Abstract
Chronic kidney disease (CKD) is a common cause of illness and death in cats. The hallmark of CKD in cats is chronic tubulointerstitial nephritis, and inflammation contributes to the progression of renal fibrosis. However, at present, it is difficult to assess directly the degree of intra-renal inflammation without renal biopsy. Measurement of inflammatory cytokine levels in urine may provide a non-invasive means of assessing intra-renal inflammation. Urine cytokine levels (urine cytokine/urine creatinine ratio) were measured in 18 healthy cats and 26 cats with CKD. When urine cytokine levels in healthy and CKD cats were compared, we found significantly higher levels of IL-8 and transforming growth factor-β1 (TGF-β1) in urine of CKD cats, along with significantly lower vascular endothelial growth factor (VEGF) levels. A significant positive correlation between serum creatinine and TGF-β1 levels was found in CKD cats. Urinary cytokine measurement may, potentially, be a useful means of assessing intra-renal inflammation, fibrosis and vascular health in cats with CKD.
Introduction
Chronic kidney disease (CKD) is a common clinical problem in geriatric cats. Prevalence increases with age and it has been reported that at least 15–30% of cats over 15 years of age are azotemic.1,2 There are many potential causes of kidney disease in cats, but, in most cats with CKD, histopathologic lesions consist of diffuse tubulointerstitial nephritis, accumulation of extracellular matrix and fibrosis.3,4 Hyperfiltration, proteinuria, tubulointerstitial inflammation, oxidative damage and induction of the renin–angiotensin–aldosterone system are major factors in humans that are thought to contribute to the process of tubulointerstitial injury.5–7 Regardless of the initial insult, once a threshold of renal damage has been reached, progression is irreversible and appears consistent in character. 5 Feline CKD may be divided into stages according to a classification scheme proposed by the International Renal Interest Society (IRIS) (www.iris-kidney.com). 8 Cats in CKD stages II–IV will generally have progressive disease. Short of renal transplant, which is expensive and not widely available, there is currently no cure for CKD in human or veterinary medicine, and medical management remains the standard of care.9,10 A better understanding of this disease process may lead to additional treatment options. Renal biopsy is a definitive method of assessing individual renal pathology, but is not without potential significant risk. 11 Recently, several studies in humans have provided evidence that urinary cytokines may be beneficial as a non-invasive assessment of renal disease and may provide an additional method by which progression of disease and response to therapy could be monitored.12–15
Cytokines and chemokines are released by damaged glomerular, mesangial and endothelial cells, activated tubular cells and infiltration inflammatory cells, and are thought to be critically involved in the progression of renal inflammation. 6 Transforming growth factor-β1 (TGF-β1) is a multifunctional cytokine that is thought to play a critical role in fibrosis in humans. 16 TGF-β1 is released from parenchymal cells and inflammatory cells, and can result in production of extracellular matrix proteins and differentiation of epithelial cells into myofibroblasts. 16 Urinary TGF-β1 levels are correlated with renal parenchymal TGF-β1 and the degree of interstitial fibrosis in humans.17,18 In one previous study in cats, urinary TGF-β1 levels were found to be elevated in cats with CKD. 19 Interleukin-8 (IL-8) is a chemokine released by white blood cells and endothelial cells in response to inflammation, which recruits neutrophils to sites of inflammation. Elevated urinary IL-8 levels have been correlated with pyelonephritis in children, as well as the subsequent degree of renal fibrosis. 15 Monocyte chemoattractant protein-1 (MCP-1) is another chemokine that is expressed at sites of injury or inflammation that functions to recruit inflammatory monocytes. 20 In humans, elevated urinary levels of MCP-1 have been associated with degree of inflammatory infiltrate, proteinuria and severity of disease.18,20 Vascular endothelial growth factor (VEGF), a potent pro-angiogenic factor important in heath and repair of renal vasculature, has been shown to be down-regulated in end-stage renal disease in humans and is thought to be a potentially useful prognostic biomarker for loss of renal function. In previous studies in humans urinary VEGF excretion is reduced in more severe kidney disease.21,22
The purpose of this study was to evaluate levels of relevant cytokines and chemokines in feline urine as a means of indirectly assessing renal inflammation, fibrosis and vascular health in cats. We hypothesized that levels of pro-inflammatory and pro-fibrotic cytokines would be increased in the urine of cats with CKD when compared with healthy cats. In addition, we hypothesized that the angiogenic factor VEGF would be decreased in the urine of cats with CKD in comparison to normal cats. As a result, measurement of urinary cytokine levels in CKD cats could provide an alternative, minimally-invasive means of assessing disease progression and/or response to therapeutic trials.
