Abstract
Practical relevance:
The majority of feline lymphoma is extranodal. While the gastrointestinal (GI) tract is the most commonly affected site, non-GI extranodal lymphomas, which are the focus of this review, account for a large proportion of lymphomas in cats. This article discusses prognostic factors for the most common of these extranodal lymphomas, both in general terms and specifically for individual sites.
Clinical challenges:
Prognostic factors remain poorly defined for feline lymphoma. Many cats with extranodal lymphoma have stage I disease at an accessible site. A major question for patients with apparently localised extranodal lymphoma is whether the tumour can be treated with localised therapy alone or requires systemic treatment as well. Again there is often no specific information available for a particular site, such as a localised intramuscular lymphoma. Instead, reliance must be placed on careful patient staging, particularly if local therapy alone is planned.
Evidence base:
Until such time as further studies looking at stage, anatomic site, histological grade and immunophenotype are available to assist treatment decision making for an individual cat with extranodal lymphoma, it seems reasonable to draw inferences from other common extranodal sites for which more specific information exists, such as nasal lymphoma.
‘Extranodal’ – a problematic nomenclature in cats
The majority of feline lymphomas affect various anatomic sites other than the lymph nodes (extranodal), and the particular extranodal anatomic site affected (subtype) impacts the expected disease behaviour and best management of the cat. The most common extranodal anatomic subtype of lymphoma reported in cats is gastrointestinal (GI) and this was recently reviewed in depth in this journal.1,2 The current review discusses the prognostic factors and treatment approaches for other extranodal lymphomas in cats, but excludes mediastinal, GI and cutaneous lymphomas, as well as extranodal sites (such as lung) where there is little specific information.
‘Extranodal’ is a somewhat difficult nomenclature in cats, since nodal involvement with lymphoma is comparatively uncommon, and most patients have involvement of extranodal sites, either alone or in combination with nodal sites. This emphasises what I find to be one of the most difficult aspects of lymphoma in cats; that is, the heterogeneous nature of this disease entity which defies broad categorisation that can be used to develop prognostic profiles for individual patients.
Attempts to categorise feline lymphoma by anatomic site have been inconsistent and usually require multicentric, miscellaneous, mixed and unclassified extranodal categories. 3 Confusion with all the systems proposed to date comes from the inclusion of renal lymphoma in the alimentary group by some authors, 4 and in the multicentric group by others, 5 while still others group all abdominally located tumours together. 6
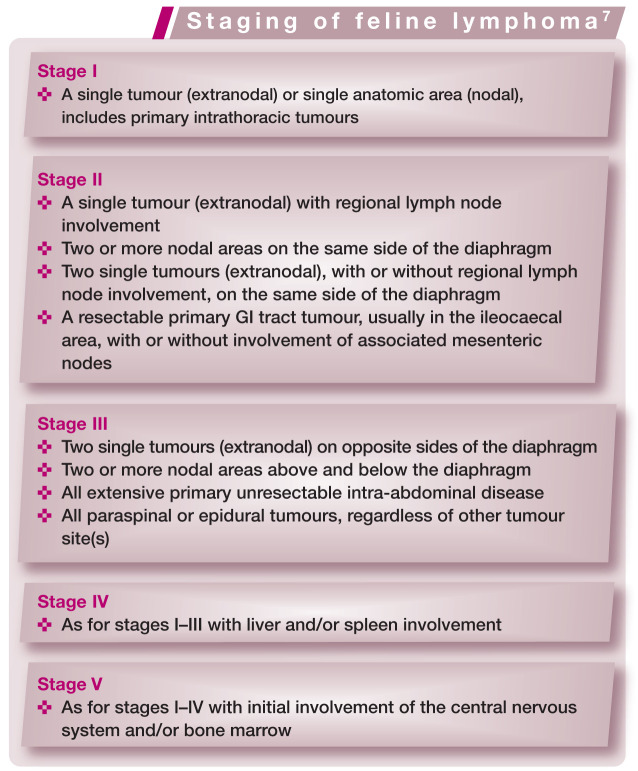
Staging of feline lymphoma
For most cancers in cats, the clinical stage of disease is important both in establishing a prognosis and in developing appropriate treatment strategies. Clinical stage refers to the overall extent of disease but is rather dif ficult to define in cats with lymphoma because of the various anatomic forms of this disease.
The stage of lymphoma, as defined by Mooney and Hayes (see box), 7 was significantly related to response to treatment in one study, where cats with stage I lymphoma had higher response rates than those in stages IV/V (93% compared with 40–60%), and cats with stage I or II lymphoma had longer survival times than those in higher stages (7.6 months compared with 3 months). 8 Not all other studies have supported the overall importance of lymphoma stage. However, those studies that do seem to indicate that the true ‘break point’ in staging for cats – where prognosis is strongly clinically affected – is stage I lymphoma.
Stage I lymphoma occurs most often in cats as extranodal disease. The anatomic form that is most commonly reported in stage I is nasal lymphoma (Figure 1), which has been shown to be associated with longer remission and survival times than those achieved when cats with other forms of lymphoma are treated.
Figure 1.
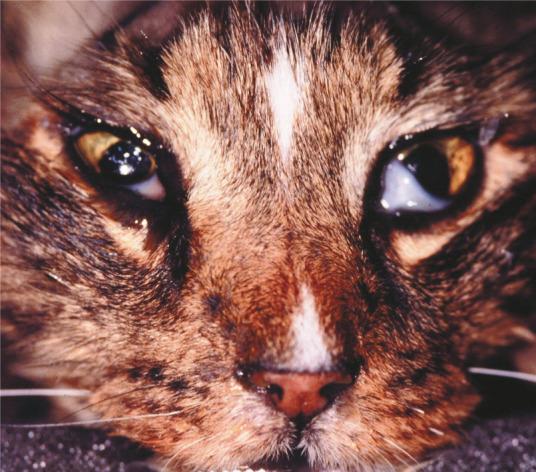
Nasal lymphoma may cause epiphora, discomfort and debility due to nasal and sinus infiltration
Anatomic categorisation and staging are separate classifications, but there is also some overlap between them. It seems that the best prognostic system for cats will include a combination of staging the extent of disease within certain anatomic categories (such as GI or renal lymphoma).
Other prognostic factors for cats with lymphoma
Substage
Cats with lymphoma that are ‘constitutionally unwell’ are classified as having substage ‘b’ lymphoma, while cats that are asymptomatic have substage ‘a’ lymphoma. The majority of patients with lymphoma affecting visceral sites are in substage b, while those with lymphoma affecting the nasal cavity and other non-visceral, extranodal sites are usually categorised as substage a. In one study, cats with substage b had a median survival of 3.5 months compared with 9.5 months for cats with substage a lymphoma. 9 This same effect of substage has been seen in multiple studies and underscores the importance of supportive care for these cats while chemotherapy is being administered.
Response to treatment
The most commonly cited prognostic factor for a cat with lymphoma (at any site) is response to treatment. While this seems intuitively obvious it is not true for all cancers. In a multi-institutional study of 90 cats, those achieving complete remission had a median survival of 8.3 months compared with 7 weeks if they achieved partial remission or did not respond to treatment. 9 These and similar results in multiple reports, including a UK study by Taylor et al of 110 cats with extranodal lymphoma (11.2 months for complete responders versus 2 months for those not achieving a complete response), 10 suggest that partial responses in cats with lymphoma are clinically irrelevant.
Unfortunately, since evaluation for this ‘prognostic factor’ requires treating the patient, it cannot practically be used to advise owners as to the suitability of therapy for their pet.
