chest radiographs |
58.4 (8.1), 60.1 (6.6) |
100: (50/50) | 50%/50% | TENS Sham TENS |
- | - | Elective coronary artery bypass | Post-op 30 min every 4 h | VAS 0–10 | Reduces pain, better pulmonary function |
| Lima, 2011, Brazil [39] | Pain, MIP, MEP |
54.2 55.1 |
20 (10/10) | 50% | TENS No TENS |
CAD | - | CABG | 30 min, 3 times a day, 3 h each | VAS 0–10 | Reduces pain; increase in respiratory muscle strength |
| Navarathnam, 1984, Australia [65] | Pulmonary function, analgesic use, atelectasis, pain | 56.4 (39–67) 52.2 (17–69) |
31 (14/17) | 86%/77% | TENS Sham TENS |
CAD, valve disease | - | CABG, AV replacement, MV replacement | - | Digital scoring system (1–5) | May be of benefit in post-op pain relief |
| Sezen, 2017, Turkey [50] | Post-op pain, complications | 55.13 (14.63), 58.86 (11.82) |
87: (43/44) | 74%/68% | TENS Sham TENS |
- | - | Thoracotomy | 8 h post-op | VAS 0–10 | No effect on hospital stay, complications; safe pain management |
| Solak, 2007, Turkey [69] | Pain, pulmonary function | 47.3 (11.7) 53.72 (12.6) |
40 (20/20) | 70%/90% | TENS PCA |
- | - | Posterolateral thoracotomy | 4 h post-op | VAS, Prince Henry score | Better pain relief than PCA |
| Stubbing, 1988, UK [70] | Analgesic use, time to oral analgesia, antiemetic use, LOS, pulmonary function |
54 (17.8) 53 (15.7) |
40 (20/20) | 65%/75% | TENS + IM papaveretum IM papaveretum alone |
- | - | Thoracotomy | For 48 h post-op | 0–4 | Lower PONV, no effect on analgesia use, peak expiratory flow rate |
| Kara, 2011, Turkey [48] | Pain, function, depression, side effects | 45.62 (10.59) 47.60 (13.75) |
54 (25/29) | 40%/55% | TENS + PCA PCA only |
- | - | Open lumbar discectomy | Twice for 30–40 min, 3–4 h interval | Horizontal 100 mm VAS | Reduces side effects, analgesic use, activity-related pain |
| McCallum, 1988, UK [64] | Morphine use | 44.6 (9.1), 45.7 (11.7) |
20: (10/10) | 50%/20% | TENS Sham TENS |
- | - | Lumbar laminectomy | 12 h prior to surgery | - | No effect on pain |
| Parseliunas, 2020, Lithuania [49] | Pain, analgesics use | 61.77 (10.84), 61.08 (12.51) |
80: (40/40) | 100% | TENS Sham TENS |
Unilateral inguinal hernia | - | Open inguinal hernia repair | Post-op | 100 mm VAS | Reduces post-op pain |
| Smedley, 1988, UK [51] | Pain, analgesic use, peak expiratory flow | 57 (21–83) 55 (24–78) |
62 (34/28) | 100% | TENS Sham TENS |
Inguinal hernia | - | Inguinal hernia repair | 48 h post-op | LAS | No differences |
| Chen, 2021, China [55] | Pain, pain attacks, N/amount analgesic drugs, changes in gene expression | 73%: 20–35 | 70 (35/35) | 0% | TENS + analgesic drugs Analgesic drugs only |
- | - | Elective C-section | 24 h post-op, 30 min each | 10 cm VAS | Reduces pain, N pain attacks, analgesic use, and expression of PNMT gene |
| Kurata, 2022, USA [47] | Opioid use, pain, patient satisfaction, LOS, adverse events | 31 (6) 32 (6) 31 (6) |
180 (60/60/60) ITT | 0% | TENS Sham TENS No TENS |
Obstetric | Prior c-section, other uterine incision | C-section | 30 min post-op, until discharge, PCA | 0–10 Likert scale | No effect on opioid use, pain, LOS |
| da Silva, 2015, Brazil [35] | Pain, analgesic use, adverse effects, quality of pain, treatment success, patient satisfaction |
25 27 |
42 (21/21) | 100% | TENS Sham TENS |
- | - | Liposuction | 30 min post-op | - | Effective in adjunction to analgesics for pain |
| Erden, 2022, Turkey [45] | Pain, patient satisfaction | 57.1 (10.88) 56.9 (10.2) |
80 (40/40) | 0% | TENS No TENS |
Breast cancer | Chronic diseases | Mastectomy | 2 times for 20 min | NRS 0–10 | Useful analgesic method |
| Ilfeld, 2021, USA [34] | Opioid use, pain, QoL |
56.8 (15.8) 55.4 (15.9) |
65 (31/34) | 52%/50% | TENS Sham TENS |
ACL injury, rotator cuff injury, hallux valgus, ankle arthrodesis, arthroplasty | - | Major foot/ankle surgery, anterior cruciate ligament reconstruction, rotator cuff repair | Up to 14 d post-op, daily | Average daily NRS 0–10 | Reduces pain and opioid use, no systemic side effects |
| Mahure, 2017, USA [59] | Anesthetic use, pain | 60.5 (11.1) 56.4 (12.2) |
37 (21/16) | 53%/44% | TENS Sham TENS |
- | - | Arthroscopic rotator cuff repair | - | VAS | Less pain, opioid use |
| Wang, 2014, China [71] | Intraoperative remifentanil use, side effects | 43.1 (15.0) 39.9 (15.7 |
60 (30/30) | 53%/63% | TEAS Sham TEAS |
- | - | Sinusotomy | 30 min before anesthesia | - | Less incidence of side-effects |
3.2. Assessment of Methodological Quality
Regarding methodological quality, 10 studies had a low risk of bias, while 29 studies were assessed as having “some concerns” regarding the risk of bias. One study had a high risk of bias. The detailed results of the quality analysis are presented in Table 2 [2,13,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71].
Table 2.
Risk of Bias table. “+” low risk of bias; “?” some concerns; “-” high risk of bias.
| First Author, Year | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Asgari 2018 [37] | + | ? | + | + | + | ? |
| Beckwee 2017 [41] | ? | + | + | + | + | ? |
| Benedetti 1997 [66] | ? | ? | + | + | + | ? |
| Bjersa 2014 [62] | + | ? | + | + | + | ? |
| Bjersa 2015 [38] | + | ? | + | + | + | ? |
| Chen 2021 [55] | ? | ? | + | + | + | ? |
| Chiu 1999 [42] | + | ? | + | + | + | ? |
| Cuschieri 1985 [63] | ? | + | + | + | + | ? |
| da Silva 2015 [35] | + | ? | ? | + | + | ? |
| Elboim-Gabyzon 2019 [43] | + | + | + | + | + | + |
| Engen 2015 [44] | ? | ? | + | + | + | ? |
| Erden 2022 [45] | + | + | - | - | + | + |
| Erden 2016 [36] | + | ? | + | + | + | ? |
| Erdogan 2005 [46] | ? | + | + | + | + | ? |
| Ferreira 2011 [40] | ? | ? | + | + | + | ? |
| Fiorelli 2011 [2] | + | + | + | + | + | + |
| Forogh 2017 [56] | + | + | + | + | + | + |
| Galli 2015 [57] | + | + | + | + | + | + |
| Gregorini 2010 [58] | + | ? | + | + | + | ? |
| Hamza 1999 [67] | + | ? | ? | + | + | ? |
| Ilfeld 2021 [34] | + | + | + | + | + | + |
| Jahangirifard 2018 [61] | ? | + | + | + | + | ? |
| Kara 2011 [48] | + | ? | + | + | + | ? |
| Kurata 2022 [47] | + | + | + | + | + | + |
| Laitinen 1991 [68] | ? | ? | + | + | + | ? |
| Lima 2011 [39] | - | ? | ? | ? | ? | - |
| Mahure 2017 [59] | + | ? | + | ? | + | ? |
| McCallum 1988 [64] | ? | + | + | + | + | ? |
| Navarathnam 1984 [65] | ? | ? | + | + | + | ? |
| Parseliunas 2020 [49] | + | + | + | + | + | + |
| Rakel 2003 [60] | ? | ? | + | + | + | ? |
| Sezen 2017 [50] | ? | ? | + | + | + | ? |
| Silva 2012 [13] | + | ? | + | + | + | ? |
| Smedley 1988 [51] | + | ? | + | + | + | ? |
| Solak 2007 [69] | ? | ? | + | + | + | ? |
| Stubbing 1988 [70] | ? | ? | + | + | + | ? |
| Wang 2014 | + | + | + | + | + | + |
| Yu 2020 [52] | + | + | + | + | + | + |
| Zhang 2017 [53] | + | ? | + | + | + | ? |
| Zhang 2018 [54] | ? | ? | + | + | + | ? |
1. Risk of bias arising from the randomization process. 2. Risk of bias arising from deviations from the intended interventions. 3. Risk of bias arising from missing outcome data. 4. Risk of bias arising from the measurement of the outcome. 5. Risk of bias arising from the selection of the reported results. 6. Overall risk of bias.