Materials and methods
Animals
This study was performed on urine samples from 57 different cats. All cats were spayed or neutered client-owned animals presenting to the Colorado State University (CSU) Veterinary Teaching Hospital (VTH) in Fort Collins, CO, USA or CatTails Feline Health Center in Colorado Springs, CO, USA. Each cat was evaluated medically before participation in the study. Healthy cats were convenience-sampled from those presenting to a veterinary clinic for an annual examination or to participate in a clinical study in which healthy cats were enrolled. Based on the animal’s clinical signs, history and serum biochemistry and urinalysis data, samples were placed in one of two categories: apparently healthy or CKD. Cats were only included in the study if urinalysis demonstrated a non-active sediment. Cats with CKD also had complete blood count, blood pressure, T4 and urine culture performed to rule out concurrent conditions. Eighteen urine samples from healthy cats were analyzed from patients that met this classification. Cats in the CKD group had known histories of stable, non-proteinuric, non-hypertensive chronic kidney disease (IRIS stage II–IV) with no known concurrent systemic illness or lower urinary tract disease. Twenty-six urine samples from CKD cats were analyzed.
Assays
Urine samples were analyzed using commercially- available antigen-capture enzyme-linked immunosorbent assays (ELISAs) for feline IL-8, IL-6, and IL-10, canine MCP-1, canine VEGF, murine TGF-β1 (R&D Systems) and multispecies TGF-β1 (Invitrogen). VEGF and TGF-β1 ELISAs (Invitrogen kit) have been utilized previously in feline urine.19,23 Owing to the high level of protein homology between feline and canine MCP-1 (89% homology at the nucleic acid level, http://blast.ncbi.nlm.nih.gov/Blast.cgi) we have proposed that feline MCP-1 could be detected using a canine MCP-1-specific ELISA. However, recombinant feline MCP-1 was not available to validate this assay in cats.
Samples were collected by cystocentesis for urinalysis, and an aliquot of urine was frozen immediately at -20°C and then stored at -80°C. Samples were thawed immediately before analysis and kept on ice. All samples were centrifuged at 2000 rpm (350 × g) for 5 mins and the supernatant was collected for analysis. ELISAs were run according to manufacturer’s instructions. Urine and serum creatinine concentrations were measured with a Roche Cobas Integra Chemistry Analyzer (Roche Diagnostics) at the CSU diagnostic laboratory. All reported cytokine concentrations were normalized for differences in urine concentration as a urine:cytokine-to-urine-creatinine ratio (cytokine:UCrR). ELISAs for IL-8, MCP-1, VEGF and TGF-β1 (R&D Systems kit) were run on urine from 18 healthy cats and 26 CKD cats. TGF-β1 ELISA (Invitrogen kit) was assayed subsequently on a subset of 27 urine samples: 12 healthy and 15 CKD. The subset of samples was chosen randomly from cats in the original group for which a second aliquot of urine was available.
In order to maximize cytokine yield in the urine samples, additional sample processing steps were performed prior to running the IL-8, MCP-1 and VEGF ELISAs. For IL-8 detection, the urine supernatant was concentrated approximately threefold using a centrifuge filter system (Amicon-Ultra 3kDa; Millipore). Volumes before and after concentration were measured to calculate the concentration factor for each sample. Filtrates were then diluted 1 to 9 in 1% bovine serum albumin (BSA). For MCP-1 detection, urine supernatant was diluted 1 to 9 in 1% BSA. For VEGF detection, urine supernatant was diluted 1 to 4 in 1% BSA.