FeLV status
Feline leukaemia virus (FeLV) is much less commonly associated with lymphoma than it was 20 years ago, with recent reported prevalence rates of 25.5% (USA 1988–1996) 9 and 20.8% (Germany 1996–2008). 11 Cats with FeLV antigen and lymphoma are more likely to be younger and to have mediastinal lymphoma. A cat with FeLV antigenaemia is less likely to have a long survival, possibly due to development of other diseases as well as lymphoma. In one study, both remission duration (4 weeks versus 5 months) and survival (5 weeks versus 6 months) were shorter for cats with lymphoma and FeLV than for cats with lymphoma uncomplicated by FeLV. 9 However, FeLV is currently an uncommon disease, and the majority of cats with lymphoma are unaffected, so the clinical importance of this factor in contemporary veterinary oncology is comparatively low.
Grade of tumour
Although the focus of this review is not GI lymphoma, it is interesting to speculate on the impact that grade of this subtype has played in prognostic studies.
A recent study classified cats with GI lymphoma by immunophenotype and by location (mucosal lymphoma where the infiltrate was confined to the epithelium and lamina propria with minimal extension into the submucosa; transmural lymphoma where the infiltrate extended markedly into the submucosa and muscularis). 12 In that study, the size of the lymphocytes was not considered as important as distribution, although most mucosal lymphomas were ‘small cell’. Cats with mucosal T cell lymphoma predominated and had a median survival of 29 months. The authors felt that mucosal T cell lymphoma matched World Health Organization enteropathy-associated T cell lymphoma (EATCL) type II in humans, which is consistent with the suspected aetiology in cats as well. The study also showed that transmural (rather than mucosal) T cell lymphoma was usually ‘large cell’ and often comprised of larger, granular lymphocytes; these cats had a worse prognosis. Cats with B cell lymphomas also had transmural involvement, but there were too few cats to make statements about prognosis for this sub-type. Both these large-cell subtypes usually had an intestinal mass effect, presumably due to the transmural involvement.
The finding that most, if not all, cats with ‘low grade’ lymphoma have T cell immunophenotype and the recognition that many previous studies did not separate patients according to the grade of their tumour, but rather by stage and anatomic site, means that the contribution of immuno phenotype to prognosis may have been misunderestimated (to quote George W Bush).
This has had ongoing consequences. One study, 9 published 15 years ago, which showed that CD-3 immunostaining did not correlate with remission or survival led to most veterinarians not pursuing immunostaining for their feline patients with lymphoma (‘It does-n’t make a difference, so why test for it?’). This in turn has hindered our understanding of the disease. We can hypothesise that most nasal lymphomas in cats are B cell derived, and that is why they have the best prognosis of any extranodal lymphoma in cats; however, since most treated cats are not immunophenotyped (only 3/97 cats in a multi-institutional retrospective study of feline nasal lymphoma, and 0/110 cats with extranodal lymphoma, had immunophenotyping performed),10,13 we can not test that hypothesis. If only to advance our knowledge of lymphoma in cats, all lymphoma samples should be immunopheno-typed as well as histologically graded.
Others
Other individual factors have been investigated for their impact on outcome in cats with lymphoma, but have not been confirmed to be prognostic when multivariate analysis is used. These include use of doxorubicin as part of the treatment protocol leading to longer remission, and a high proliferative fraction of cats’ tumour cells leading to longer survival times. 9 One small study found that elevations in serum lactate dehydrogenase (LDH) were associated with poor prognosis, 14 which mirrors the same effect of serum LDH levels in human lymphoma patients, but this test is rarely now included in veterinary serum chemistry profiles.
Specific extranodal sites for lymphoma in cats
The study by Taylor et al categorised extranodal lymphomas as nasal, laryngeal, pharyngeal, tracheal, pulmonary, renal, CNS, ocular, retrobulbar and cutaneous. 10 The multicentric category used in some studies may include some cats with extranodal disease, and some cats with one extranodal site may have another involved (eg, renal and CNS lymphoma). I have followed that scheme, but have combined laryngeal, pharyngeal and tracheal lymphoma, as to my eye they appear to have a similar response to therapy. It may be that they should be combined with nasal lymphoma as ‘upper respiratory tract’, but future studies will be needed to determine that grouping.
Nasal lymphoma
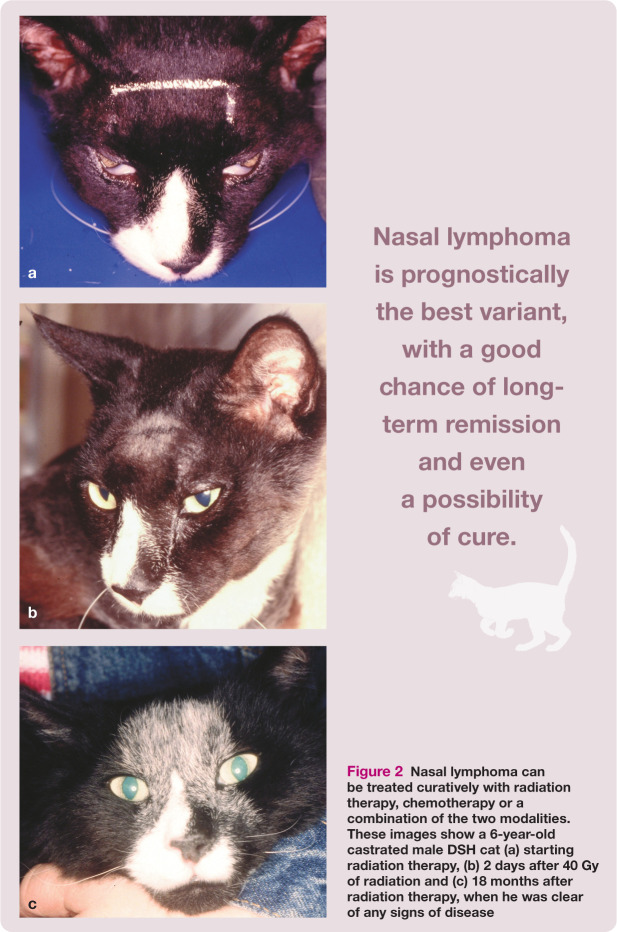
The time lag between owner recognition of initial signs and presentation to the veterinarian is longer for cats with nasal lymphoma than for most other forms of lymphoma – 2 months in the Taylor et al study. 10 Lymphoma in cats normally carries a guarded prognosis; however, nasal lymphoma is prognostically the best variant, with a good chance of long-term remission and even a possibility of cure (Figure 2). Response rates to treatment (chemotherapy and/or radiation therapy) average 66–75%, with reported median survival times of 12–30 months,13,15 even when chemo therapy is delivered intraperitoneally. 16 Taylor et al’s study reported a complete response rate to chemotherapy of 73% (32/44 cats), with the longest median survival time among all cats with extranodal lymphoma, of over 24 months. 10 By contrast, those cats that fail to respond to treatment have short survival times of approximately 3 months. 17
Figure 2.
