Abstract
Four cats with feline gastrointestinal eosinophilic sclerosing fibroplasia (FGESF) are described. Clinical signs included decreased appetite, weight loss, vomiting and diarrhea. Bloodwork abnormalities included mild neutrophilia (n = 2) and hyperglobulinemia with concurrent hyperproteinemia (n = 2). Ultrasonographically, a total of five solitary masses with mural thickening and loss of layering were identified in the stomach, duodenum, jejunum and colon. In one cat a second, separate lesion was diagnosed 3 weeks following surgical resection of one mass. Histopathologically, lesions were characterized by collagen trabeculae and mixed inflammatory cell infiltrates, predominantly eosinophils. Multiple areas of necrosis were also noted, which contained bacteria in 2/4 cats. In two cats, changes consistent with FGESF were also noted in the liver. All cats had surgical resection of their lesions. Two cats are still living at time of publication (43 and 24 months post-surgery). FGESF should be considered as a differential for intestinal masses in cats.
Introduction
Feline gastrointestinal eosinophilic sclerosing fibroplasia (FGESF) is a newly proposed term for a recently classified nodular, non-neoplastic, densely fibroproliferative, eosinophil and mast cell replete inflammatory response in cats. 1 The acronym FGESF is preferred over FIESF to emphasize the potential gastric involvement in this disease process. 2
The pathologic appearance of FGESF has been described.1 –3 Grossly, it appears commonly as an ulcerated mural mass with a necrotic center.1,3 Histologically, FGESF lesions exhibit a characteristic branching, trabecular pattern of dense sclerotic collagen separated by large populations of spindle cells. The lesions contain a mixed population of inflammatory cells with a predominance of eosinophils and mast cells, and fewer neutrophils, lymphocytes and plasma cells.1,3 Both grossly and histologically, the appearance of the lesion can mimic neoplasia. The highly sclerotic collagen trabeculae in these lesions resembles osteoid and these lesions can be mistaken for osteosarcoma. 1 Furthermore, the presence of large numbers of mast cells can lead to a diagnosis of sclerosing mast cell tumor; one of the discriminating factors is the large population of fibroblasts identified in FGESF lesions.4–6 Although these fibroblasts have been identified by one group as neoplastic mast cells with a spindle morphology, 4 most pathologists agree that they are not mast cells or neoplastic,1,2,5,6 which coincides with the long survival time in most cats.1,2
Intracellular bacteria have been found in 56% of the cats with gastrointestinal eosinophilic sclerosing fibroplasia. 1 Most frequently, Gram-positive methicillin-resistant Staphylococcus aureus has been cultured. 3 Regional lymphadenopathy is another common finding with these lesions. Microscopic evaluation of these lymph nodes reveals eosinophilic inflammation and/or fibrosis.1,3
The pathogenesis of feline gastrointestinal eosinophilic sclerosing fibroplasia lesions is unknown. Several mechanisms, such as penetrating wounds from a migrating foreign body, genetic eosinophil dysregulation, herpesvirus infection and food hypersensitivity have been proposed. 1
Thus far, there is minimal data that details clinicopathological or diagnostic imaging findings associated with FGESF. 2 The goals of this article are to report the imaging and clinical features seen in four cats with a confirmed diagnosis of FGESF.
Case histories
Cat 1
An 11-year-old male cat presented with a 1-year history of decreased appetite, constipation, weight loss and vomiting. At the time of presentation, the patient was being treated for presumptive inflammatory bowel disease with oral amoxicillin/clavulonic acid (Clavamox; Pfizer) (12.5 mg/kg q12h), oral prednisone (0.5 mg/kg q24h) and a hypoallergenic diet (z/d; Hill’s, HP23; Purina). A complete blood count (CBC) and blood chemistry were unremarkable (Table 1). Thoracic radiographs were within normal limits. On abdominal radiographs, there was segmental intestinal dilation and a fluid and gas distended stomach, suggestive of mechanical obstruction. On abdominal ultrasound, the jejunum had a 2 cm, focal, poorly echogenic circumferential wall thickening (0.5 cm) with luminal narrowing and abnormal wall layering. Focal distension of small bowel was present oral to the thickening, indicating a partial obstruction. A jejunal lymph node was mildly enlarged. Abdominal exploratory surgery revealed a 3 cm segment of thickened jejunum with reduced lumen and distended small intestine oral to the stricture. Resection and anastomosis at the jejunal site was performed. Histopathology confirmed FGESF of the intestine and lymph node follicular hyperplasia.