3.3. Pain at Rest
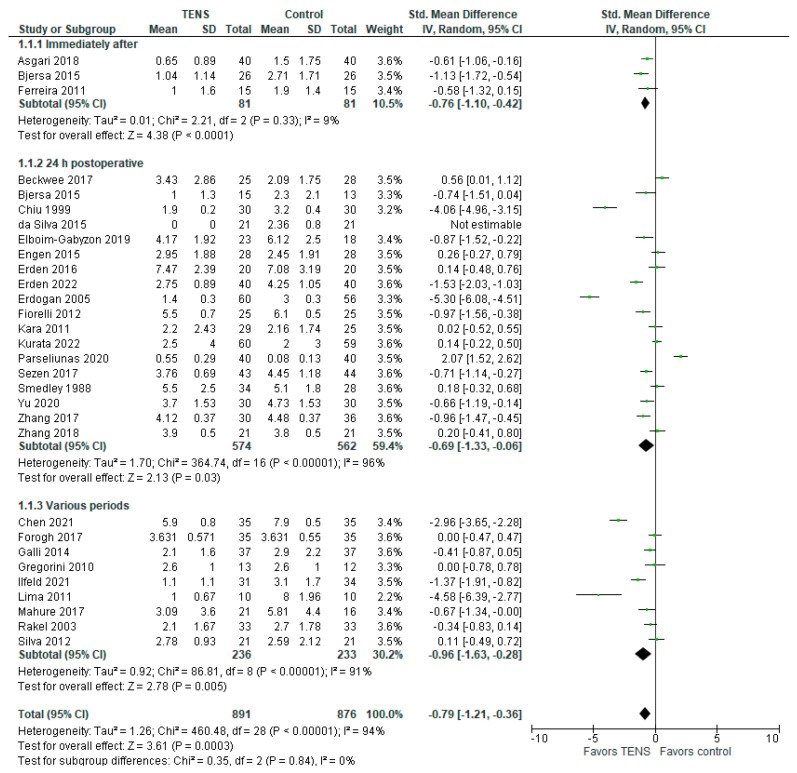
The forest plot in Figure 2 illustrates the pain intensity at rest measured immediately after surgery, 24 h post-surgery, and at various intervals. The overall model effect favors TENS over the control, indicating a standardized mean difference (SMD) on a 0-10 scale with a 95% CI of −0.79 [−1.21, −0.36], with a p-value less than 0.00001. However, it is important to note that the model shows substantial heterogeneity (I2 = 94%). The TENS group comprises 891 patients, while the control group consists of 876 patients. One study [72] was excluded from the meta-analysis due to the absence of a reported sample standard deviation.
Figure 2.
Pain at rest [2,13,34,35,36,37,38,39,40,41,42,44,45,47,48,49,50,51,52,53,54,55,56,57,58,59,60].
Subgroup analysis further reinforces the superiority of TENS over the control across all three subgroups (‘immediately after surgery’, ’24 h after surgery’, and ‘various periods’), although with considerable heterogeneity for the latter two (I2 = 96% and I2 = 91%, respectively). In the ‘immediately after surgery’ subgroup, the SMD with a 95% CI is −0.76 [−1.10, −0.42], with a highly significant p-value < 0.0001, I2 = 9%. In the primary (24 h postoperative) subgroup, the SMD with a 95% CI is −0.69 [−1.33, −0.06], with a p-value of 0.03, I2 = 96%. The third subgroup, covering varied measurement times, such as ‘after TENS’ [13], ’12 h postoperative’ [59], ‘postoperative day 3’ [58], ‘postoperative day 7’ [34], ‘4 weeks postoperative’ [56], and instances with no provided information [39,55,57,60], shows a significant SMD with a 95% CI of −0.96 [−1.63, −0.28], with a p-value of 0.005, I2 = 91%. These results indicate statistically significant improvements in pain intensity for the TENS group in all the measured periods.
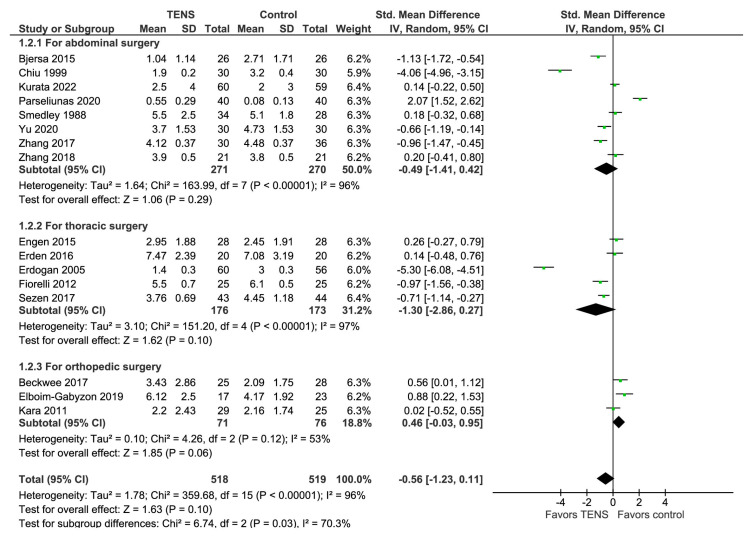
3.4. Pain at Rest for Specific Types of Surgeries 24 h PO
The pain intensity at rest, measured 24 h after three different types of surgeries (abdominal, thoracic, and orthopedic), is presented in the forest plot below (Figure 3). The overall effect of the model shows no significant difference between TENS and control (SMD on a 0–10 scale with a 95% CI = −0.56 [−1.23, 0.11], p-value = 0.10), and the model shows substantial heterogeneity with the value of I2 = 96%. However, the result is sensitive to the exclusion of the study by Parseliunas (2020), in which case the model favors the TENS group. The total number of patients in the TENS group is 518, and 519 in the control group. Two studies [35,45], representing the results for plastic and breast surgery, respectively, were excluded.
Figure 3.
Pain at rest 24 h after different surgeries [2,36,38,41,42,43,44,46,47,48,49,50,51,52,53,54].
In terms of subgroup analysis, the model shows no significant difference between the TENS and control groups. The ‘For abdominal surgery’ subgroup yielded an SMD of −0.49 [−1.41, 0.42], p-value = 0.29; the ‘For thoracic surgery’ subgroup yielded an SMD of −1.30 [−2.86, 0.27], p-value = 0.10; and the “For orthopedic surgery” subgroup yielded an SMD of 0.46 [−0.03, 0.95], p-value = 0.06.