Statistical analysis
Cytokine:UCrR ratios were compared between groups using non-parametric (Mann–Whitney) unpaired, two-tailed t-tests, with Prism5 software (GraphPad). Manual elimination of prominent outliers was performed on some data sets to confirm the results were not skewed by their presence. Cytokine:UCrR level and serum creatinine concentration data were log-transformed and compared using Pearson correlations with Prism5 software.
Results
Signalment and relevant clinical pathology data
The mean age of the healthy cats was 6.5 years (median 5.8 years, range 8 months–13 years). The mean serum creatinine of these cats was 1.3 mg/dl (median 1.4 mg/dl, range 0.8–1.5 mg/dl). There were 10 female cats and 8 male cats included in the healthy group. The mean age of the CKD cats was 13.8 years (median 13.5 years, range 6–19 years). The mean serum creatinine of these cats was 3.3 mg/dl (median 2.7 mg/dl, range 1.6–8.3 mg/dl). There were 14 female cats and 12 male cats included in the CKD group. The CKD group was significantly older than the apparently healthy group (P < 0.0001). The serum creatinine concentration was also significantly higher in the CKD group than the healthy group (P < 0.0001). The mean for CKD urine creatinine concentration was 99.5 mg/dl (median 87.7 mg/dl, range 52.4–201.3 mg/dl) and was significantly lower than the urine creatinine concentration of the healthy group (mean 298.8, median 309.3 mg/dl, range 174–416 mg/dl) (P < 0.0001).
Urine cytokine levels
We found that commercially-available (R&D Systems) antigen-capture ELISAs for feline IL-6, feline IL-10 and murine TGF-β1 (R&D Systems kit) could not detect urine concentrations of these cytokines in feline urine samples. The minimum concentrations of detection for each of the four other cytokines evaluated were 16 pg/ml for IL-8, VEGF and TGF-β1 (Invitrogen kit), and 7 pg/ml for MCP-1. In the samples included in this study, MCP-1 could be detected in all but three of the urine samples (two CKD samples and one healthy sample,). Concentrations of IL-8, VEGF and TGF-β1 (Invitrogen kit) were detected in all urine samples.
A summary of cytokine:UCrR results is presented in Table 1. We found that IL-8:UCrR levels were significantly higher in CKD urine samples than in healthy urine samples (P < 0.0001), even when high outliers were excluded from the statistical analysis (P < 0.0001) (Figure 1). TGF-β1:UCrR was also significantly higher in CKD urine samples than in healthy urine samples (P = 0.0002) (Figure 2). Conversely, VEGF:UCrR was significantly lower in CKD cat urine versus healthy cat urine (P = 0.0003) (Figure 3). A significant difference in MCP-1:UCrR levels between healthy urine samples and CKD urine samples could not be detected (Figure 4).
Table 1.
Summary of urinary cytokine:urine creatinine ratio results in normal cats and cats with chronic kidney disease
| Normal cats |
CKD cats |
|||||
|---|---|---|---|---|---|---|
| Mean ± SD | Median | Range | Mean ±SD | Median | Range | |
| IL–8 (pg/mg) | 4.0 ± 2.8 | 3.2 | 1.7−13.5 | 9.5 ± 5.4 | 8.7 | 3.2− 30.4 |
| MCP–1 (pg/mg) | 103 ± 87.8 | 74.7 | 0−284.3 | 205.6 ± 215.5 | 124.2 | 0−837.4 |
| TGF–β1 (pg/mg) | 988.3 ± 427.1 | 870.9 | 488−1770 | 6814 ± 2006 | 6698 | 3601−9831 |
| VEGF (pg/mg) | 636.1 ± 326 | 525.3 | 129.4−1569 | 300.9 ± 161.4 | 239.9 | 35.3−593.7 |
IL-8 = interleukin-8, MCP-1 = monocyte chemoattractant protein-1, TGF-β1 = transforming growth factor-β1, VEGF = vascular endothelial growth factor
Figure 1.