Table 1.
Blood chemistry and complete blood count findings at presentation
| Cat 1 | Cat 2 | Cat 3 | Cat 4 | Reference interval | |
|---|---|---|---|---|---|
| Albumin | 3.6 g/dl | 1.9 g/dl | 3.3 g/dl | N/a | 2.2–4.3 g/dl |
| Globulin | 3.6 g/dl | 9.3 g/dl | 6.1 g/dl | 4.0 | 2.6–5.8 g/dl |
| Total protein | 7.2 g/dl | 11.2 g/dl | 9.4 g/dl | N/a | 6.3–8.5 g/dl |
| ALP | 33 U/l | 5 U/l | 158 U/l | 28 | 10–96 U/l |
| ALT | 43 U/l | 14 U/l | 1010 U/l | 69 | 25–186 U/l |
| AST | 20 U/l | 25 U/l | 379 U/l | 23 | 5–46 U/l |
| Total bilirubin | 0.3 mg/dl | 0.1 mg/dl | 2.3 mg/dl | N/a | 0.0–0.4 mg/dl |
| WBC | 7.1 × 103/µl | 18.4 × 103/µl | 16.8 × 103/µl | 5.7 × 103/µl | 4.7–19.6 × 103/µl |
| Neutrophils | 4.3 × 103/µl | 15.0 × 103/µl | 13.6 × 103/µl | 4.0 × 103/µl | 2.0–13.4 × 103/µl |
| Lymphocytes | 1.1 × 103/µl | 1.1 × 103/µl | 0.7 × 103/µl | 1.4 × 103/µl | 1.1–8.0 × 103/µl |
| Monocytes | 0.5 × 103/µl | 0.2 × 103/µl | 0.3 × 103/µl | 0.2 × 103/µl | 0.1–1.0 × 103/µl |
| Eosinophils | 1.1 × 103/µl | 1.1 × 103/µl | 2.2 × 103/µl | 0.4 × 103/µl | 0.2–1.7 × 103/µl |
| Basophils | 0.2 × 103/µl | 0.2 × 103/µl | N/a | 0.1 × 103/µl | 0.0–1.0 × 103/µl |
| Intralesional bacteria | No | Yes | Yes | No | – |
ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, T bili = total bilirubin; WBC = white blood cells
Histologically, the lesion was characterized by multiple areas of epithelial necrosis with mixed inflammatory cell infiltrates, predominated by eosinophils throughout the submucosal and muscular layers. Additionally, there were fibroblasts interspersed within sclerotic collagen trabeculae. No infectious organisms were identified on hematoxylin and eosin (H&E) stains.
Antibiotics and the hypoallergenic diet were discontinued, and oral prednisone (1 mg/kg PO q24h) was restarted 2 weeks later. Three weeks after surgery, the patient presented for lethargy and inappetence. A CBC showed mild eosinophilia (1.81 × 103/µl, 15.5%) with a normal total white blood cell count. Blood chemistry was normal. Abdominal ultrasound identified the cat’s previous surgery site, which was within normal range for a recent postoperative enterectomy site. 7 However, a focal area of small intestinal thickening (1.1 cm) with altered layering and a mildly enlarged jejunal lymph node (0.5 cm) was identified at a different site. Fine needle aspirates of the intestinal thickening performed under ultrasound guidance were of low cellularity and non-diagnostic. The prednisone was continued at the same dose (1 mg/kg PO q24h) and the patient was also sent home with a 5-day course of fenbendazole granules (Panacur; Intervet) (100 mg/kg PO q24h).