3.5. Pain while Walking (POD 1, POD 2)
The forest plot in Figure 4 illustrates the pain intensity while walking measured 24 h and 48 h after surgery. The overall effect of the model does not favor TENS over the control (SMD with a 95% CI: 0.61 [−1.52, 2.74], p-value = 0.57). This result is sensitive to the exclusion of the study by Elboim-Gabyzon et al., 2019 [43]. The model shows considerable heterogeneity (I2 = 96%), which is likely attributed to the limited number of included studies. Parseliunas et al., 2020 [49] reported pain while walking values for postoperative day 1 (POD 1), whereas Elboim-Gabyzon et al., 2019 [43] reported them for POD 2.
Figure 4.
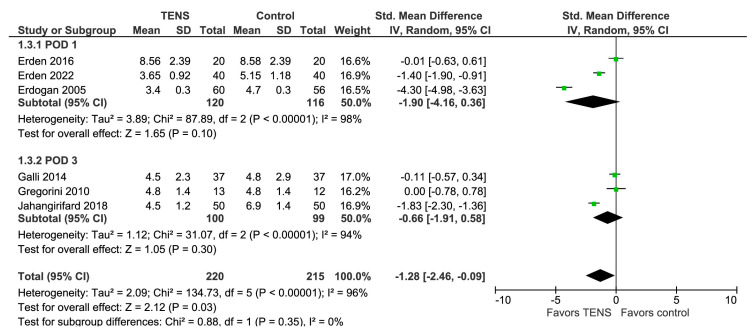
3.6. Pain at Coughing (POD 1, POD 3)
The overall effect of the model favors the TENS group over the control group (SMD with a 95% CI: −1.28 [−2.46, −0.09], p-value = 0.03) (Figure 5). This result is statistically significant but sensitive to the exclusion of some studies [45,46,61]. Subgroup analysis reveals no significant difference between the groups. It is important to note that one study [57] did not provide the time of measurements, and as a result, we included it in the POD 3 subgroup.
Figure 5.
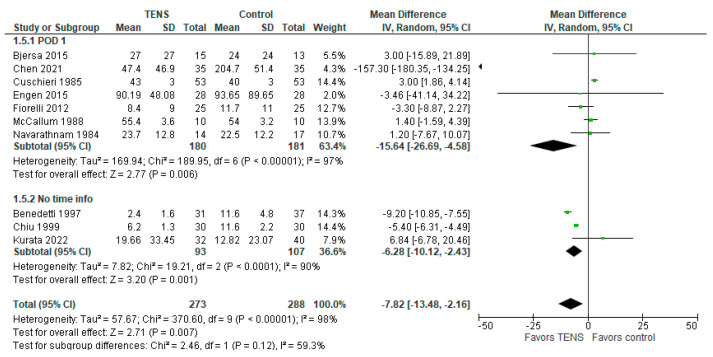
3.7. Morphine Requirements (mg)
One study [66] reported ketorolac intake (mg), and we adjusted the values by multiplying them by 0.4, following the recommendation of the American Pain Society in 2003 and 2008 (https://cdn-links.lww.com/permalink/jpsn/a/jpsn_4_2_2015_04_23_manworren_jpsn-d-14-00050r2_sdc1.pdf (accessed on 15 December 2022)). Another study [38] reported IV oxycodone (mg) consumption, and we used a conversion factor of 1.5. Finally, one study [62] reported IV morphine consumption at 24 h; however, there was not enough information about the units (mL in the Table, but mg in the text), so we did not include this study in the analysis (the results were not sensitive to the values from this study).
Some studies [42,47,66] did not explicitly report the time of measurement, so we included them in the ‘No time info’ subgroup. The majority of the studies reported morphine requirements within 24 h after surgery (‘POD 1′ subgroup). One study [61] was excluded due to the absence of information about the sample standard deviation.
The overall effect of the model favors TENS over control (MD with a 95% CI: −7.82 [−13.48, −2.16], p-value < 0.00001) (Figure 6). However, this result is sensitive to the exclusion of a study by Chen 2021 [55]. Broken down, on POD1, the use of TENS decreased morphine use by 15.64 mg [−26.69, −4.58], p-value = 0.006, I2 = 97%. In the “no time” subgroup, morphine use was decreased by 6.28 mg (−6.28 [−10.12, −2.43], p-value = 0.001, I2 = 90%) in the TENS group.
Figure 6.
Postoperative morphine requirements (mg) [2,38,42,44,47,55,63,64,65,66].
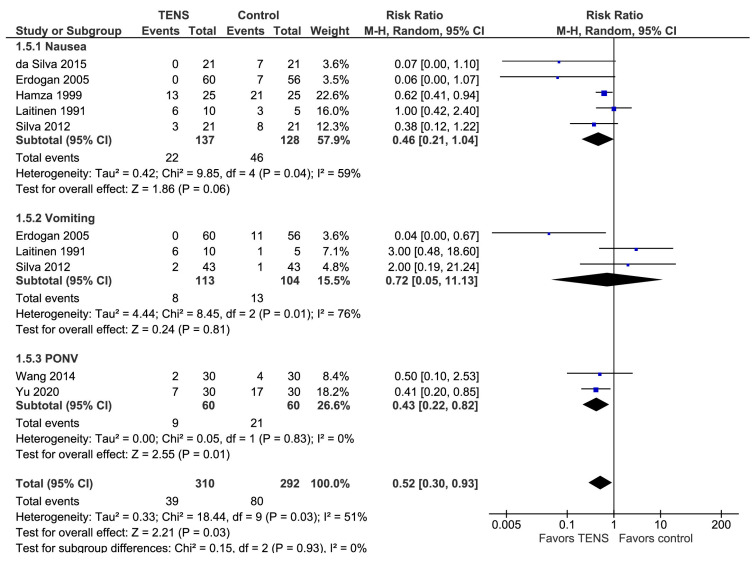
3.8. Postoperative Nausea and Vomiting
The overall effect of the model favors the TENS group over the control group (the risk ratio (RR) with a 95% CI: 0.52 [0.30, 0.93], p-value = 0.03; I2 = 51%) (Figure 7). It should be noted that the studies primarily reported the incidences of PONV on postoperative day 1 (POD 1) and postoperative day 2 (POD 2), but several studies did not explicitly report the time of measurement [13,35,46].
Figure 7.
The subgroup analysis indicates that the model favors the TENS group over the control group in two subgroups, namely ‘nausea’ and ‘PONV’.
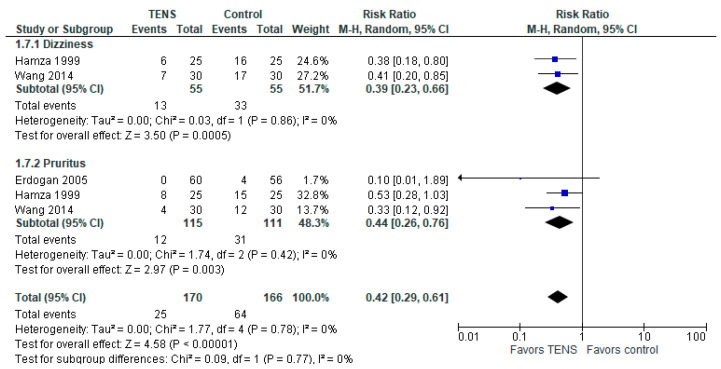
3.9. Other Adverse Events (Dizziness and Pruritus)
The overall effect of the model favors TENS over control (RR with a 95% CI: 0.42 [0.29, 0.61], p-value < 0.00001, I2 = 0%) (Figure 8). The model supports TENS over control in both subgroups: ‘dizziness’ and ‘pruritus’. Specifically, for dizziness, the RR for the TENS group is 0.39 [0.23, 0.66], p-value = 0.0005, I2 = 0%, 2 studies, 110 patients. For pruritus, the RR for the TENS group is 0.44 [0.26, 0.76], p-value = 0.003, I2 = 0%, 3 studies, 226 patients.
Figure 8.