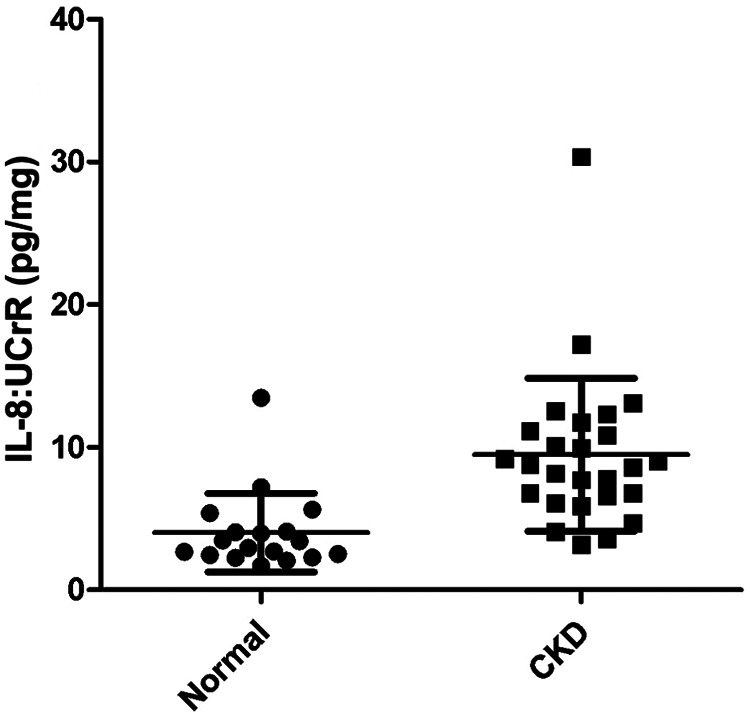
Urinary interleukin-8 (IL-8):urine creatinine ratio (UCrR) of healthy (n = 18) and chronic kidney disease (CKD) (n = 26) cats as measured by ELISA. Urine from CKD cats had a significantly higher IL-8:UCrR than urine from healthy cats (Mann–Whitney: P < 0.0001)
Figure 2.
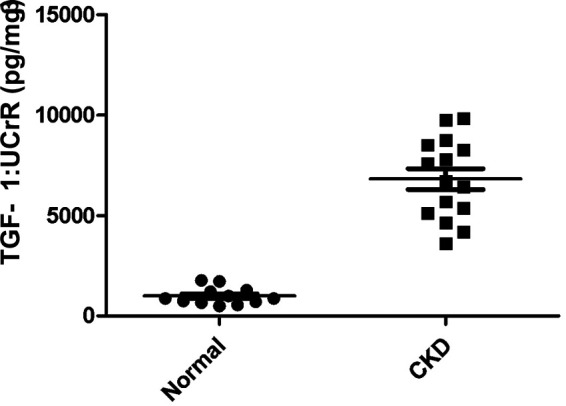
Urinary transforming growth factor (TGF)-β1:urine creatinine ratio (UCrR) of healthy (n = 12) and chronic kidney disease (CKD) cats (n = 15), as measured by ELISA. Urine from CKD cats had a significantly higher TGF-β1:UCrR than urine from apparently healthy cats (Mann–Whitney: P = 0.0002)
Figure 3.
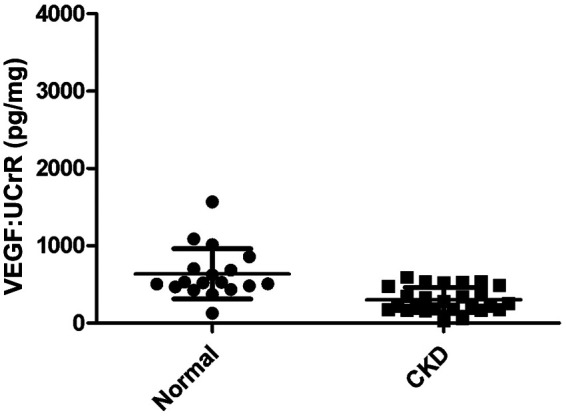
Urinary vascular endothelial growth factor (VEGF):urine creatinine ratio (UCrR) of healthy (n = 18) and chronic kidney disease (CKD) cats (n = 26), as measured by ELISA. Urine from CKD cats had a significantly lower VEGF:UCrR than urine from apparently healthy cats (Mann–Whitney: P = 0.0003)
Figure 4.