Two months after surgery, the cat exhibited no clinical signs and CBC and blood chemistry were normal. No ultrasound was performed at that time. Four months after surgery, the previously noted resection and anastomosis site, and small intestinal thickening were no longer identified on ultrasound, but a 1.2 × 0.8 cm echogenic lesion with loss of layering was noted within the gastric wall. A fine needle aspirate of the gastric mass was consistent with eosinophilic inflammation. The prednisone was continued at the same dose.
Seven months after surgery, the patient represented for a 3-week history of weight loss, inappetence, vomiting and hematemesis. A CBC was unremarkable and a complete blood chemistry and abdominal radiographs were within normal limits. On abdominal ultrasound, the gastric mass had increased in size (2.1 cm × 1.3 cm) and a central focal hyperechoic lesion was noted within the mass. In addition, the irregular mucosal margin and the concave surface of the mass were suggestive of extensive ulceration. The jejunal lymph nodes were heterogeneous and mildly enlarged (0.6 cm).
The cat underwent a second surgery and two 3–4 cm hemorrhagic ulcerated masses that deformed the gastric serosal surface were identified — one near the cardia, the other in the fundus. Additionally, multiple 2–3 cm irregular firm lesions were present along the jejunal mesenteric border, proximal ascending colon and ileocolic junction. Owing to the extent of the disease, the owners elected euthanasia. Histopathology of the gastric masses was similar to the previously resected jejunal mass and consistent with FGESF. The fundic mass and jejunal lesions identified at the second surgery also contained aggregates of round cells consistent with lymphosarcoma. No bacterial organisms were identified on H&E stains and no further diagnostics were performed.
Cat 2
A 5-year-old male cat presented with a 3-day history of large bowel diarrhea and a chronic history of vomiting daily. A CBC showed a mild neutrophilia with a normal white blood cell count (Table 1). The patient’s blood chemistry showed mild hypoalbuminemia, hyperglobulinemia and hyperproteinemia (Table 1). Thoracic radiographs were within normal limits. On abdominal ultrasound, the colon was fluid-filled and a 6 cm mixed echogenicity mass of was associated with the proximal descending colonic wall (Figure 1). There were several hyperechoic areas present within the mass and one small (0.5 cm in diameter) round anechoic area. The colonic wall adjacent to the mass was mildly thickened with loss of layering. The fat along the descending colon was increased in echogenicity and had several small and poorly echogenic nodules at that location. Fine needle aspirates of the colonic mass showed mixed cell inflammation, predominantly made up of vacuolated macrophages, with lesser numbers of eosinophils, neutrophils and lymphocytes. Increased numbers of atypical plasma cells were also identified. Ultrasound-guided Tru-cut colonic biopsies were performed, which revealed multifocal histiocytic inflammation with bacteria and round cell proliferation, suspicious for a mast cell tumor. Abdominal exploratory revealed a large colonic mass (Figure 2) with enlarged lymph nodes at the root of the mesentery; an end-to-end resection and anastomosis and lymph node biopsy was performed.
Figure 1.
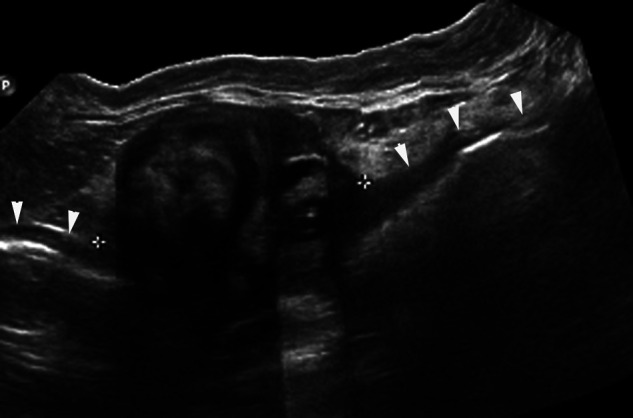
Panoramic ultrasonographic image (cat 2). In the longitudinal plane, a mixed echogenic mass (calipers) is present within the proximal descending colon. Several ill-defined hyperechoic and hypoechoic regions are identified within the left aspect of the mass. The right aspect of the mass has a well-defined circular region with well-defined regions of hyper- and hypoechogenicity. The adjacent ventral colonic wall is outlined by arrowheads and is irregularly thickened
Figure 2.