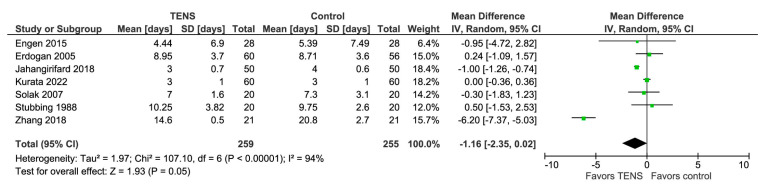
3.10. Hospital Stay Duration (Days)
The model shows no significant difference between TENS and control (MD with a 95% CI: −1.16 [−2.35, 0.02], p-value = 0.05; I2 = 94%) (Figure 9). The result is sensitive to the exclusion of any of these three studies: Erdogan 2005, Solak 2007, or Stubbing 1998, in which case, the model favors TENS.
Figure 9.
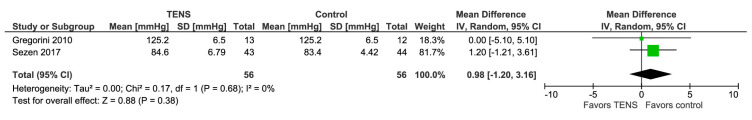
3.11. Blood Pressure Postoperatively (mmHg)
The model indicates no significant difference between TENS and control (MD with a 95% CI: 0.98 [−1.20, 3.16], p-value = 0.38; I2 = 0%) (Figure 10). Sezen et al., 2017 [50] reported the blood pressure values for postoperative day 1 (POD 1), and Gregorini et al., 2010 [58] reported these for postoperative day 3 (POD 3).
Figure 10.
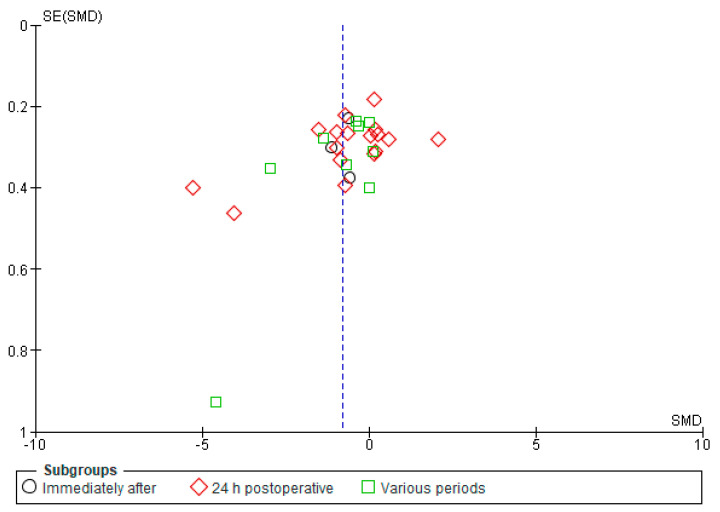
3.12. Publication Bias
Our findings from the analyses using the funnel plots and Egger’s regression test did not indicate substantial evidence of publication bias in the studies included in our meta-analysis regarding pain intensity.
The funnel plot below for the pain intensity at rest (Figure 11) demonstrates a spread of study outcomes that resembles a slightly asymmetric distribution. This slight asymmetry could be attributed to the nature of the random effects model, accounting for potential heterogeneity among the included studies.
Figure 11.
Funnel plot for pain at rest, the random effects model.
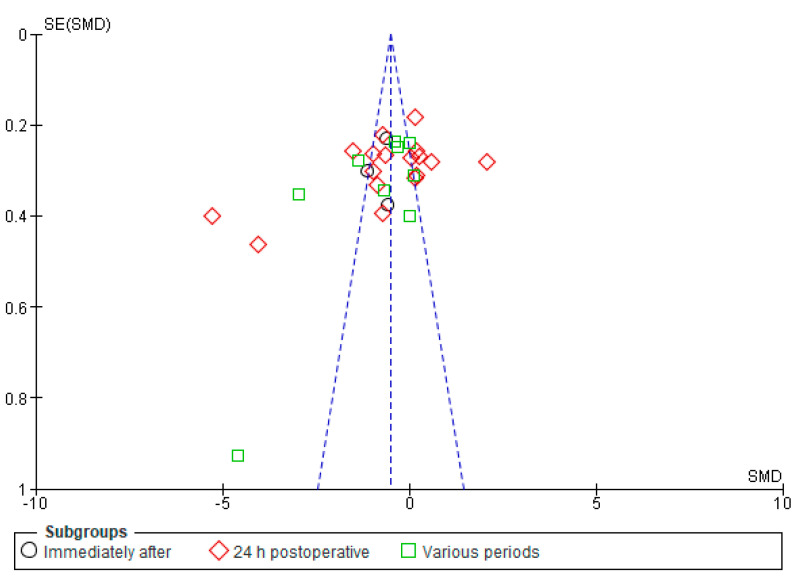
In contrast, the funnel plot under the fixed effect model (Figure 12) illustrates a more symmetric distribution of study outcomes. However, it is important to note that the fixed effect model assumes homogeneity across studies, which might not accurately represent the true variability seen in the data.
Figure 12.
Funnel plot for pain at rest, the fixed effects model.
3.13. Certainty of Evidence
Table 3 provides the certainty of the evidence for five outcomes (pain at rest at 24 h, morphine consumption at 24 h, PONV, postoperative adverse events, and hospital length of stay). The certainty of evidence ranges from “very low” to “moderate”. The evidence profile (Table 4) contains information regarding the quality of evidence evaluation and the summary of findings for each of the studied outcomes.
Table 3.
Summary of findings. ⨁⨁⨁⨁ high quality of evidence, ⨁⨁⨁⊖ moderate quality of evidence, ⨁⨁⊖⊖ low quality of evidence, ⨁⊖⊖⊖ very low quality of evidence. Population: Patients undergoing various surgeries. Settings: In-hospital. Intervention: Use of TENS. Comparison: No TENS [2,13,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71].
| Outcomes | Risk Ratio (95% CI) | (Standardized) Mean Difference [95% CI] | N of Participants (Studies) | Certainty of the Evidence (GRADE) |
|---|---|---|---|---|
| Pain at rest, 24 h (0–10) | - | −0.69 [−1.33, −0.06] | 1136 (18) | ⨁⨁⊖⊖ Very low a |
| Morphine requirements, 24 h (mg) | - | −15.64 [−26.69, −4.58] | 361 (7) | ⨁⊖⊖⊖ Very low b |
| Postoperative nausea | 0.46 [0.21, 1.04] | - | 265 (5) | ⨁⨁⊖⊖ Low c |
| Pruritus | 0.44 [0.26, 0.76] | - | 226 (3) | ⨁⨁⨁⊖ Moderate d |
| Hospital stay duration (days) | - | −1.16 [−2.35, 0.02] | 514 (7) | ⨁⨁⊖⊖ Low e |
a For three studies, the randomization method was unclear. Four studies were not blinded, and for two, blinding was unclear. There was considerable heterogeneity, wide variance of point estimates, and some confidence intervals did not overlap. b Four studies did not specify randomization procedures. One study was not blinded, and one study did not mention blinding. There was considerable heterogeneity, wide variance of point estimates, and some confidence intervals did not overlap. The confidence interval of the pooled effect crossed the no-difference line. c For two studies, the randomization method was unclear. Two studies were not blinded, and for two studies, blinding was unclear. There was moderate heterogeneity. The overall risk ratio was lower than 0.5; therefore, the outcome was upgraded. d For one study, the randomization method was unclear, for another, blinding was unclear. The overall risk ratio was lower than 0.5. e Four studies did not properly describe the randomization process. One study was not blinded, and two did not mention blinding. There was considerable heterogeneity and a wide variance of point estimates.
Table 4.