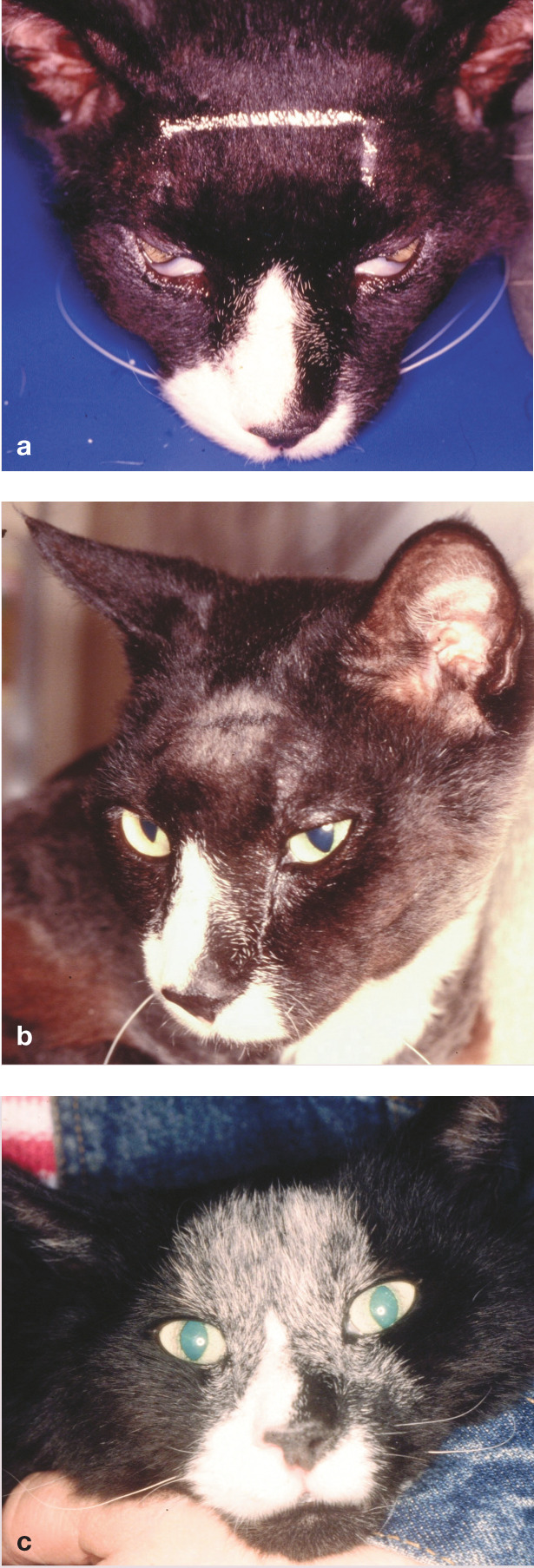
Nasal lymphoma can be treated curatively with radiation therapy, chemotherapy or a combination of the two modalities. These images show a 6-year-old castrated male DSH cat (a) starting radiation therapy, (b) 2 days after 40 Gy of radiation and (c) 18 months after radiation therapy, when he was clear of any signs of disease
Cats with nasal lymphoma often have favourable overall prognostic factors for lymphoma. Their disease is often localised (stage I), they are often still eating well (substage a) and are usually FeLV negative (10% affected in one study). 11 Most cats with nasal lymphoma appear to have B cell lymphoma (71% B cell, 16% T cell and 13% mixed of 50 cats; 71% B cell and 29% T cell of 35 cats),18,19 although the prognostic effect of immunophenotype has not yet been examined.
Specific to nasal lymphoma, negative prognostic factors in two studies included anorexia, anaemia and involvement of the cribriform plate on computed tomography. 20 While cats that had a complete clinical response, cats that received radiotherapy alone or with chemotherapy, and cats receiving >32 Gy (total radiotherapy dosage) had longer survival times, 13 only complete clinical response and anaemia were significant on multivariate analysis. The data is nevertheless useful information when making decisions for individual patients.
Laryngeal/pharyngeal/tracheal lymphoma
Lymphoma of the larynx, pharynx and trachea is uncommon. In the Taylor et al study of extranodal feline lymphoma, the larynx was the affected organ in 11/110 cats; an additional cat had pharyngeal involvement and a further four had tracheal lymphoma. 10 Male cats appear more likely to be affected (nearly 90% of the small number where information was available) and most affected cats are older (median age 10 years).
Dyspnoea is the most common sign and this can progress to acute respiratory distress and cyanosis. Other respiratory signs such as cough, gagging, stertor, aphonia and voice changes have been reported. All cats (4/4) with tracheal lymphoma in one study were dyspnoeic. 21
In cats reported with laryngeal lymphoma, most had masses that protruded into the laryngeal lumen, while fewer had diffuse laryngeal thickening. 22 Approximately 25% also had retropharyngeal or cervical lymph node involvement. 22 Some had nasal and tracheal involvement, and one cat had intestinal and lymph node involvement. 23 Many cats appeared to have localised stage I disease, but staging was not always complete in the reports. It is wise to stage the patient carefully, particularly if local therapy alone is planned.
Response rates to chemotherapy are high for this group of cats: 2/2 cats had complete remission for 225 and 264 days, 24 and all cats treated with chemotherapy and/or radiotherapy in another study had complete remission for between 3 and 19 months after treatment. 21 The response rate for cats with laryngeal lymphoma was 87.5% (7/8 cats) in Taylor et al’s study, and a further five cats with tracheal and pharyngeal lymphoma were in a group that had a >70% complete response rate (but not reported separately, unfortunately). 10 It seems that surgical debulking (if possible), or perhaps radiation therapy, to relieve the acute respiratory signs, followed by chemotherapy is the treatment of choice for this disease.
The high response rate, common occurrence of stage I disease, and presence of nasal, laryngeal and tracheal lymphoma together in some patients suggests nasal and other upper respiratory tract lymphoma should be considered as a single entity. Further studies on immunophenotyping and response rates may help to determine if this is clinically appropriate.
Renal lymphoma
Cats with renal lymphoma are usually older and FeLV negative, although in one Australian study 54% (15/28) cats with renal lymphoma were feline immunodeficiency virus (FIV) positive. 25 The prognostic impact of FIV status was not explored separately for this group of cats. Renal involvement with lymphoma is always bilateral (Figure 3), and it is uncommon to have renal lymphoma without other organ or system involvement. In studies where full staging was reported, only 18/68 cats (26%) had lymphoma confined to the kidneys.3,26,27 For 20 of the remaining 50 cats, other sites of lymphoma were limited to the abdominal cavity (mesenteric nodes alone or with involvement of liver, spleen or GI tract). The rest of the cats had involvement of multiple sites including peripheral or sternal lymph nodes, lungs, heart, bone marrow, eyes, skin or CNS. One report suggested that the risk of lymphoma relapse in the CNS was higher in cats with renal lymphoma (56% of 18 cats); 26 however, no other study has confirmed that association. It has also been reported that cats with spinal lymphoma often have renal involvement, so it is difficult to know whether the association is unidirectional or simply reflects the heterogeneity of extranodal lymphoma in cats.
Figure 3.
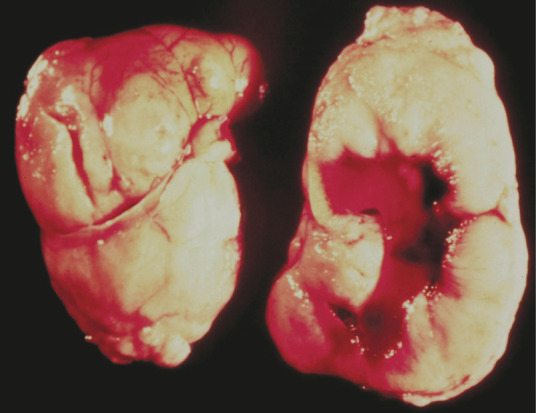
Renal lymphoma is always bilateral, causing enlarged and/or irregular kidneys, as seen in this necropsy specimen. Courtesy of Dr Gordon Theilen
Renal lymphoma appears to be mainly B cell in origin. Of 44 cats with renal lymphoma, 38 were B cell, five were T cell and one was a null cell lymphoma, but this was not examined for or correlated to prognosis.9,28
The most common clinical signs seen in cats with renal lymphoma are related to acute renal insufficiency due to cortical infiltration by lymphoma cells. Cats are depressed, anorectic, have lost weight and are usually polydipsic and polyuric despite clinical dehydration. The time between owner recognition of initial signs and presentation to the veterinarian is usually only 2–6 weeks.10,29 The abnormal renal values are likely to be a consequence of lymphoma infiltration; if patients are treated promptly, and if they achieve a complete remission with treatment, functional recovery of the renal cortex occurs. 26 However, since affected cats are often older, some patients may have underlying renal insufficiency.