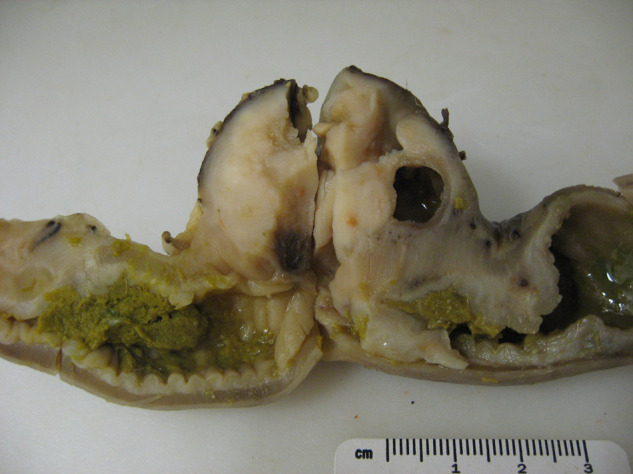
Gross pathologic cut-section of colonic mass which correlates to the ultrasound image in Figure 1 (cat 2). A focal, well-encapsulated, eccentric mural mass is present within the colon. The well-defined cavitation in the right side of the mass correlates with the circular region containing well-defined areas of hyper- and hypoechogenicity in the ultrasound image (Figure 1), and histologically represents an area of necrosis. There is focal narrowing of the colonic lumen secondary to the mass and there is thickening of the adjacent colonic wall
Histologically, the mass contained extensive necrosis and inflammation eosinophils, neutrophils, macrophages and mast cells, with colonies of bacterial cocci. There were also trabeculae of dense collagen separated by fibroplasia, which expanded the colonic wall.
Based on the nomenclature that was current at the time, the biopsies were interpreted initially as mast cell neoplasia. Based on this diagnosis, the cat was started on single agent oral therapy Lomustine (CCNU, 60 mg/m2 PO q6 weeks). The cat received one dose, after which the owner discontinued treatment. After the entity of FGESF was described, 1 the case was reclassified as eosinophilic sclerosing fibroplasia as it more appropriately described the histologically appearance of the mass. No additional therapy was instituted at that time.
Two months after surgery, a CBC was normal. On abdominal ultrasound, mild focal thickening of the wall was noted at the surgical site, which is considered within normal limits. One jejunal lymph node was mildly enlarged (0.9 cm). Six months after surgery, the cat had no gastrointestinal clinical signs and the intestinal surgical site was unchanged. At that time, blood chemistry, including albumin, globulin and total proteins was within normal limits. At the time of writing this article (43 months post-surgery), there has been no evidence of recurrence of the colonic mass on ultrasound.
Cat 3
A 7-year-old neutered male cat presented with occasional vomiting and retching. On physical examination, the cat was icteric and slightly thin. A CBC showed mild eosinophilia, neutrophilia and lymphopenia, with a normal white blood cell count (Table 1). On a blood chemistry panel there were elevated liver enzymes, including alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and serum bilirubinemia with mild hyperproteinemia and hyperglobinulinemia (Table 1). Thoracic radiographs were within normal limits. Abdominal radiographs revealed diffuse hepatomegaly with rounded liver lobe margins. On abdominal ultrasound there was gallbladder distension with intrahepatic duct dilation, suggestive of chronic extrahepatic biliary tract obstruction. The descending duodenum was thickened with loss of layering and contained an echogenic intraluminal mass. A fine-needle aspirate of this mass was of poor cellularity and was non-diagnostic. Surgery revealed a 4 cm diameter mural duodenal mass that extended to the common bile duct at the level of the duodenal papilla. The location precluded complete resection and the owners elected humane euthanasia.
The duodenum, common bile duct and pylorus all exhibited histologic change consistent with FGESF: focally thickened walls with expansion of the submucosa that progressed to transmural effacement with mucosal ulceration and necrosis. Regions of necrosis contained many eosinophils and fewer neutrophils occasionally surrounded clusters of bacterial cocci. Fewer mast cells, lymphocytes and plasma cells were also present. Some areas contained densely packed fibroblasts next to branching sclerotic collagen trabeculae.