The evidence profile (the quality of evidence evaluation and the summary of findings for each of the studied outcomes).
| Pain at Rest, 24 h | Morphine Requirements, 24 h (mg) | Postoperative Nausea and Vomiting | Pruritus | Hospital Stay Duration (Days) | |
| Risk of Bias | Very Serious | Very Serious | Very Serious | Serious | Very Serious |
| Lack of allocation concealment | Some concerns | Some concerns | Some concerns | No | Some concerns |
| Lack of blinding | Some concerns | Some concerns | Some concerns | No | Some concerns |
| Incomplete accounting of patients and outcome events | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns |
| Selective outcome reporting | No | No | No | No | No |
| Other limitations | No | No | No | No | No |
| Inconsistency | Very serious | Very serious | Serious | Not serious | Serious |
| I2 (unexplained heterogeneity of results) | Considerable | Considerable | Moderate | None | Considerable |
| Wide variance of point estimates | No | Yes | Yes | No | Yes |
| Confidence intervals (CIs) do not overlap | Yes | Yes | No | No | No |
| Indirectness | Not serious | Not serious | Not serious | Serious | Not serious |
| Differences in population | No | No | No | No | No |
| Differences in interventions | No | No | No | No | No |
| Differences in outcome measures | No | No | No | Yes | No |
| Indirect comparisons | No | No | No | No | No |
| Imprecision | Not serious | Serious | Not serious | Not serious | Not serious |
| Few patients | Not serious | Not serious | Not serious | Not serious | Not serious |
| Wide confidence interval (CI) | Not serious | Serious | Not serious | Not serious | Not serious |
| Upgrading | None | None | None | RR < 0.5 | None |
| RR > 2 or RR < 0.5 RR > 5 or RR < 0.2 |
No | No | No | RR < 0.5 | No |
| Dose-response gradient | No | No | No | No | No |
| Effect of plausible residual confounding | No | No | No | No | No |
4. Discussion
Our meta-analysis revealed positive associations between TENS and various postoperative improvements, including reduced immediate and early postoperative pain, as well as diminished pain at coughing on days 1 and 3. Furthermore, TENS demonstrated effectiveness in decreasing morphine requirements, overall PONV, dizziness, pruritus, and, possibly, hospital length of stay. However, TENS did not show a significant impact on pain during walking. Similarly, sub-analysis based on the type of surgery did not reveal differences in pain scores.
A previously conducted large meta-analysis supports our findings regarding the pain-alleviating effect of TENS. The authors found that TENS reduced acute pain (surgical and non-surgical combined together) by −1.02 [−1.24, −0.79] on a ten-point scale, and postoperative pain by −0.92 [−1.15, −0.69] [27]. All studies combined (92 samples with 4841 participants reporting acute/chronic pain, (non)procedural, etc.) produced similar results: Pain scores in the TENS group were −0.96 [−1.14, −0.78] lower than in the placebo arm [27]. The researchers concluded that while TENS provided analgesic effects, the type of pain, the diagnosis, and the procedure did not affect the impact [27].
Unlike our study, meta-analyses concentrating on specific surgeries observed a statistically significant pain reduction in the TENS group compared to controls. A meta-analysis comprising 559 patients observed lower pain scores on POD one, two, and three in the TENS group compared to the placebo following inguinal hernia repair [24]. In a gynecological study, TENS was found to provide a pain-relieving effect comparable to that of opioids [25]. The use of TENS reduced pain scores at 12, 24, and 48 h following total knee arthroplasty (TKA), according to a meta-analysis of five studies comprising 472 patients [22]. Similarly, TENS reduced pain scores on the first five postoperative days following lung surgeries [23].
Regarding opioid consumption, a meta-analysis of 21 studies found that TENS reduced the postoperative use of opioids by more than 25% [21]. The TENS group consumed fewer opioids in the post-analgesia care unit than the opioid-only group following gynecological surgeries [25]. TENS reduced opioid use at 12, 24, and 48 h following TKA [22]. Similarly, lower pain scores at rest and on coughing were observed in the TENS group after cardiothoracic procedures [26].
Thus, while previous literature demonstrates the pain-relieving and opioid-sparing effects of TENS in the postoperative period following specific surgeries or generally for acute pain management, our study contributes insights to the existing literature on TENS by providing a comprehensive analysis of its effectiveness in postoperative pain management across various surgical contexts. This approach allows for a more generalized evaluation of TENS efficacy in postoperative pain control, offering valuable insights applicable to a wide array of clinical scenarios. Our study examines a comprehensive set of postoperative outcomes, including immediate and early postoperative pain, pain at different time points, pain during specific activities (coughing and walking), as well as morphine requirements, adverse events, and hospital length of stay. This holistic approach provides a more nuanced understanding of TENS’s impact on various aspects of postoperative recovery.
This meta-analysis observed a considerable heterogeneity in most outcomes. However, such heterogeneity was anticipated beforehand, given the differences in the durations of interventions, patient characteristics, control groups, surgical procedures, and outcome measures. Variations in TENS protocols, including differences in electrode placement, stimulation parameters, and treatment duration, introduced another source of heterogeneity. Although this heterogeneity posed a challenge in terms of combining and interpreting data, given that the effectiveness of TENS may vary across different contexts, a random effects model was used to account for the between-study variability. Furthermore, sub-group analysis was undertaken wherever possible to obtain more homogeneous results. Sensitivity analyses were also performed to help explore the sources of heterogeneity.
An important limitation of this research was the lack of extended follow-up information, which would have allowed for an assessment of the long-term advantages or potential complications associated with the use of TENS. Furthermore, the quality of the meta-analysis was contingent on the quality of the studies included. As evident from the Cochrane risk of bias, a number of studies had “some concerns” regarding the risk of bias, which subsequently affected the certainty of the evidence. Finally, challenges related to synthesizing data, such as differences in outcome measurement scales or reporting formats, complicated the aggregation of results and the conduct of a comprehensive analysis, as some studies had to be excluded due to these issues.
The implications of our meta-analysis extend both clinically and practically. For clinicians, our findings suggest that incorporating transcutaneous electrical nerve stimulation (TENS) into postoperative pain management protocols can offer tangible benefits, particularly in alleviating early postoperative pain and opioid requirements, and reducing adverse events. This information empowers healthcare providers to make informed decisions about the inclusion of TENS in multimodal analgesia strategies, enhancing overall patient care. Therefore, the study may serve as a guide for clinicians considering TENS as an adjunctive therapy in postoperative care.
From the research point of view, the study identified the need for investigations into the long-term effects of TENS and its impact on specific surgical contexts. Researchers could focus on conducting well-designed, prospective studies with extended follow-up periods to understand the sustained benefits and potential delayed adverse effects of TENS.
5. Conclusions
When considering all types of surgeries, the meta-analysis shows that TENS reduces pain intensity at rest (immediately after surgery and 24 h after surgery), pain intensity during coughing, morphine consumption, the incidence of PONV, and other adverse events, such as PONV, dizziness, and pruritus. We did not find a significant difference between the TENS group and the control group in reducing pain during walking. The subgroup analysis does not show significant differences between the TENS group and controls in pain severity at rest for thoracic, abdominal, or orthopedic surgeries.