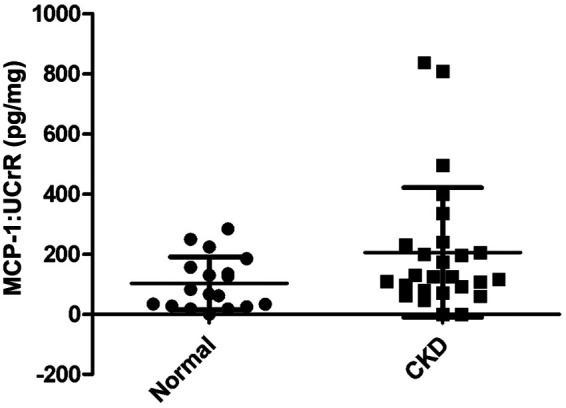
Urinary monocyte chemoattractant protein-1 (MCP-1):urine creatinine ratio (UCrR) of healthy (n =18) and chronic kidney disease (CKD) (n = 26) cats, as measured by ELISA. A significant difference in urinary MCP-1:UCrR levels was not detected between healthy and CKD cats
Urine cytokine to serum creatinine correlations
Pearson correlations were calculated between cytokine: UCrR and serum creatinine for each urinary cytokine analyzed in the CKD cat group. TGF-β1:UCrR was correlated positively with serum creatinine in CKD cats (P = 0.008, r = 0.65) (Figure 5). MCP-1, IL-8 and VEGF were not correlated significantly with serum creatinine concentrations in CKD cats.
Figure 5.
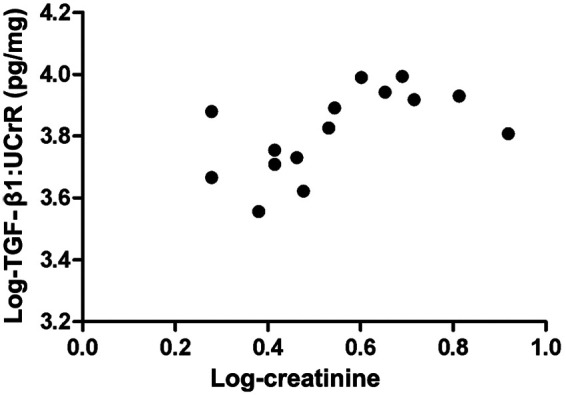
Correlation between urinary transforming growth factor (TGF)-β1 and serum creatinine in cats with chronic kidney disease (CKD). Log-transformed TGF-β1:urine creatinine ratio (UCrR) and serum creatinine concentrations from CKD cats (n = 15) show a significant positive correlation (Pearson correlation: P < 0.008, r = 0.65)
Discussion
The aim of this study was to assess the use of urinary cytokines as a potential indirect method of assessing renal inflammation, fibrosis and vascular health in cats with CKD. Results from this study indicate that cats with CKD have a statistically significant elevation in UCrR of IL-8 and TGF-β1 when compared with healthy cats. CKD cats were also documented to have a statistically significant decrease in urinary levels of VEGF when compared with healthy cats. Urinary TGF-β1 levels were found to correlate positively with serum creatinine concentrations in CKD cats.