Cat 4
A 9-year-old female spayed cat presented with a 3-month history of constipation and vomiting. On physical examination, thickened intestinal loops were palpated. A CBC showed a normal white blood cell count (Table 1). Blood chemistry was unremarkable (Table 1). Abdominal ultrasound revealed two discrete hyperechoic nodules (1.2 and 1.6 cm in diameter) with a thin hypoechoic rim in the right lateral aspect of the liver. Several jejunal segments and part of the descending colon were thickened (up to 5.6 mm), especially the muscularis layer. One jejunal segment was associated with an amorphous, poorly echogenic mass with hyperechoic regions that extended over 2.5 cm in length (thickness over 1 cm) and was contiguous to the asymmetrically thickened intestinal muscular layer (Figure 3).
Figure 3.
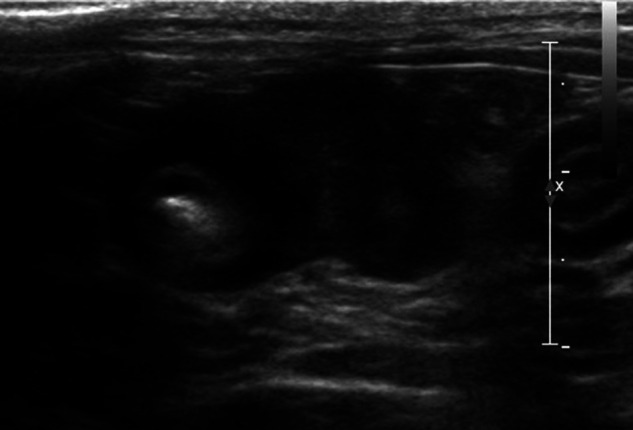
Ultrasonographic image (cat 4). Transverse sonogram of a thickened jejunal segment with an eccentric, inhomogeneous mural mass measuring 1 cm in thickness. Note the marked muscular layer thickening of the affected intestinal segment, as well as the adjacent intestinal loop (to the right of the calipers)
Ultrasound-guided biopsies and fine needle aspirates were performed of the intestinal mass, thickened muscular layer and multiple jejunal segments, all of which were consistent with eosinophilic inflammation and fibrosis. No treatment was initiated at that time.
Repeat abdominal ultrasound 5 months later showed progressive enlargement of the asymmetrical jejunal mass with slightly progressive thickening of the muscular layer. The entire liver and the liver nodules were also mildly enlarged.
At surgery, a 4-cm mid-jejunal segment was resected and the right lateral liver lobe removed. Both samples, along with a biopsy of a jejunal lymph node were submitted for histopathology. On microscopic evaluation, the jejunum and the liver were effaced and expanded by anastomosing trabeculae and bundles of dense collagen lined by fibroblasts and separated by a dense population of larger spindle cells (Figures 4 and 5). Moderate numbers of eosinophils, mixed with fewer neutrophils, lymphocytes and plasma cells were present, and dispersed infiltrates of mast cells (as opposed to sheets). The jejunal lymph node was within normal limits. No intra-lesional bacteria were reported. The jejunal mass and liver lesions were consistent with FGESF. At the time of writing this article (24 months post-surgery), the cat was still doing well.
Figure 4.
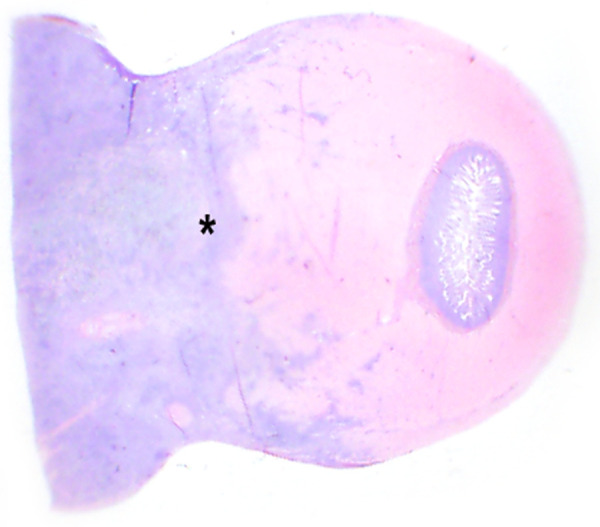
Subgross photomicrograph of a surgical biopsy of a jejunal mass (cat 4). The poorly demarcated, dense, basophilic mass (at left) regionally disrupts and effaces the intestinal wall (asterisk)
Figure 5.