Author Contributions
Conceptualization, methodology: D.V.; software: F.N. and Y.G.A.; formal analysis: F.N. and Y.G.A.; writing—original draft preparation: D.V., Y.G.A., N.S., K.T. and M.A.; writing—review and editing: D.V., Y.G.A., M.A., K.T., F.N. and R.T.; quality assessment: M.A., N.S. and K.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data can be requested from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported in part by Nazarbayev University Faculty Development Competitive Research grant nos. SOM2021005 (021220FD2851) and 11022021FD2906. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Waller-Wise R. Transcutaneous Electrical Nerve Stimulation: An Overview. J. Perinat. Educ. 2022;31:49–57. doi: 10.1891/J-PE-D-20-00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiorelli A., Morgillo F., Milione R., Pace M.C., Passavanti M.B., Laperuta P., Aurilio C., Santini M. Control of Post-Thoracotomy Pain by Transcutaneous Electrical Nerve Stimulation: Effect on Serum Cytokine Levels, Visual Analogue Scale, Pulmonary Function and Medication. Eur. J. Cardio-Thorac. Surg. 2012;41 doi: 10.1093/ejcts/ezr108. [DOI] [PubMed] [Google Scholar]
- 3.Bisgaard T., Kehlet H., Rosenberg J. Pain and Convalescence after Laparoscopic Cholecystectomy. Eur. J. Surg. 2001;167:84–96. doi: 10.1080/110241501750070510. [DOI] [PubMed] [Google Scholar]
- 4.Viderman D., Aubakirova M., Abdildin Y.G. Transversus Abdominis Plane Block in Colorectal Surgery: A Meta-Analysis. Front. Med. 2022;8:802039. doi: 10.3389/fmed.2021.802039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viderman D., Ben-David B., Sarria-Santamera A. Analysis of bupivacaine and ropivacaine-related cardiac arrests in regional anesthesia: A systematic review of case reports. Rev. Española Anestesiol. Y Reanim. (Engl. Ed.) 2021;68:472–483. doi: 10.1016/j.redar.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Kim S.S., Niu X., Elliott I.A., Jiang J.P., Dann A.M., Damato L.M., Chung H., Girgis M.D., King J.C., Hines O.J., et al. Epidural Analgesia Improves Postoperative Pain Control but Impedes Early Discharge in Patients Undergoing Pancreatic Surgery. Pancreas. 2019;48:719–725. doi: 10.1097/MPA.0000000000001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-David B., Kaligozhin Z., Viderman D. Quadratus Lumborum Block in Management of Severe Pain after Uterine Artery Embolization. Eur. J. Pain Lond. Engl. 2018;22:1032–1034. doi: 10.1002/ejp.1171. [DOI] [PubMed] [Google Scholar]
- 8.Kikuchi S., Kuroda S., Nishizaki M., Matsusaki T., Kuwada K., Kimura Y., Kagawa S., Morimatsu H., Fujiwara T. Comparison of the Effects of Epidural Analgesia and Patient-Controlled Intravenous Analgesia on Postoperative Pain Relief and Recovery after Laparoscopic Gastrectomy for Gastric Cancer. Surg. Laparosc. Endosc. Percutan. Tech. 2019;29:405–408. doi: 10.1097/SLE.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 9.Viderman D., Dautova A., Sarria-Santamera A. Erector Spinae Plane Block in Acute Interventional Pain Management: A Systematic Review. Scand. J. Pain. 2021;21:671–679. doi: 10.1515/sjpain-2020-0171. [DOI] [PubMed] [Google Scholar]
- 10.Viderman D., Aubakirova M., Umbetzhanov Y., Kulkaeva G., Shalekenov S.B., Abdildin Y.G. Ultrasound-guided erector spinae plane block in thoracolumbar spinal surgery: A systematic review and meta-analysis. Front. Med. 2022;9:932101. doi: 10.3389/fmed.2022.932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdildin Y., Tapinova K., Nugumanova M., Viderman D. Transversus Abdominis Plane Block in Adult Open Liver Surgery Patients: A Systematic Review with Meta-Analysis of Randomized Controlled Trials. J. Visc. Surg. 2023;160:253–260. doi: 10.1016/j.jviscsurg.2022.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Chou R., Gordon D.B., De Leon-Casasola O.A., Rosenberg J.M., Bickler S., Brennan T., Carter T., Cassidy C.L., Chittenden E.H., Degenhardt E., et al. Management of Postoperative Pain: A Clinical Practice Guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J. Pain. 2016;17:131–157. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Silva M.B., de Melo P.R., de Oliveira N.M.L., Crema E., Fernandes L.F.R.M. Analgesic Effect of Transcutaneous Electrical Nerve Stimulation after Laparoscopic Cholecystectomy. Am. J. Phys. Med. Rehabil. 2012;91:652–657. doi: 10.1097/PHM.0b013e318246638f. [DOI] [PubMed] [Google Scholar]
- 14.Tokuda M., Tabira K., Masuda T., Nishiwada T., Shomoto K. Effect of Modulated-Frequency and Modulated-Intensity Transcutaneous Electrical Nerve Stimulation after Abdominal Surgery: A Randomized Controlled Trial. Clin. J. Pain. 2014;30:565–570. doi: 10.1097/AJP.0b013e31829ea151. [DOI] [PubMed] [Google Scholar]
- 15.Borges M.R., de Oliveira N.M.L., Antonelli I.B.S., Silva M.B., Crema E., Fernandes L.F.R.M. Transcutaneous Electrical Nerve Stimulation Is Superior than Placebo and Control for Postoperative Pain Relief. Pain Manag. 2020;10:235–246. doi: 10.2217/pmt-2019-0063. [DOI] [PubMed] [Google Scholar]
- 16.Mannheimer J.S., Lampe G.N. Clinical Transcutaneous Electrical Nerve Stimulation. F A Davis Company; Philadelphia, PA, USA: 1984. [Google Scholar]
- 17.Sluka K.A., Walsh D. Transcutaneous Electrical Nerve Stimulation: Basic Science Mechanisms and Clinical Effectiveness. J. Pain. 2003;4:109–121. doi: 10.1054/jpai.2003.434. [DOI] [PubMed] [Google Scholar]
- 18.Woolf C.J., Mitchell D., Barrett G.D. Antinociceptive Effect of Peripheral Segmental Electrical Stimulation in the Rat. Pain. 1980;8:237–252. doi: 10.1016/0304-3959(88)90011-5. [DOI] [PubMed] [Google Scholar]
- 19.Melzack R., Wall P.D. Pain Mechanisms: A New Theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 20.Kalra A., Urban M.O., Sluka K.A. Blockade of Opioid Receptors in Rostral Ventral Medulla Prevents Antihyperalgesia Produced by Transcutaneous Electrical Nerve Stimulation (TENS) J. Pharmacol. Exp. Ther. 2001;298:257–263. [PubMed] [Google Scholar]
- 21.Bjordal J.M., Johnson M.I., Ljunggreen A.E. Transcutaneous Electrical Nerve Stimulation (TENS) Can Reduce Postoperative Analgesic Consumption. A Meta-Analysis with Assessment of Optimal Treatment Parameters for Postoperative Pain. Eur. J. Pain Lond. Engl. 2003;7:181–188. doi: 10.1016/S1090-3801(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 22.Li J., Song Y. Transcutaneous Electrical Nerve Stimulation for Postoperative Pain Control after Total Knee Arthroplasty: A Meta-Analysis of Randomized Controlled Trials. Medicine. 2017;96:e8036. doi: 10.1097/MD.0000000000008036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J., Dan Y., Yixian Y., Lyu M., Zhong J., Wang Z., Zhu Y., Liu L. Efficacy of Transcutaneous Electronic Nerve Stimulation in Postoperative Analgesia after Pulmonary Surgery: A Systematic Review and Meta-Analysis. Am. J. Phys. Med. Rehabil. 2020;99:241–249. doi: 10.1097/PHM.0000000000001312. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe J., Izumi N., Kobayashi F., Miki A., Sata N. Efficacy and Safety of Transcutaneous Electrical Nerve Stimulation in Patients Undergoing Inguinal Hernia Repair: A Systematic Review and Meta-Analysis. JMA J. 2023;6:371–380. doi: 10.31662/jmaj.2023-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piasecki A., Ögren C., Thörn S.-E., Olausson A., Svensson C.J., Platon B., Wolf A., Andréll P. High-Frequency, High-Intensity Transcutaneous Electrical Nerve Stimulation Compared with Opioids for Pain Relief after Gynecological Surgery: A Systematic Review and Meta-Analysis. Scand. J. Pain. 2023;24:20230068. doi: 10.1515/sjpain-2023-0068. [DOI] [PubMed] [Google Scholar]