Combination chemotherapy with a doxorubicin-containing regimen represents the current standard of care and is usually recommended as the basis of treatment for cats with any form of lymphoma. However, the presence of underlying renal insufficiency needs to be accounted for when selecting treatment protocols, as doxorubicin is a potential renal toxin in cats. 30 Personally, I am happy to use doxorubicin if there is a return to normal renal function after treatment with other drugs. However, for older patients, where underlying renal function may not be robust, I would be very reluctant to recommend doxorubicin due to the potential for renal toxicity.
Taylor et al found that 15/24 (62.5%) cats with renal lymphoma achieved complete clinical response with chemotherapy for a median of 7 months. There was no difference in outcomes whether a patient received vincristine, cyclophosphamide and prednisone therapy, or the same therapy with doxorubicin as well, but toxicities were not carefully evaluated. 10 This is a very similar outcome to that seen in a group of 28 cats treated with a combination of vincristine, L-asparaginase, prednisone, cyclophosphamide and methotrexate (± cyto-sine arabinoside), with >75% clinical response in 61% of patients for a median of 4 months. 26
CNS lymphoma
In two studies only 4/54 cats with CNS lymphoma had brain involvement.31,32 In contrast, another study found that 15/18 cats with CNS lymphoma had signs of intracranial disease. 33 One possibility for the differing presentation is that there has been a reduction in the number of cats with spinal lymphoma (Figures 4 and 5), possibly due to the high association of spinal lymphoma with FeLV infection and the low numbers of cats now affected by that virus. Certainly, anec dotally, I feel we see fewer cats with spinal lymphoma than 20 years ago. Counter to my impression though, Taylor et al’s study implied (from clinical signs) that most CNS lymphoma involved the spine. 10
Figure 4.
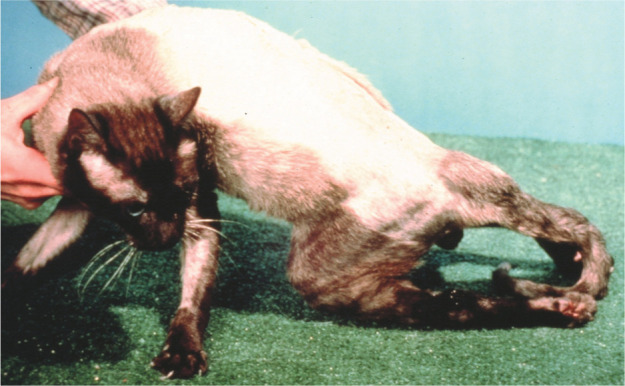
A Siamese cat with spinal lymphoma causing hind limb paralysis. This is still considered the most common cause of posterior paresis in cats. Courtesy of Dr John Berg
Figure 5.
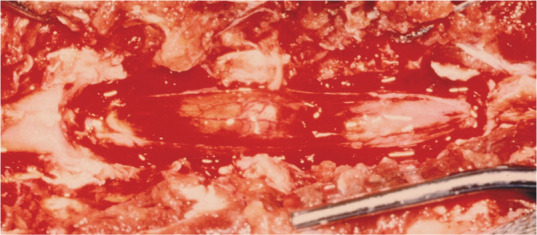
At surgery, extradural lymphoma can be cytoreduced, and spinal pressure relieved, but it is common for multiple segments to be affected, limiting the ability of surgery alone as a therapy. Chemotherapy and/or radiotherapy is necessary. Courtesy of Dr John Berg
Chemotherapy alone has been relatively ineffective in recent studies, with only 3/8 cats treated having a complete response;10,24 in one study this was the lowest response rate for any extranodal anatomic variant of lymphoma. 10 However, long survival is possible in complete responders.
Radiation therapy is probably the treatment of choice for CNS lymphoma, particularly if there is a localised lesion. The concurrent use of chemotherapy is warranted, however, because of the high rate of involvement of other anatomic sites. In one study, 11/16 cats also had lymphoma on a bone marrow aspirate, 34 and in another study some cats were even shown to have circulating lymphoblasts. 35 As mentioned earlier, the kidney is another very common secondary site of involvement, seen in 22/42 cats with spinal lymphoma.32,34,36
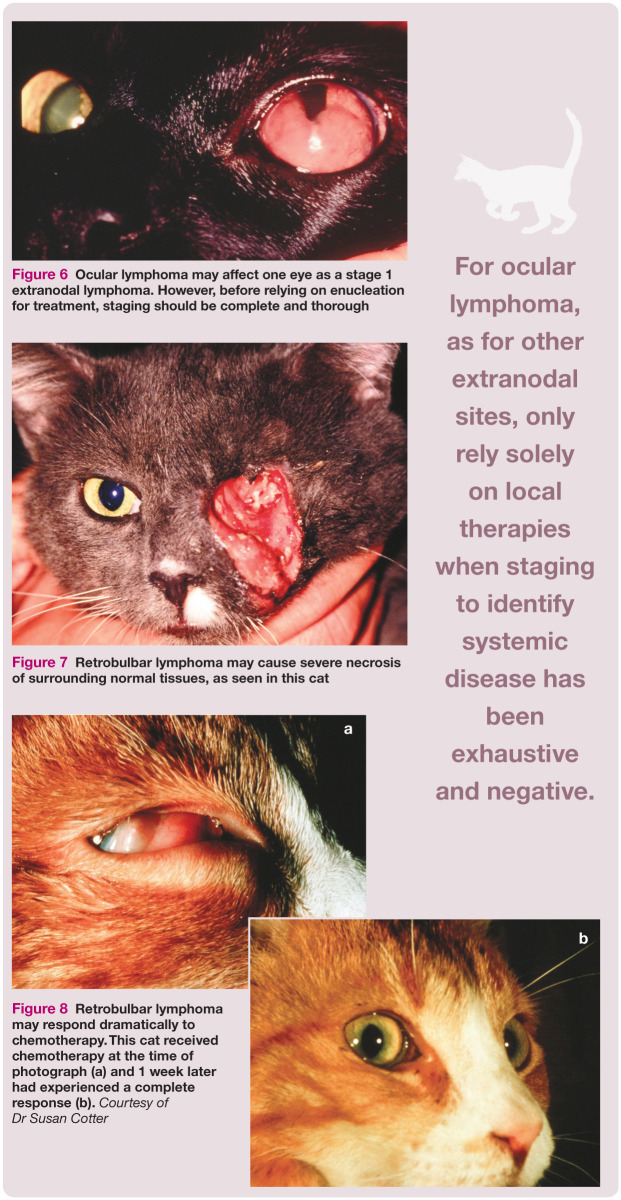
Ocular lymphoma
Ocular manifestations of lymphoma (Figure 6) are an uncommon clinical entity. Unilateral or bilateral ocular lymphoma may precede systemic disease in some cats, although it is more common to see multicentric lymphoma at the same time as ocular disease. In one study, approximately 35% of cats with ocular lymphoma had bilateral eye involvement, and 40% of cats with ocular lymphoma were FeLV positive. 37 Similar to nasal lymphoma, cats with ocular lymphoma are often in substage a.
Figure 6.