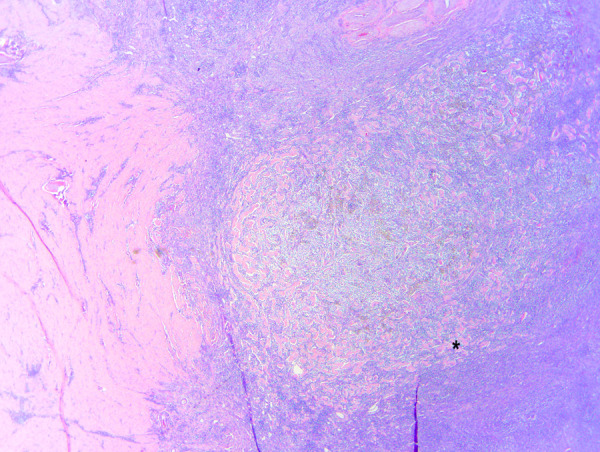
Photomicrograph of a surgical biopsy of a jejunal mass (cat 4) (10×). The mass infiltrates intestinal smooth muscle at left. The mass is densely cellular and basophilic, and contains thick, sclerotic collagen trabeculae (asterisk)
Discussion
In this small case series, FGESF appeared ultrasonographically as a focal mass with loss of layering in four cats. The lesions exhibited mixed echogenicity with hyperechoic regions probably corresponding to the fibrotic zones described histopathologically. Mild regional lymphadenopathy was present in all cats.
Despite its gross and ultrasonographic appearance, which can mimic neoplasia, it has been proposed that eosinophilic sclerosing fibroplasia is inflammatory in origin based on its behavior and histological appearance.1,2 To ensure diagnostic consistency, biopsy specimens were reviewed by two pathologists, one of whom (LC) is the author of the original article detailing the unique entity of FGESF. 1 On ultrasound, FGESF lesions appear as a mass that distorts the wall architecture, which is usually supportive of a neoplastic process.8–11 However, severe granulomatous diseases have been reported to share similar findings.12–14 Focal wall thickening with loss of layering is most commonly observed with neoplasia and these features have been described in most of the common neoplasias affecting the gastrointestinal tract in cats — lymphosarcoma, adenocarcinoma and mast cell tumors.8,10,11,15 Intestinal mast cell disease commonly involves the small intestines and cats may exhibit non-circumferential, eccentric wall thickening with altered or loss of intestinal layering.11,16 Occasionally, mast cell disease can also cause diffuse thickening of the intestinal tract. 16 Like eosinophilic sclerosing fibroplasia, mast cell disease has occasionally been associated with peripheral eosinophilia. 17 Similar to mast cell disease, lymphosarcoma may also present as either nodular or diffuse disease, with transmural thickening associated with altered to absent layering.8,15 The most common solitary site is the ileocolic junction.8,15 In contrast to round cell tumors, adenocarcinoma often appears as a focal (ring-like) lesion with circumferential bowel wall thickening with loss of layers and subsequent mechanical ileus. 10 As the ultrasonographic appearance of neoplasia and FGESF overlap, FGESF cannot be diagnosed based on ultrasound alone. The only particular feature that was present in the cats of this series is the presence of hyperechoic areas, likely representing fibrotic regions which are not usually seen in neoplasia.
Our study population consisted of three adult male (3/4) cats that presented with gastrointestinal signs; these findings are similar to those reported previously.1–3 Peripheral eosinophilia was only noted in 1/4 cats (Table 1), which is less frequent than reported previously (58%). 1 Neutrophilia (2/4), lymphopenia (1/4), hyperproteinemia (2/3) and hyperglobulinemia (2/4) were also noted.