Cats with CKD are evaluated using a clinical scoring system that is based on serum creatinine, known as IRIS staging. 8 Although determination of serum creatinine is clinically available widely, it is a relatively insensitive marker of renal function. 24 Glomerular filtration rate (GFR) is a much more sensitive and direct measure of renal function than serum creatinine, but GFR is not as widely available, is expensive to perform and often requires sedation. Consequently, progression of disease and response to therapy is most commonly assessed clinically using serial serum creatinine measurements. Moreover, both GFR and serum creatinine measurements are only a marker of renal function and do not necessarily reflect the underlying disease process present in the kidney. Renal biopsy does allow accurate assessment of the degree of inflammation, fibrosis and vascular compromise within the kidney, but it is not without risk and therefore is not as routinely performed in CKD cats as it is in human medicine. 11 Therefore, the identification of an alternative, non-invasive method of assessing kidney disease in these patients, such as measurement of urinary cytokine levels, is appealing. Abnormal urinary cytokine levels are not a definitive representation of the cytokine concentrations present in the kidney or the degree of pathology present; however, studies in other species have found that the correlation of urinary cytokine levels to changes in renal parenchymal is strong, providing convincing rationale for their use as a non-invasive means of assessing intra-renal inflammation and fibrosis.18,20
The pathophysiology of the progression of CKD is complex and multifactorial.5,6 Regardless of the inciting cause, end-stage kidney disease appears to have similar features, even between species. As renal parenchymal cells become damaged (by various means), they release various inflammatory mediators, which mediate the recruitment of inflammatory cells to the kidney. Varying degrees of tubulointerstitial inflammation are present as a result and these inflammatory cells further exacerbate the situation by releasing additional inflammatory mediators.5–7 Eventually, cells damaged by the original insult or continued inflammation are lost or transdifferentiate into myofibroblasts. During this entire process, extracellular matrix accumulates. Fibrosis is an end result in a process that is critically dependent on TGF-β1 of renal origin. 18 It is thought that this intra-renal environment is significantly pro-inflammatory and pro-fibrotic.5–7 In this study we found that urinary levels of IL-8 and TGF-β1 were elevated in CKD cats. This is clinically significant for two reasons: (i) directing therapies at specific aspects of renal inflammation and fibrosis is an appealing new therapeutic modality and (ii) if urinary cytokine levels do represent pathologic changes in the feline kidney as they do in other species, the ability to serially and non-invasively measure the response to therapy using urinary cytokines will greatly facilitate feline clinical studies.
Researchers have argued recently that hypoxia is a critical component of CKD progression and a key therapeutic target.5,22 Distortion and destruction of peritubular capillary blood supply by extracellular matrix and fibrosis is a characteristic histologic feature of CKD. The resulting hypoxia leads to further tubular cell death and dysfunction. VEGF is integral in this process and it appears that late-stage renal patients may have a compromised VEGF levels. 22 Our findings are consistent with previous studies in this regard, as urinary VEGF levels were decreased in CKD cats. Whether this is a protective mechanism or whether VEGF also functions as a pro-inflammatory cytokine in CKD is unclear. 22 VEGF does, however, appear to have significant value as a prognostic indicator and a marker for response to therapy in humans with CKD.12,22 The availability of such a tool in feline patients would be very valuable.