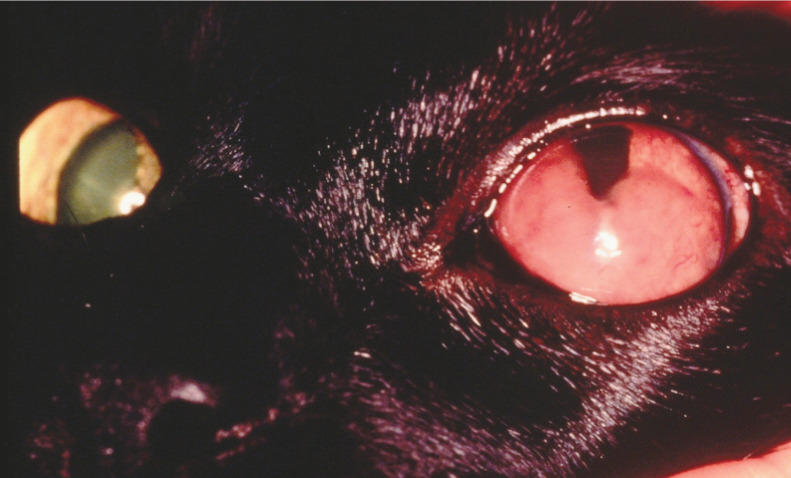
Ocular lymphoma may affect one eye as a stage 1 extranodal lymphoma. However, before relying on enucleation for treatment, staging should be complete and thorough
In the largest study to date, of 50 eyes of cats affected with ocular lymphoma, the most commonly affected structure was the uvea, either as a nodular iris mass, or diffuse infiltration of the iris. 37 The cornea is less commonly infiltrated with lymphoma, but secondary corneal changes may occur. Retinal infiltration with lymphoma was seen in only five cats, but 15 had a secondary retinal detachment. Glaucoma, secondary to tumour infiltration or anterior synechiae, was seen in 10 cats.
Ocular involvement may extend to affect the retrobulbar space (Figures 7 and 8) causing exophthalmos. Secondary inflammatory changes are common.
Figure 7.
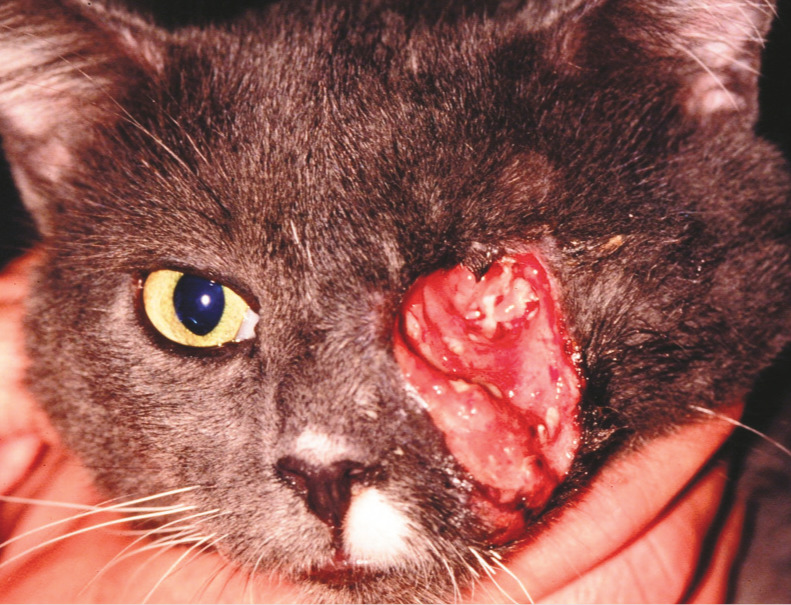
Retrobulbar lymphoma may cause severe necrosis of surrounding normal tissues, as seen in this cat
Figure 8.
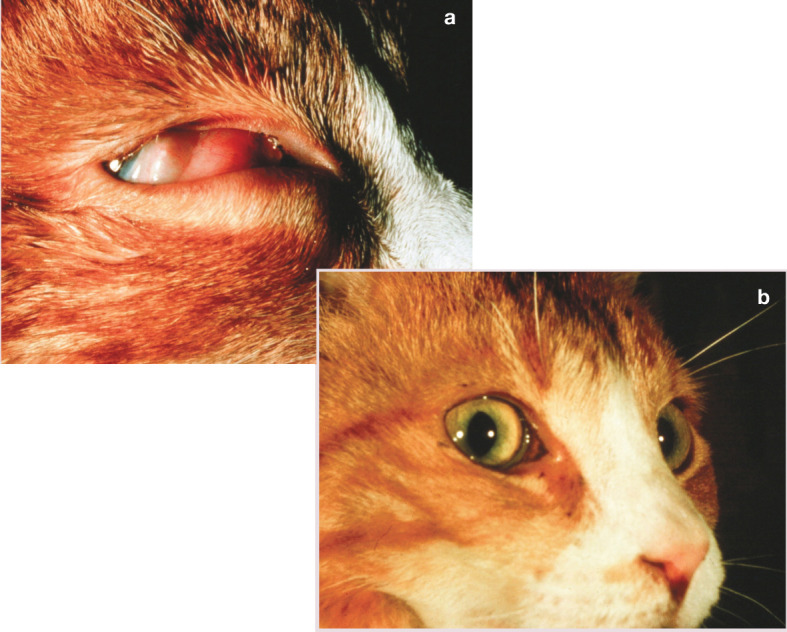
Retrobulbar lymphoma may respond dramatically to chemotherapy. This cat received chemotherapy at the time of photograph (a) and 1 week later had experienced a complete response (b). Courtesy of Dr Susan Cotter
Enucleation alone (some received corticosteroids) was the therapy for 10 cats in the above-mentioned study, and their average survival was 14 months. 37 Just as for other extranodal sites of lymphoma, you should always assume lymphoma is a systemic disease and only rely solely on local therapies when staging to identify systemic disease has been exhaustive and negative.
Choosing the best treatment
Survival times for untreated cats with lymphoma tend to be very short, although not all reports have classified patients by the site of their lymphoma. The average survival for cats that were untreated, or only treated supportively, in two early studies ranged between 2 weeks (15 cats) 38 and 5 days (16 cats). 39 In another group of 39 untreated cats, only two cats lived longer than 4 months. 40 Some untreated cats that have localised and sub-stage a lymphoma anecdotally can have longer survival times than this, but progression is inevitable.
When is local therapy alone appropriate?
When extranodal lymphoma is localised to a single anatomic site (stage I), local therapy (surgery or radiation therapy) may be appropriate. Given that it is rare to find stage I extranodal lymphoma in an anatomic location where surgery is an option, most local therapy is, in practice, radiation.
Radiation doses for treatment of lymphoma can be quite low, as lymphoid cells do not need to be dividing to be killed by radiation therapy (unlike carcinomas and sarcomas). This means that side effects may often be quite limited in lymphoma patients. Radiation therapy was noted to cause short-term responses in feline lymphoma as early as the 1960s, 41 and considerable work has been done in treating feline nasal lymphoma with radiation therapy since then, as summarised earlier. Radiation therapy for extranodal sites other than nasal lymphoma has only been documented in one small series of cats. 42 In that report, 2/3 cats with retrobulbar lymphoma, as well as one cat with oral, one with subcutaneous and one with mediastinal lymphoma, all showed a complete response to therapy. 42 The responses lasted for a median of 24 months. Based on my own experience, I would concur that radiation is the local treatment of choice for any cat with stage I lymphoma that is not easily resectable.
Electrochemotherapy combines the localised administration of chemotherapy with the application of permeabilising electric pulses in order to increase the uptake of the chemotherapeutic agents. This technique is not widely available, but has been reported to be successful in treating cats with localised extranodal lymphoma, using bleomycin. 43
When is systemic therapy warranted?
There is little specific information regarding the responses of cats with extranodal lymphoma to chemotherapy. One small study found that once a complete remission was achieved, cats with extranodal, non-GI lymphoma had a better prognosis (median complete remission 8.7 months) than cats with GI lymphoma (<2 months), but not as good as those with mediastinal lymphoma (17.2 months). 24 The findings of Taylor et al’s study of chemotherapy in 110 cats 10 are discussed above in relation to the individual extranodal lymphoma sites.
COP
The COP (cyclophosphamide, vincristine and prednisone) protocol is the most widely used protocol for treatment of cats with any form of lymphoma. In an early US report, 38 cats treated with COP had an overall complete clinical response rate of 74% (30/38 cats). 44 The duration of complete response ranged from 2–42 months. The complete response rate varied from 100% for multicentric lymphoma to 50% for extranodal lymphoma. Both groups contained individuals that lived longer than a year.