In our series ultrasound-guided fine needle aspirates of the masses and lymph nodes were not diagnostic, which is similar to a previous study. 1 Though ultrasound-guided fine needle aspirates were not useful in this small case series, ultrasound-guided core biopsies might be a useful procedure to further define this lesion.
Bacteria were identified in two cats in this study (Table 1); this is consistent with a single case report 2 and a previous case series in which bacteria were found in 56% of lesions. In the same article, the incidence of bacteria rose to 100% in lesions that were distal to the ileocecocolic junction. 1 In another study describing eosinophilic infiltrative lesions in 27 cats, intralesional bacteria (Gram-positive cocci) were identified in all the cats. 3 Whether these bacteria are a cause or consequence of the lesion is unknown. Additional stains, such as Gram stains, and molecular techniques, such as fluorescence in situ hybridization (FISH), may help to reliably identify bacteria, as well as elucidate their role in FGESF lesions.
The prognosis of gastrointestinal eosinophilic sclerosing fibroplasia is variable and, in light of previous articles, lesion location and treatment may play a significant role in outcome. 1 It has been found that 67% of cats alive at the time of publication had ileocecocolic or colonic masses compared with 33% that had small intestinal lesions. 1 Cats treated with antibiotics and surgery, or surgery alone had a significantly shorter survival time than cats that were treated with surgery and prednisone. 1 Of the cats that were still living, mean survival time was 9.4 months with a range of 1 month to 2 years. 1 In a single case report, the cat was doing well 9 months post-surgery. 2 In our study, the survival time is 7 months, 43 months, 1 month and 24 months, respectively. Two cats are still alive at time of publication (43 and 24 months). Cat 3 had a non-resectable proximal duodenal mass and, subsequently, had the shortest survival time. Cat 1 underwent surgery and had been treated with prednisone both before and after diagnosis, and had a survival time of 7 months, though the concurrent diagnosis of lymphosarcoma limits the conclusions that can be drawn from this case. It is unknown whether FGESF predisposed cat 1 to lymphoma or if they are unrelated. However, in the two case series,1,4 none of the cats had concurrent lymphoma, suggesting that, if present, the association may be rare. Ancillary diagnostics, such as immunohistochemistry or polymerase chain reaction for antigen receptor rearrangement (PARR), were not performed. However, the FGESF lesion in cat 1 is histologically identical to the 25 and 50 feline case series.1,4
Local spread of disease may have an effect on the ability to resect disease at surgery and, ultimately, may negatively impact survival rates. Cat 3 had spread of FGESF into adjacent organs and was euthanased at surgery. Cat 4 had focal disease in the right liver lobe and is still doing well 24 months post-surgery. However, multicentric FGESF has not been reported previously and its effect on survival data is not available.
Conclusions
Feline eosinophilic sclerosing fibroplasia shares ultrasonographic features with neoplasia and no clinicopathologic features of FGESF are unique to confidently differentiate it as a separate disease entity. Therefore, we recommend this newly described entity be added to a list of differential diagnoses in cats with focal and inhomogeneous intestinal masses, especially if fine-needle aspirates or biopsies reveal the presence of eosinophilic inflammation, mast cells and fibroblasts. Based on our results and the location of the lesions, surgical biopsy and/or resection of the mass is recommended, as FGESF may carry a good prognosis.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case series.
The authors do not have any potential conflicts of interest to declare.