- 26.Cardinali A., Celini D., Chaplik M., Grasso E., Nemec E.C. Efficacy of Transcutaneous Electrical Nerve Stimulation for Postoperative Pain, Pulmonary Function, and Opioid Consumption Following Cardiothoracic Procedures: A Systematic Review. Neuromodul. Technol. Neural Interface. 2021;24:1439–1450. doi: 10.1111/ner.13302. [DOI] [PubMed] [Google Scholar]
- 27.Johnson M.I., Paley C.A., Jones G., Mulvey M.R., Wittkopf P.G. Efficacy and Safety of Transcutaneous Electrical Nerve Stimulation (TENS) for Acute and Chronic Pain in Adults: A Systematic Review and Meta-Analysis of 381 Studies (the Meta-TENS Study) BMJ Open. 2022;12:e051073. doi: 10.1136/bmjopen-2021-051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; London, UK: 2023. [(accessed on 1 December 2022)]. Version 6.4 (Updated August 2023) Available online: www.training.cochrane.org/handbook. [Google Scholar]
- 30.Luo D., Wan X., Liu J., Tong T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 31.Wan X., Wang W., Liu J., Tong T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.-Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 33.Guyatt G.H., Oxman A.D., Schünemann H.J., Tugwell P., Knottnerus A. GRADE Guidelines: A New Series of Articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011;64:380–382. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Ilfeld B.M., Plunkett A., Vijjeswarapu A.M., Hackworth R., Dhanjal S., Turan A., Cohen S.P., Eisenach J.C., Griffith S., Hanling S., et al. Percutaneous Peripheral Nerve Stimulation (Neuromodulation) for Postoperative Pain: A Randomized, Sham-Controlled Pilot Study. Anesthesiology. 2021;135:95–110. doi: 10.1097/ALN.0000000000003776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.da Silva M.P., Liebano R.E., Rodrigues V.A., Abla L.E.F., Ferreira L.M. Transcutaneous Electrical Nerve Stimulation for Pain Relief after Liposuction: A Randomized Controlled Trial. Aesthetic Plast. Surg. 2015;39:262–269. doi: 10.1007/s00266-015-0451-6. [DOI] [PubMed] [Google Scholar]
- 36.Erden S., Senol Celik S. The Effect of Transcutaneous Electrical Nerve Stimulation on Post-Thoracotomy Pain. Contemp. Nurse. 2015;51:163–170. doi: 10.1080/10376178.2016.1166971. [DOI] [PubMed] [Google Scholar]
- 37.Asgari Z., Tavoli Z., Hosseini R., Nataj M., Tabatabaei F., Dehghanizadeh F., Haji-Amoo-Assar H., Sepidarkish M., Montazeri A. A Comparative Study between Transcutaneous Electrical Nerve Stimulation and Fentanyl to Relieve Shoulder Pain during Laparoscopic Gynecologic Surgery under Spinal Anesthesia: A Randomized Clinical Trail. Pain Res. Manag. 2018;2018:9715142. doi: 10.1155/2018/9715142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bjerså K., Jildenstaal P., Jakobsson J., Egardt M., Fagevik Olsén M. Adjunct High Frequency Transcutaneous Electric Stimulation (TENS) for Postoperative Pain Management during Weaning from Epidural Analgesia Following Colon Surgery: Results from a Controlled Pilot Study. Pain Manag. Nurs. Off. J. Am. Soc. Pain Manag. Nurses. 2015;16:944–950. doi: 10.1016/j.pmn.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Lima P.M.B., de Brito Farias R.T.F., Carvalho A.C.A., da Silva P.N.C., Ferraz Filho N.A., de Brito R.F. Estimulação elétrica nervosa transcutânea após cirurgia de revascularização miocárdica. Braz. J. Cardiovasc. Surg. 2011;26:591–596. doi: 10.5935/1678-9741.20110049. [DOI] [Google Scholar]
- 40.Ferreira F.C., Issy A.M., Sakata R.K. Assessing the Effects of Transcutaneous Electrical Nerve Stimulation (TENS) in Post-Thoracotomy Analgesia. Rev. Bras. Anestesiol. 2011;61:561–567. doi: 10.1016/S0034-7094(11)70067-8. [DOI] [PubMed] [Google Scholar]
- 41.Beckwée D., Bautmans I., Lefeber N., Lievens P., Scheerlinck T., Vaes P. Effect of Transcutaneous Electric Nerve Stimulation on Pain after Total Knee Arthroplasty: A Blind Randomized Controlled Trial. J. Knee Surg. 2018;31:189–196. doi: 10.1055/s-0037-1602134. [DOI] [PubMed] [Google Scholar]
- 42.Chiu J.H., Chen W.S., Chen C.H., Jiang J.K., Tang G.J., Lui W.Y., Lin J.K. Effect of Transcutaneous Electrical Nerve Stimulation for Pain Relief on Patients Undergoing Hemorrhoidectomy: Prospective, Randomized, Controlled Trial. Dis. Colon Rectum. 1999;42:180–185. doi: 10.1007/BF02237124. [DOI] [PubMed] [Google Scholar]
- 43.Elboim-Gabyzon M., Andrawus Najjar S., Shtarker H. Effects of Transcutaneous Electrical Nerve Stimulation (TENS) on Acute Postoperative Pain Intensity and Mobility after Hip Fracture: A Double-Blinded, Randomized Trial. Clin. Interv. Aging. 2019;14:1841–1850. doi: 10.2147/CIA.S203658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engen D.J., Carns P.E., Allen M.S., Bauer B.A., Loehrer L.L., Cha S.S., Chartrand C.M., Eggler E.J., Cutshall S.M., Wahner-Roedler D.L. Evaluating Efficacy and Feasibility of Transcutaneous Electrical Nerve Stimulation for Postoperative Pain after Video-Assisted Thoracoscopic Surgery: A Randomized Pilot Trial. Complement. Ther. Clin. Pract. 2016;23:141–148. doi: 10.1016/j.ctcp.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Erden S., Yurtseven Ş., Demir S.G., Arslan S., Arslan U.E., Dalcı K. Effects of Transcutaneous Electrical Nerve Stimulation on Mastectomy Pain, Patient Satisfaction, and Patient Outcomes. J. Perianesthesia Nurs. 2022;37:485–492. doi: 10.1016/j.jopan.2021.08.017. [DOI] [PubMed] [Google Scholar]
- 46.Erdogan M., Erdogan A., Erbil N., Karakaya H.K., Demircan A. Prospective, Randomized, Placebo-Controlled Study of the Effect of TENS on Postthoracotomy Pain and Pulmonary Function. World J. Surg. 2005;29:1563–1570. doi: 10.1007/s00268-005-7934-6. [DOI] [PubMed] [Google Scholar]
- 47.Kurata N.B., Ghatnekar R.J., Mercer E., Chin J.M., Kaneshiro B., Yamasato K.S. Transcutaneous Electrical Nerve Stimulation for Post-Cesarean Birth Pain Control: A Randomized Controlled Trial. Obstet. Gynecol. 2022;140:174–180. doi: 10.1097/AOG.0000000000004798. [DOI] [PubMed] [Google Scholar]
- 48.Kara B., Baskurt F., Acar S., Karadibak D., Ciftci L., Erbayraktar S., Gokmen A.N. The Effect of TENS on Pain, Function, Depression, and Analgesic Consumption in the Early Postoperative Period with Spinal Surgery Patients. Turk. Neurosurg. 2011;21:618–624. doi: 10.5137/1019-5149.JTN.4985-11.0. [DOI] [PubMed] [Google Scholar]