Indeed, in human medicine, urinary cytokines are used widely in the assessment of prognosis, staging and response to experimental therapies. In one study, low urinary VEGF levels in glomerulonephritis patients improved once therapy was initiated. 12 Patients that had no clinical improvement continued to have decreased VEGF levels. Similarly, urinary TGF-β1 levels were found to be increased in hypertensive patients and these levels decreased once anti-hypertensive therapy was initiated. 13 The detection of statistically significant differences in urinary VEGF and TGF-β1 levels in CKD-affected cats when compared with healthy cats suggests that urinary cytokine measurements may also be a valuable tool in feline patients for assessing response to therapeutic treatments, particularly in a clinical trial setting. Limitations of this study include the possible presence of undetected factors that affected urinary cytokine levels unrelated to renal disease. Although previous studies have shown that urinary cytokine levels in humans appear to correlate with renal parenchymal levels and are thought to be of renal origin,18,25 the possibility that other non-renal systemic disease could influence urinary concentrations cannot be ruled out, as all cytokines analyzed are small enough to be filtered through the glomerulus (http://blast.ncbi.nlm.nih.gov). In humans, urinary IL-8 has been demonstrated to be elevated with active inflammatory intestinal disease, as well as other systemic inflammatory conditons. 26 However, the effect of inflammatory disease on urinary cytokines is likely variable between cytokines and diseases, as another human study demonstrated that urinary MCP-1 and TGF-β1 were specific to lupus nephritis and not affected by the presence of systemic lupus — a noted inflammatory condition. 25 In this study, we attempted to avoid the influence of systemic diseases on urinary cytokine levels by excluding cats with any clinical or physical indicators of systemic inflammatory conditions. Regardless, undiagnosed inflammatory disease could still have existed in some cases. Renal-related effects, such as proteinuria and hypertension, have also been reported to have an effect on urinary cytokine levels in humans and cats, and so, for similar reasons, these cats were excluded from analysis in this study.13,20,23 Additionally, as there was a statistically significant difference between the age of the apparently healthy group and cats with CKD, it is possible that age could have an unknown effect on urinary cytokine levels. Further studies will be necessary to determine if this is a relevant factor. There may also be components in urine (ie, a matrix effect) that may interfere with sample analysis. In this study, we found that sample dilution altered the cytokine measurements, particularly with the MCP-1 ELISA, suggesting the presence of a matrix effect. Other authors have reported a similar issue with urinary IL-6 in dogs. 27 Finally, the correlation between urinary cytokine levels and severity of disease, currently based on serum creatinine measurement, includes the insensitivity inherent in using creatinine levels as an indicator of renal function. 24
Conclusions
Future directions for evaluating the utility of measuring urinary cytokine levels in cats with CKD include definitively correlating urinary cytokine levels with renal parenchymal cytokine levels, and assessing the effect of other systemic or inflammatory illness, lower urinary tract disease, proteinuria, hypertension and age on urinary cytokine levels, as well as conducting longitudinal studies to better understand urinary cytokine levels over time with progression of disease.
Acknowledgments
The authors wish to acknowledge Amber Caress for technical assistance and Dr Jim zumBrunnen for statistical assistance.
Footnotes
Funding: This study was supported by grants from Bohemian Foundation and Frankie’s Fund for Feline Stem Cell Research.
The authors do not have any potential conflicts of interest to declare.
Accepted: 20 August 2012
References
- 1. Lulich JP, O’Brien TD, Osborne CA, Polzin DJ. Feline renal failure:questions, answers, questions. Compend Contin Educ Vet 1992; 14: 127–152. [Google Scholar]
- 2. Boyd LM, Langston C, Thompson K, et al. Survival in cats with naturally occurring chronic kidney disease (2000–2002). J Vet Intern Med 2008; 22: 1111–1117. [DOI] [PubMed] [Google Scholar]
- 3. Lawler DF, Evans RH, Chase K, et al. The aging feline kidney: a model mortality antagonist? J Feline Med Surg 2006; 8: 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DiBartola SP, Rutgers HC, Zack PM, Tarr MJ. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973–1984). J Am Vet Med Assoc 1987; 190: 1196–1202. [PubMed] [Google Scholar]