Accepted: 18 September 2012
References
- 1. Craig L, Hardam E, Hertzke D, Flatland B, Rohrbach B, Moore R. Feline gastrointestinal eosinophilic sclerosing fibroplasia. Vet Pathol 2009; 46: 63–70. [DOI] [PubMed] [Google Scholar]
- 2. Sihvo H, Outi S, Vainionpaa M. Pathology in practice. Severe chronic multifocal intramural fibrosing and eosinophilic enteritis, with occasional intralesion bacteria, consistent with feline gastrointestinal eosinophilic sclerosing fibroplasia (FIESF). J Am Vet Med Assoc 2011; 238: 585–587. [DOI] [PubMed] [Google Scholar]
- 3. Ozaki K, Yamagami T, Nomura K, Haritani M, Tsutsumi Y, Narama I. Abscess-forming inflammatory granulation tissue with Gram-positive cocci and prominent eosinophil infiltration in cats: possible infection of methicillin-resistant Staphylococcus. Vet Pathol 2003; 40: 283–287. [DOI] [PubMed] [Google Scholar]
- 4. Halsey CHC, Powers BE, Kamstock DA. Feline intestinal sclerosing mast cell tumour: 50 cases (1997–2008). Vet Comp Oncol 2010; 8: 72–79. [DOI] [PubMed] [Google Scholar]
- 5. Gamble D. Feline intestinal sclerosing mast cell tumour: 50 cases (1997–2008) 2010; 8: 72–79’by CHC Halsey, BE Powers and DA Kamstock Letter to the editor# 2. [Letter, rebuttal] Vet Comp Oncol 2010; 8: 235–236.20691031 [Google Scholar]
- 6. Schulman F, Lipscomb T. Feline intestinal sclerosing mast cell tumour: 50 cases (1997–2008) [Letter] Vet Comp Oncol 2010; 8: 234–235. [DOI] [PubMed] [Google Scholar]
- 7. Matthews AR, Penninck DG, Webster CR. Postoperative ultrasonographic appearance of uncomplicated enterotomy or enterectomy sites in dogs. Vet Radiol Ultrasound 2008; 49: 477–483. [DOI] [PubMed] [Google Scholar]
- 8. Grooters A, Biller D, Ward H, Miyabayashi TGC. Ultrasonographic appearance of feline alimentary lymphoma. Vet Radiol Ultrasound 1994; 35: 468–472. [Google Scholar]
- 9. Penninck D, Smyers B, Webster CR, Rand W, Moore AS. Diagnostic value of ultrasonography in differentiating enteritis from intestinal neoplasia in dogs. Vet Radiol Ultrasound 2003; 44: 570–575. [DOI] [PubMed] [Google Scholar]
- 10. Rivers BJ, Walter PA, Feeney DA, Johnston GR. Ultrasonographic features of intestinal adenocarcinoma in five cats. Vet Radiol Ultrasound 1997; 38: 300–306. [DOI] [PubMed] [Google Scholar]
- 11. Sato AF, Solano M. Ultrasonographic findings in abdominal mast cell disease: a retrospective study of 19 patients. Vet Radiol Ultrasound 2004; 45: 51–57. [DOI] [PubMed] [Google Scholar]
- 12. Graham JP, Newell SM, Roberts GD, Lester NV. Ultrasonographic features of canine gastrointestinal pythiosis. Vet Radiol Ultrasound 2000; 41: 273–277. [DOI] [PubMed] [Google Scholar]
- 13. Penninck D, d’Anjou M-A. Gastrointestinal tract. In: Penninck D, d’Anjou M-A. (eds). Atlas of Small animal ultrasonography. Ames: Blackwell, 2008, pp 281–318. [Google Scholar]
- 14. Penninck D, Mitchell SL. Ultrasonographic detection of ingested and perforating wooden foreign bodies in four dogs. J Am Vet Med Assoc 2003; 223: 206–209. [DOI] [PubMed] [Google Scholar]
- 15. Penninck D, Moore AS, Tidwell AS, Matz M, Freden G. Ultrasonography of alimentary lymphosarcoma in the cat. Vet Radiol Ultrasound 1994; 35: 299–304. [Google Scholar]
- 16. Laurenson M, Skorupski K, Moore P, Zwingenberger A. Ultrasonography of intestinal mast cell tumors in the cat. Vet Radiol Ultrasound 2011; 52: 330–334. [DOI] [PubMed] [Google Scholar]
- 17. Bortnowski H, Rosenthal R. Gastrointestinal mast cell tumors and eosinophilia in two cats. J Am Anim Hosp Assoc 1992; 28: 271–275. [Google Scholar]