Later reports of the use of the COP protocol in the USA did not confirm the same efficacy. A further 38 cats treated in the 1990s differed from the earlier cats in being less likely to be FeLV positive or have mediastinal lymphoma. Eighteen of the 38 cats (47%) had a complete response. The median response duration was less than 3 months, and no cat had a remission longer than 6 months. Of the cats with extranodal lymphoma, one cat with nasal and one with peripheral nerve lymphoma responded, but only 2/9 with renal lymphoma responded. 45 In contrast, two European studies found that COP (given either intravenously 13 or intraperitoneally 14 ) gave a similar overall complete clinical response rate (about 70%) to that seen in earlier US studies, but extranodal sites were not reported separately. Taylor et al’s study found no difference between remission rates and duration of complete remission for cats with extranodal lymphoma that were treated with COP or COP plus methotrexate and doxorubicin (± L-asparaginase): 72.7% versus 64%, respectively. 10
Doxorubicin ± COP
It is difficult to define the contribution of doxorubicin to the treatment of non-low grade feline lymphoma, as studies of single agent protocols show very little efficacy. In two studies, single agent doxorubicin as an induction agent produced a complete response in fewer than 30% of cats.46,47 A further study found that doxorubicin was an ineffective rescue agent in cats with lymphoma that failed to respond to or had relapsed after chemotherapy with other drugs. 48 By contrast, doxorubicin appears effective when used as a component of multiagent chemotherapy. In a study in which cats that had achieved a complete response to COP were randomised to either continue COP maintenance, or to receive doxorubicin alone, those that received only COP had a median remission of 3 months while cats receiving doxorubicin had a median remission of 9.5 months. 45 The only long-term survivors in the study received both doxorubicin and COP. These findings were supported by a multi-institutional study. 9
L-asparaginase
L-asparaginase was evaluated for single agent efficacy in the treatment of cats with multicentric lymphoma. Only 30% of cats responded (15% complete remission), which is considerably lower than the published efficacy in dogs. 49 The two cats showing complete response had multicentric T cell lymphoma. Regardless, there is probably an advantage to using L-asparaginase in the treatment of cats with lymphoma, particularly if following the logic that doxorubicin as a single agent in cats is poorly effective, but improves length of remission and survival in cats that can achieve remission; the same may be true for L-asparaginase. Also, since it is not myelosuppressive, L-asparaginase can be combined with other chemotherapy drugs.
L-asparaginase is an expensive drug, and there is considerable wastage when treating a feline patient. Based on a study that showed a less than 10% reduction in activity over 7 days, 50 personally, I will use L-asparaginase for 7 days as long as it has been stored refrigerated, and is not cloudy; this allows for two administrations a week apart in a protocol. Beyond that time, efficacy is less certain and, despite the costs, I consider the risk of using an ineffective drug to be greater than the cost-saving is worth.
MOPP
MOPP (mechlorethamine, vincristine, prednisone and procarbazine) is a protocol that combines two alkylating agents with vincristine and prednisone. Although only reported in abstract form, a 28-day cycling MOPP protocol was given to 23 cats, most of which had GI lymphoma, when other chemotherapy failed to give a remission, and was found to be effective. 51 Anorexia in cats receiving the MOPP protocol is most likely to resolve when procarbazine is reduced to alternate-day administration, or given concurrently with metoclopramide.
In another study presented only in abstract form, MOPP was administered in an alternating protocol with doxorubicin and cyclophosphamide as first-line therapy. In this study, 35 cats with lymphoma were categorised as having multicentric (15), extranodal (12), GI (six) or mediastinal (one) lymphoma and the complete response rate was 52%. 52 While still not published in the peer-reviewed literature, mechlorethamine and procarbazine clearly have a role to play in the treatment of lymphoma in cats. Although mechlor ethamine is not widely available, I have used procarbazine and anecdotally have seen responses in many cats with lymphoma.
Footnotes
Funding: The author received no specific grant from any funding agency in the public, commercial or notfor-profit sectors for the preparation of this article.
The author does not have any potential conflicts of interest to declare.
Key Points
Non-GI extranodal lymphomas account for a large proportion of lymphoma in cats.
-
The most common groupings are:
- – nasal/upper respiratory tract
- – renal
- – central nervous system
- – ocular
Many other rare, extranodal sites have been reported.
Prognostic factors are still poorly defined for extranodal feline lymphoma and the clinician is encouraged to consider the stage, clinical substage, anatomic site, histological grade and immunophenotype in all patients with this disease.
Since there is often no specific information available as to the best treatment options for extranodal lymphoma arising in a particular site, it may be reasonable to draw inferences from other, more common extranodal sites with often localised disease (such as nasal lymphoma) to help in decision making.
If cats with extranodal lymphoma can be confirmed (through thorough staging) to have stage I disease at an accessible site, local therapies can be both palliative and therapeutic, and even potentially curative.
For most patients with extranodal lymphoma, however, chemotherapy is necessary.
Case notes
Pusskins, a 16-year-old castrated male Tonkinese weighing 4.9 kg, has a history of progressive early stage renal failure, and in the 2 months before referral to you developed left fore limb lameness. This was originally felt by the referring veterinarian to be due to degenerative joint disease, but when it was observed to be progressive computed tomography (CT) was undertaken. The CT scan revealed an intramuscular mass, cytology from which was suggestive for lymphoma. A thoracic CT scan and abdominal ultrasonography showed no conclusive evidence of lymphoma (cytology from a hepatic nodule revealed only hepatocytes), and a complete blood count was normal. Serum chemistry showed a mild elevation in albumin and urea, and urine specific gravity was 1.015.
Amputation was performed and the diagnosis with immunohistochemistry was high grade, B cell lymphoma. On arrival at your clinic Pusskins requires sedation for handling and, according to his owners, is difficult to medicate orally at home.
WHAT IS YOUR ASSESSMENT?
What further staging is required?
What prognostic factors exist for this patient?
What treatment options should be considered?
What are the practical considerations, and how might they affect your recommendations?
What are the risks for anticancer chemotherapy in this patient?
Discussion of questions posed on page 386 in relation to Pusskins, a 16-year-old Tonkinese referred for treatment of intramuscular lymphoma.
What further staging is required? FeLV/FIV testing should be considered for completeness as it may be prognostic.
What prognostic factors exist for this patient? Pusskins has a stage I high grade lymphoma; he is systemically well (substage a), his immunophenotype is B cell (the prognostic effect of this is uncertain) and he is not anaemic. As the major question is whether his tumour could be treated with localised therapy, and since there is no specific information available for the intramuscular site, the best answer would probably come from another common extranodal site with often localised, B cell lymphoma – the nasal cavity. Although obviously not exactly the same, it may be reasonable to draw inferences that could help in decision making. Specific to nasal lymphoma, studies have shown that complete clinical response to therapy (better) and anaemia (worse) were significant prognostic factors, 20 and cats that received radiation therapy had longer survival times, 13 suggesting that local tumour control, in addition to chemotherapy, had an impact on survival.
What treatment options should be considered? As the disease appears to be stage I, local therapy alone may be sufficient to control Pusskins’ lymphoma; the risk is that other sites of disease may have escaped detection despite the extensive staging performed. For that reason, chemotherapy should be considered. Combination chemotherapy with a doxorubicin-containing regimen represents the current standard of care for cats with any form of lymphoma. The disadvantage is the risk of doxorubicin nephrotoxicity, which is progressive even after therapy is ceased. Pusskins is an older cat and he has early renal failure. In the light of the possible effect of doxorubicin on his renal function, particularly since it may entail anaesthesia to administer, it would be wise to err on the side of caution and not use doxorubicin in the protocol.