- 49.Parseliunas A., Paskauskas S., Kubiliute E., Vaitekunas J., Venskutonis D. Transcutaneous Electric Nerve Stimulation Reduces Acute Postoperative Pain and Analgesic Use after Open Inguinal Hernia Surgery: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Pain. 2021;22:533–544. doi: 10.1016/j.jpain.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Sezen C.B., Akboga S.A., Celik A., Kalafat C.E., Tastepe A.I. Transcutaneous Electrical Nerve Stimulation Effect on Postoperative Complications. Asian Cardiovasc. Thorac. Ann. 2017;25:276–280. doi: 10.1177/0218492317703838. [DOI] [PubMed] [Google Scholar]
- 51.Smedley F., Taube M., Wastell C. Transcutaneous Electrical Nerve Stimulation for Pain Relief Following Inguinal Hernia Repair: A Controlled Trial. Eur. Surg. Res. 1988;20:233–237. doi: 10.1159/000128766. [DOI] [PubMed] [Google Scholar]
- 52.Yu X., Zhang F., Chen B. The Effect of TEAS on the Quality of Early Recovery in Patients Undergoing Gynecological Laparoscopic Surgery: A Prospective, Randomized, Placebo-Controlled Trial. Trials. 2020;21:43. doi: 10.1186/s13063-019-3892-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang C., Xiao Z., Zhang X., Guo L., Sun W., Tai C., Jiang Z., Liu Y. Transcutaneous Electrical Stimulation of Somatic Afferent Nerves in the Foot Relieved Symptoms Related to Postoperative Bladder Spasms. BMC Urol. 2017;17:58. doi: 10.1186/s12894-017-0248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang B., Xu F., Hu P., Zhang M., Tong K., Ma G., Xu Y., Zhu L., Chen J.D.Z. Needleless Transcutaneous Electrical Acustimulation: A Pilot Study Evaluating Improvement in Post-Operative Recovery. Am. J. Gastroenterol. 2018;113:1026–1035. doi: 10.1038/s41395-018-0156-y. [DOI] [PubMed] [Google Scholar]
- 55.Chen W., Liu C., Yang Y., Tian L. The Effect of Transcutaneous Electrical Nerve Stimulation (TENS) on Pain Control and Phenylethanolamine-N-Methyltransferase (PNMT) Gene Expression after Cesarean Section. Cell. Mol. Biol. 2021;67:153–157. doi: 10.14715/cmb/2021.67.3.23. [DOI] [PubMed] [Google Scholar]
- 56.Forogh B., Aslanpour H., Fallah E., Babaei-Ghazani A., Ebadi S. Adding High-Frequency Transcutaneous Electrical Nerve Stimulation to the First Phase of Post Anterior Cruciate Ligament Reconstruction Rehabilitation Does Not Improve Pain and Function in Young Male Athletes More than Exercise Alone: A Randomized Single-Blind Clinical Trial. Disabil. Rehabil. 2019;41:514–522. doi: 10.1080/09638288.2017.1399294. [DOI] [PubMed] [Google Scholar]
- 57.Galli T.T., Chiavegato L.D., Liebano R.E. Effects of TENS in Living Kidney Donors Submitted to Open Nephrectomy: A Randomized Placebo-Controlled Trial. Eur. J. Pain Lond. Engl. 2015;19:67–76. doi: 10.1002/ejp.521. [DOI] [PubMed] [Google Scholar]
- 58.Gregorini C., Cipriano Junior G., de Aquino L.M., Branco J.N.R., Bernardelli G.F. Short-Duration Transcutaneous Electrical Nerve Stimulation in the Postoperative Period of Cardiac Surgery. Arq. Bras. Cardiol. 2010;94:325–331. doi: 10.1590/s0066-782x2010000300011. [DOI] [PubMed] [Google Scholar]
- 59.Mahure S.A., Rokito A.S., Kwon Y.W. Transcutaneous Electrical Nerve Stimulation for Postoperative Pain Relief after Arthroscopic Rotator Cuff Repair: A Prospective Double-Blinded Randomized Trial. J. Shoulder Elbow Surg. 2017;26:1508–1513. doi: 10.1016/j.jse.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 60.Rakel B., Frantz R. Effectiveness of Transcutaneous Electrical Nerve Stimulation on Postoperative Pain with Movement. J. Pain. 2003;4:455–464. doi: 10.1067/S1526-5900(03)00780-6. [DOI] [PubMed] [Google Scholar]
- 61.Jahangirifard A., Razavi M., Ahmadi Z.H., Forozeshfard M. Effect of TENS on Postoperative Pain and Pulmonary Function in Patients Undergoing Coronary Artery Bypass Surgery. Pain Manag. Nurs. 2018;19:408–414. doi: 10.1016/j.pmn.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 62.Bjerså K., Andersson T. High Frequency TENS as a Complement for Pain Relief in Postoperative Transition from Epidural to General Analgesia after Pancreatic Resection. Complement. Ther. Clin. Pract. 2014;20:5–10. doi: 10.1016/j.ctcp.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Cuschieri R.J., Morran C.G., McArdle C.S. Transcutaneous Electrical Stimulation for Postoperative Pain. Ann. R. Coll. Surg. Engl. 1985;67:127–129. [PMC free article] [PubMed] [Google Scholar]
- 64.McCallum M.I., Glynn C.J., Moore R.A., Lammer P., Phillips A.M. Transcutaneous Electrical Nerve Stimulation in the Management of Acute Postoperative Pain. Br. J. Anaesth. 1988;61:308–312. doi: 10.1093/bja/61.3.308. [DOI] [PubMed] [Google Scholar]
- 65.Navarathnam R.G., Wang I.Y., Thomas D., Klineberg P.L. Evaluation of the Transcutaneous Electrical Nerve Stimulator for Postoperative Analgesia Following Cardiac Surgery. Anaesth. Intensive Care. 1984;12:345–350. doi: 10.1177/0310057X8401200411. [DOI] [PubMed] [Google Scholar]
- 66.Benedetti F., Amanzio M., Casadio C., Cavallo A., Cianci R., Giobbe R., Mancuso M., Ruffini E., Maggi G. Control of Postoperative Pain by Transcutaneous Electrical Nerve Stimulation after Thoracic Operations. Ann. Thorac. Surg. 1997;63:773–776. doi: 10.1016/S0003-4975(96)01249-0. [DOI] [PubMed] [Google Scholar]
- 67.Hamza M.A., White P.F., Ahmed H.E., Ghoname E.A. Effect of the Frequency of Transcutaneous Electrical Nerve Stimulation on the Postoperative Opioid Analgesic Requirement and Recovery Profile. Anesthesiology. 1999;91:1232–1238. doi: 10.1097/00000542-199911000-00012. [DOI] [PubMed] [Google Scholar]
- 68.Laitinen J., Nuutinen L. Failure of Transcutaneous Electrical Nerve Stimulation and Indomethacin to Reduce Opiate Requirement Following Cholecystectomy. Acta Anaesthesiol. Scand. 1991;35:700–705. doi: 10.1111/j.1399-6576.1991.tb03375.x. [DOI] [PubMed] [Google Scholar]
- 69.Solak O., Turna A., Pekcolaklar A., Metin M., Sayar A., Solak O., Gürses A. Transcutaneous Electric Nerve Stimulation for the Treatment of Postthoracotomy Pain: A Randomized Prospective Study. Thorac. Cardiovasc. Surg. 2007;55:182–185. doi: 10.1055/s-2006-924631. [DOI] [PubMed] [Google Scholar]
- 70.Stubbing J.F., Jellicoe J.A. Transcutaneous Electrical Nerve Stimulation after Thoracotomy. Pain Relief and Peak Expiratory Flow Rate—A Trial of Transcutaneous Electrical Nerve Stimulation. Anaesthesia. 1988;43:296–298. doi: 10.1111/j.1365-2044.1988.tb08977.x. [DOI] [PubMed] [Google Scholar]
- 71.Wang H., Xie Y., Zhang Q., Xu N., Zhong H., Dong H., Liu L., Jiang T., Wang Q., Xiong L. Transcutaneous Electric Acupoint Stimulation Reduces Intra-Operative Remifentanil Consumption and Alleviates Postoperative Side-Effects in Patients Undergoing Sinusotomy: A Prospective, Randomized, Placebo-Controlled Trial. Br. J. Anaesth. 2014;112:1075–1082. doi: 10.1093/bja/aeu001. [DOI] [PubMed] [Google Scholar]
- 72.Platon B., Andréll P., Raner C., Rudolph M., Dvoretsky A., Mannheimer C. High-Frequency, High-Intensity Transcutaneous Electrical Nerve Stimulation as Treatment of Pain after Surgical Abortion. Pain. 2010;148:114–119. doi: 10.1016/j.pain.2009.10.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data can be requested from the corresponding author.