- 5. Nangaku M, Fujita T. Activation of the renin-angiotensin system and chronic hypoxia of the kidney. Hypertens Res 2008; 31: 175–184. [DOI] [PubMed] [Google Scholar]
- 6. Harris RC, Neilson EG. Toward a unified theory of renal progression. Annu Rev Med 2006; 57: 365–380. [DOI] [PubMed] [Google Scholar]
- 7. Siragy HM, Carey RM. Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. Am J Nephrol 2010; 31: 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elliott J, Watson ADJ. Chronic kidney disease: staging and management. In: Bonagura JD, Twedt DC. (eds). St Louis, MO: Saunders Elsevier, 2009: 883–892. [Google Scholar]
- 9. Plotnick A. Feline chronic renal failure: long-term medical management. Compend Contin Educ Vet 2007; 29: 342–344. [PubMed] [Google Scholar]
- 10. US Renal Data System (USRDS). Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institutes of Diabetes and Digestive and Kidney Diseases, 2010. [Google Scholar]
- 11. Vaden SL, Levine JF, Lees GE, et al. Renal biopsy: a retrospective study of methods and complications in 283 dogs and 65 cats. J Vet Intern Med 2005; 19: 794–801. [DOI] [PubMed] [Google Scholar]
- 12. Honkanen EO, Teppo AM, Gronhagen-Riska C. Decreased urinary excretion of vascular endothelial growth factor in idiopathic membranous glomerulonephritis. Kidney Int 2000; 57: 2343–2349. [DOI] [PubMed] [Google Scholar]
- 13. Scaglione R, Argano C, Corrao S, et al. Transforming growth factor beta1 and additional renoprotective effect of combination ACE inhibitor and angiotensin II receptor blocker in hypertensive subjects with minor renal abnormalities: a 24-week randomized controlled trial. J Hypertens 2005; 23: 657–664. [DOI] [PubMed] [Google Scholar]
- 14. Tam FW, Sanders JS, George A, et al. Urinary monocyte chemoattractant protein-1 (MCP-1) is a marker of active renal vasculitis. Nephrol Dial Transplant 2004; 19: 2761–2768. [DOI] [PubMed] [Google Scholar]
- 15. Sheu JN, Chen SM, Meng MH, Lue KH. The role of serum and urine interleukin-8 on acute pyelonephritis and subsequent renal scarring in children. Pediatr Infect Dis J 2009; 28: 885–890. [DOI] [PubMed] [Google Scholar]
- 16. Branton MH, Kopp JB. TGF-beta and fibrosis. Microbes Infect 1999; 1: 1349–1365. [DOI] [PubMed] [Google Scholar]
- 17. Murakami K, Takemura T, Hino S, Yoshioka K. Urinary transforming growth factor-beta in patients with glomerular diseases. Pediatr Nephrol 1997; 11: 334–336. [DOI] [PubMed] [Google Scholar]
- 18. Bobkova IN, Chebotareva NV, Kozlovskaia LV, et al. Urine excretion of a monocytic chemotaxic protein-1 and a transforming growth factor beta 1 as an indicator of chronic glomerulonephritis progression. Ter Arkh 2006; 78: 9–14. [PubMed] [Google Scholar]
- 19. Arata S, Ohmi A, Mizukoshi F, et al. Urinary transforming growth factor beta 1 in feline chronic renal failure. J Vet Med Sci 2005; 67: 1253–1255. [DOI] [PubMed] [Google Scholar]
- 20. Eardley KS, Zehnder D, Quinkler M, et al. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int 2006; 69: 1189–1197. [DOI] [PubMed] [Google Scholar]
- 21. Bobkova IN, Kozlovskaia LV, Rameeva AS, et al. Clinical implication of urine test for markers of endothelial dysfunction and angiogenesis factors in assessment of tubulointerstitial fibrosis in chronic glomerulonephritis. Ter Arkh 2007; 79: 10–15. [PubMed] [Google Scholar]
- 22. Mayer G. Capillary rarefaction, hypoxia, VEGF and angiogenesis in chronic renal disease. Nephrol Dial Transplant 2011; 26: 1132–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chakrabarti S, Syme HM, Elliott J. Urinary vascular endothelial growth factor as a marker of renal hypoxia in cats. J Vet Int Med 2011; 25: 720. [Google Scholar]
- 24. Kerl ME, Cook CR. Glomerular filtration rate and renal scintigraphy. Clin Tech Small Anim Pract 2005; 20: 31–38. [DOI] [PubMed] [Google Scholar]
- 25. Torabinejad S, Mardani R, Habibagahi J, et al. Urinary monocyte chemotactic protein-1 and transforming growth factor-β in systemic lupus erythematosus. Indian J Nephrol 2012; 22: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taha AS, Grant V, Kelly RW. Urinalysis for interleukin-8 in the non-invasive diagnosis of acute and chronic inflammatory diseases. Postgrad Med J 2003; 79: 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wood M, Breitschwerdt E, Vaden SL, Nordone S. Immunologic testing of dog urine requires neutralization of matrix effects to allow for accurate measurement of IL-6 concentrations. J Vet Int Med 2010; 24: 770. [Google Scholar]