-
What are the practical considerations, and how might they affect your recommendations? For cats that cannot be handled safely for intravenous injection (ie, pose a risk to personnel safety and a risk of extravasation), I find isoflurane given either by mask (probably not possible in Pusskins) or in a cage works quickly and with little in the way of after-effects. While they are maintained under anaesthesia on a mask, their bloodwork can be checked in-house, and therapy can be administered as needed. The process is more cumbersome, but actually takes less time (and stress) than trying to fight with these patients each time.
If that is not acceptable to his owners, then another option is oral chemotherapy, but this does not sound as if it would be possible either in Pusskins’ case.
A third alternative would be to use a protocol based on drugs given by subcutaneous injection, and one could be developed if Pusskins’ owners would prefer that option. However, there are not many drugs that are active for feline lymphoma that can be given by this route.
A final option (one that I have not used myself) would be an intraperitoneal vincristine and cyclophosphamide protocol, with transdermal prednisone. While that sounds uncomfortable, a recent European publication claimed identical or better remission rates, with no abdominal discomfort, when such a protocol was administered. 16 However, that may still not be possible without anaesthesia in a cat like Pusskins.
What are the risks for anticancer chemotherapy in this patient? Supportive care may be another challenging aspect of Pusskins’ treatment if chemotherapy is given. With chemotherapy, antiemetics (metoclopramide) and appetite stimulants (eg, megestrol acetate or cyproheptadine orally) may be appropriate, in addition to good general nursing care, in order to improve nutritional status. In my opinion, although other authorities differ, the use of prophylactic antibiotics is warranted, particularly when administering a myelosuppressive drug for the first time. I feel that preventing potentially life-threatening sepsis in a patient, rather than treating when they become unwell, will improve quality of life and reduce risks for hospitalisation. Further, the use of prophylactic trimethoprim–sulfadiazine has been shown to be protective in dogs. 57 In cats, this drug is poorly tolerated (induces salivation and palatibility is poor); as an alternative, I like marbofloxacin (Zeniquin; Pfizer), which has good Gram-negative activity and is orally administered. However, if supportive care cannot be administered by Pusskins’ owners, then it will also increase his risks for complications with chemotherapy.
References
- 1. Barrs VR, Beatty JA. Feline alimentary lymphoma: 1. Classification, risk factors, clinical signs and non-invasive diagnostics. J Feline Med Surg 2012; 14: 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barrs VR, Beatty JA. Feline alimentary lymphoma: 2. Further diagnostics, therapy and prognosis. J Feline Med Surg 2012; 14: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gabor LJ, Malik R, Canfield PJ. Clinical and anatomical features of lymphosarcoma in 118 cats. Aust Vet J 1998; 76: 725–732. [DOI] [PubMed] [Google Scholar]
- 4. Meincke JE, Hobbie WV, Jr, Hardy WD, Jr. Lymphoreticular malignancies in the cat: clinical findings. J Am Vet Med Assoc 1972; 160: 1093–1098. [PubMed] [Google Scholar]
- 5. Hardy WD, Jr. Hematopoietic tumors of cats. J Am Anim Hosp Assoc 1981; 17: 921–940. [Google Scholar]
- 6. Slayter MV, Farver TB, Schneider R. Feline malignant lymphoma: log linear multiway frequency analysis of a population involving the factors of sex and age of animal and tumor cell type and location. Am J Vet Res 1984; 45: 2178–2181. [PubMed] [Google Scholar]
- 7. Mooney SC, Hayes AA. Lymphoma in the cat: an approach to diagnosis and management. Semin Vet Med Surg (Small Anim) 1986; 1: 51–57. [PubMed] [Google Scholar]
- 8. Mooney SC, Hayes AA, MacEwen EG, Matus RE, Geary A, Shurgot BA. Treatment and prognostic factors in lymphoma in cats: 103 cases (1977–1981). J Am Vet Med Assoc 1989; 194: 696–699. [PubMed] [Google Scholar]
- 9. Vail DM, Moore AS, Ogilvie GK, Volk LM. Feline lymphoma (145 cases): proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats. J Vet Intern Med 1998; 12: 349–354. [DOI] [PubMed] [Google Scholar]
- 10. Taylor SS, Goodfellow MR, Browne WJ, Walding B, Murphy S, Tzannes S, et al. Feline extranodal lymphoma: response to chemotherapy and survival in 110 cats. J Small Anim Pract 2009; 50: 584–592. [DOI] [PubMed] [Google Scholar]
- 11. Stutzer B, Simon K, Lutz H, Majzoub M, Hermanns W, Hirschberger J, et al. Incidence of persistent viraemia and latent feline leukaemia virus infection in cats with lymphoma. J Feline Med Surg 2011; 13: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moore PF, Rodriguez-Bertos A, Kass PH. Feline gastrointestinal lymphoma: mucosal architecture, immunophenotype, and molecular clonality. Vet Pathol 2012; 49: 658–668. [DOI] [PubMed] [Google Scholar]
- 13. Haney SM, Beaver L, Turrel J, Clifford CA, Klein MK, Crawford S, et al. Survival analysis of 97 cats with nasal lymphoma: a multi-institutional retrospective study (1986–2006). J Vet Intern Med 2009; 23: 287–294. [DOI] [PubMed] [Google Scholar]
- 14. Hadden AG, Cotter SM, Rand W, Moore AS, Davis RM, Morrissey P. Efficacy and toxicosis of VELCAP-C treatment of lymphoma in cats. J Vet Intern Med 2008; 22: 153–157. [DOI] [PubMed] [Google Scholar]
- 15. Teske E, van Straten G, van Noort R, Rutteman GR. Chemotherapy with cyclophosphamide, vincristine, and prednisolone (COP) in cats with malignant lymphoma: new results with an old protocol. J Vet Intern Med 2002; 16: 179–186. [DOI] [PubMed] [Google Scholar]
- 16. Teske E, van Lankveld AJ, Rutteman GR. Intraperitoneal antineoplastic drug delivery: experience with a cyclophosphamide, vincristine and prednisolone protocol in cats with malignant lymphoma. Vet Comp Oncol. Epub ahead of print 10 April 2012. DOI: 10.1111/j.1476-5829.2012.00329.x [DOI] [PubMed] [Google Scholar]
- 17. North SM, Meleo K, Mooney S, Mauldin GN. Radiation therapy in the treatment of nasal lymphoma in cats. Proceedings of the 14th Annual Veterinary Cancer Society Conference 1994; p 21. [Google Scholar]
- 18. Little L, Patel R, Goldschmidt M. Nasal and nasopharyngeal lymphoma in cats: 50 cases (1989–2005). Vet Pathol 2007; 44: 885–892. [DOI] [PubMed] [Google Scholar]
- 19. Mukaratirwa S, van der Linde-Sipman JS, Gruys E. Feline nasal and paranasal sinus tumours: clinicopathological study, histomorphological description and diagnostic immunohistochemistry of 123 cases. J Feline Med Surg 2001; 3: 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sfiligoi G, Theon AP, Kent MS. Response of nineteen cats with nasal lymphoma to radiation therapy and chemotherapy. Vet Radiol Ultrasound 2007; 48: 388–393. [DOI] [PubMed] [Google Scholar]
- 21. Brown MR, Rogers KS, Mansell KJ, Barton C. Primary intratracheal lymphosarcoma in four cats. J Am Anim Hosp Assoc 2003; 39: 468–472. [DOI] [PubMed] [Google Scholar]
- 22. Saik JE, Toll SL, Diters RW, Goldschmidt MH. Canine and feline laryngeal neoplasia: a 10 year survey. J Am Anim Hosp Assoc 1986; 22: 359–365. [Google Scholar]
- 23. Carlisle CH, Biery DN, Thrall DE. Tracheal and laryngeal tumors in the dog and cat: literature review and 13 additional patients. Vet Radiol 1991; 32: 229–235. [Google Scholar]
- 24. Simon D, Eberle N, Laacke-Singer L, Nolte I. Combination chemotherapy in feline lymphoma: treatment outcome, tolerability, and duration in 23 cats. J Vet Intern Med 2008; 22: 394–400. [DOI] [PubMed] [Google Scholar]
- 25. Gabor LJ, Love DN, Malik R, Canfield PJ. Feline immunodeficiency virus status of Australian cats with lymphosarcoma. Aust Vet J 2001; 79: 540–545. [DOI] [PubMed] [Google Scholar]
- 26. Mooney SC, Hayes AA, Matus R, MacEwen EG. Renal lymphoma in cats: 28 cases (1977–1984). J Am Vet Med Assoc 1987; 191: 1473–1477. [PubMed] [Google Scholar]
- 27. Weller RE, Stann SE. Renal lymphosarcoma in the cat. J Am Anim Hosp Assoc 1983; 19: 363–367. [Google Scholar]
- 28. Gabor LJ, Canfield PJ, Malik R. Immunophenotypic and histological characterization of 109 cases of feline lymphosarcoma. Aust Vet J 1999; 77: 436–441. [DOI] [PubMed] [Google Scholar]
- 29. Osborne CA, Johnson KH, Kurtz HJ, Hanlon GF. Renal lymphoma in the dog and cat. J Am Vet Med Assoc 1971; 158: 2058–2070. [PubMed] [Google Scholar]
- 30. Cotter SM, Kanki PJ, Simon M. Renal disease in five tumor-bearing cats treated with adria-mycin. J Am Anim Hosp Assoc 1985; 21: 405–409. [Google Scholar]
- 31. Zaki FA, Hurvitz AI. Spontaneous neoplasms of the central nervous system of the cat. J Small Anim Pract 1976; 17: 773–782. [DOI] [PubMed] [Google Scholar]
- 32. Lane SB, Kornegay JN, Duncan Jr, Oliver JE, Jr. Feline spinal lymphosarcoma: a retrospective evaluation of 23 cats. J Vet Intern Med 1994; 8: 99–104. [DOI] [PubMed] [Google Scholar]
- 33. Noonan M, Kline KL, Meleo K. Lymphoma of the central nervous system: a retrospective study of 18 cats. Compend Contin Educ Pract Vet 1997; 19: 497–504. [Google Scholar]
- 34. Spodnick GJ, Berg J, Moore FM, Cotter SM. Spinal lymphoma in cats: 21 cases (1976–1989). J Am Vet Med Assoc 1992; 200: 373–376. [PubMed] [Google Scholar]
- 35. Ogilvie GK. Extradural lymphoma in a cat. Veterinary Medicine Report 1988; 1: 57–61. [Google Scholar]
- 36. Northington JW, Juliana MM. Extradural lymphosarcoma in six cats. J Small Anim Pract 1978; 19: 409–416. [DOI] [PubMed] [Google Scholar]
- 37. Peiffer RL, Jr, Wilcock BP. Histopathologic study of uveitis in cats: 139 cases (1978–1988). J Am Vet Med Assoc 1991; 198: 135–138. [PubMed] [Google Scholar]
- 38. Squire RA. Feline lymphoma. A comparison with the Burkitt tumor of children. Cancer 1966; 19: 447–453. [Google Scholar]
- 39. Ladiges WC, Zeidner NS. An overview of feline cancer therapy. Feline Pract 1980; 10: 38–43. [Google Scholar]
- 40. Crighton GW. Clinical aspects of lymphosarcoma in the cat. Vet Rec 1968; 83: 122–126. [DOI] [PubMed] [Google Scholar]
- 41. Carpenter JL, Holzworth J. Treatment of leukemia in the cat. J Am Vet Med Assoc 1971; 158: 1130–1131. [PubMed] [Google Scholar]
- 42. Elmslie RE, Ogilvie GK, Gillette EL, McChesney-Gillette S. Radiotherapy with and without chemotherapy for localized lymphoma in 10 cats. Vet Radiol 1991; 32: 277–280. [Google Scholar]
- 43. Spugnini EP, Citro G, Mellone P, Dotsinsky I, Mudrov N, Baldi A. Electrochemotherapy for localized lymphoma: a preliminary study in companion animals. J Exp Clin Cancer Res 2007; 26: 343–346. [PubMed] [Google Scholar]
- 44. Cotter SM.Treatment of lymphoma and leukemia with cyclophosphamide, vincristine, and prednisone: II. treatment of cats. J Am Anim Hosp Assoc 1983; 19: 166–172. [Google Scholar]
- 45. Moore AS, Cotter SM, Frimberger AE, Wood CA, Rand WM, L’Heureux DA. A comparison of doxorubicin and COP for maintenance of remission in cats with lymphoma. J Vet Intern Med 1996; 10: 372–375. [DOI] [PubMed] [Google Scholar]
- 46. Peaston AE, Maddison JE. Efficacy of doxorubicin as an induction agent for cats with lymphosarcoma. Aust Vet J 1999; 77: 442–444. [DOI] [PubMed] [Google Scholar]
- 47. Kristal O, Lana SE, Moore AS, Ogilvie GK, Cotter SM. Single agent chemotherapy with doxorubicin for feline lymphoma. Proceedings of 18th Annual Veterinary Cancer Society Conference 1998; p 25. [Google Scholar]
- 48. Oberthaler KT, Mauldin E, McManus PM, Shofer FS, Sorenmo KU. Rescue therapy with doxorubicin-based chemotherapy for relapsing or refractory feline lymphoma: a retrospective study of 23 cases. J Feline Med Surg 2009; 11: 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. LeBlanc AK, Cox SK, Kirk CA, Newman SJ, Bartges JW, Legendre AM. Effects of L-asparaginase on plasma amino acid profiles and tumor burden in cats with lymphoma 2. J Vet Intern Med 2007; 21: 760–763. [DOI] [PubMed] [Google Scholar]
- 50. Stecher AL, de Deus PM, Polikarpov I, Abrahao-Neto J. Stability of L-asparaginase: an enzyme used in leukemia treatment. Pharm Acta Helv 1999; 74: 1–9. [DOI] [PubMed] [Google Scholar]
- 51. Mauldin GE, Mooney SC, Mauldin GN. MOPP chemotherapy for cats with refractory lymphoma. Proceedings of the 17th Annual Veterinary Cancer Society Conference 1997; p 98. [Google Scholar]
- 52. Rassnick KM, Kristal O, Northrup NC, Chretin JD, Cotter SM, Erb HN, et al. MOPP-AC for the treatment of lymphoma in cats. Proceedings of the 21st Annual Veterinary Cancer Society Conference 2001, p 10. [Google Scholar]
- 53. Kiselow MA, Rassnick KM, McDonough SP, Goldstein RE, Simpson KW, Weinkle TK, et al. Outcome of cats with low-grade lymphocytic lymphoma: 41 cases (1995–2005). J Am Vet Med Assoc 2008; 232: 405–410. [DOI] [PubMed] [Google Scholar]
- 54. Lingard AE, Briscoe K, Beatty JA, Moore AS, Crowley AM, Krockenberger M, et al. Low-grade alimentary lymphoma: clinicopathological findings and response to treatment in 17 cases. J Feline Med Surg 2009; 11: 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stein TJ, Pellin M, Steinberg H, Chun R. Treatment of feline gastrointestinal small-cell lymphoma with chlorambucil and glucocorticoids. J Am Anim Hosp Assoc 2010; 46: 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dutelle AL, Bulman-Fleming JC, Lewis CA, Rosenberg MP. Evaluation of lomustine as a rescue agent for cats with resistant lymphoma. J Feline Med Surg 2012; 14: 694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chretin JD, Rassnick KM, Shaw NA, Hahn KA, Ogilvie GK, Kristal O, et al. Prophylactic trimetho-prim-sulfadiazine during chemotherapy in dogs with lymphoma and osteosarcoma: a double-blind, placebo-controlled study. J Vet Intern Med 2007; 21: 141–148. [DOI] [PubMed] [Google Scholar]