Abstract
Previous publications on ischaemic myelopathy in cats are limited to single case reports and small case series. The overall prognosis appears poor, with 42% of cats being euthanased. In this study the clinical outcome of 19 cats with a presumptive diagnosis of ischaemic myelopathy [based on clinical and magnetic resonance imaging (MRI) findings] was evaluated retrospectively. The degree of neurological dysfunction at the time of presentation was similar to previously reported cases, ranging from ambulatory paresis to plegia with intact nociception. The most common lesion localisations (based on MRI) were to the C1–C5 (30%) and C6–T2 (30%) spinal cord segments, with the T3–L3 and L4–S1 spinal cord segments accounting for 25% and 15%, respectively. Potential inciting or predisposing causes for development of spinal infarction were identified in 12 cats, including physical exertion, trauma, general anaesthesia, renal disease, hyperthyroidism, hypertension and hypertrophic cardiomyopathy. The median time to recovery of ambulation was 3.5 days (3–19 days). Four cats (21%) were euthanased within 2 months of diagnosis. The remaining 15 (79%) cats had a favourable outcome. Follow-up ranged from 6 months to 10 years and 4 months, with a median of 3 years and 1 month. Even when plegia was present at the time of presentation, all surviving cats with long-term, owner-derived follow-up were reported to return to a normal quality of life, suggesting that the long-term prognosis for recovery from presumed ischaemic myelopathy is favourable in the majority of cats.
Introduction
Ischaemic myelopathy (IM) results from obstruction of the spinal vasculature and subsequent ischaemic necrosis of dependent regions of spinal cord parenchyma. 1 Fibrocartilaginous material histologically and histochemically identical to the nucleus pulposus of the intervertebral disc has been reported as the most common cause of IM in dogs and cats. Therefore, this condition has been referred to as fibrocartilaginous embolic myelopathy (FCEM). However, IM may also result from embolisation of material other than fibrocartilage, including thrombi or bacterial, parasitic, neoplastic or fat emboli. 1 In addition, underlying medical conditions that may predispose to embolisation or thrombosis, including cardiomyopathy, chronic renal failure, hypertension and hyperadrenocorticism, should be considered and investigated in animals with spinal cord or cerebral infarction.1,2
The clinical presentation of IM is typically characterised by peracute (less than 6 h) to acute (7–24 h) onset of non-progressive and non-painful (after the first 24–48 h) myelopathy that is often asymmetric. However, progression of neurological signs for up to 5 days has been reported in a cat with histologically-confirmed FCEM. This was hypothesised to result from progressive ischaemia and inflammation. 3 Definitive diagnosis of IM requires histopathological confirmation; however, a presumptive antemortem diagnosis is based on typical clinical presentation, exclusion of other aetiologies and magnetic resonance imaging (MRI) findings suggestive of IM. 1
MRI features of IM have been described in humans, dogs and cats,4–7 and include a well-demarcated focal intramedullary lesion that is hyperintense to normal grey matter on T2-weighted FSE images and iso- to hypointense on T1-weighted FSE images. Lesion enhancement following intravenous administration of a contrast medium is inconsistent and is typically seen 5–7 days following the ischaemic event. Lesions predominantly affect the spinal cord grey matter and are often lateralised. Patients imaged in the first 72 h following the ischaemic event may have no evidence of abnormal parenchymal signal intensity on conventional MRI.4,8
Cerebrospinal fluid (CSF) analysis may be normal or reveal non-specific abnormalities, including xanthochromia, mild-to-moderate pleocytosis (7–84 white blood cells/µl) and elevated protein concentration (0.5–2.39 g/l) in dogs with IM.1,4 In previously reported feline cases of IM, CSF pleocytosis has been reported to be neutrophilic, with a reported median nucleated cell count of 23 cells/µl (range 10–495 cells/µl) and elevated total protein concentrations (range 0.35–1.93 g/l; median 1.34 g/l).3,9,10
Treatment is primarily based on physiotherapy and supportive care. While IM and its clinical course have been well described in large studies in dogs, only case reports and small case series have been published in cats. In dogs, a successful outcome has been reported in 58% 11 to 84% 12 of cases, with an improved outcome noted in the more recent studies. In dogs, the mean and median times for recovery of unassisted ambulation have been reported to be similar at 12 days 13 and 11 days, 12 respectively. However, despite the relatively high recovery rate reported in recent studies in dogs, by combining the results of feline publications 8/19 cats (42%) with IM were euthanased, suggesting a poor outcome in this species.3,7,9,10,14–18
To our knowledge, there is no large case series evaluating the clinical outcome of cats with a presumptive diagnosis of IM based on clinical presentation and high-field MRI. The objective of this study was to evaluate the clinical outcome of presumptive IM in cats retrospectively.
Materials and methods
The medical databases at two UK referral veterinary hospitals were searched to identify cats presumptively diagnosed with IM (based on the history and clinical, MRI and, when available, CSF analysis findings consistent with those described previously in animals with IM1,3–6,9,10,13) between 1 January 2000 and 31 December 2011. Inclusion criteria were a history of peracute (6 h) or acute (<24 h) onset myelopathy, 1.5 Tesla MRI of the spine performed within 10 days of onset, complete medical records and long-term follow-up information of a minimum of 6 months in surviving cats. Cases were excluded if decreased nucleus pulposus volume or evidence of loss of annulus fibrosus integrity were evident in the intervertebral disc ventral to the intramedullary lesion.
The following information was retrieved from the medical records: breed, age, sex, time from onset of clinical signs to presentation, presence of pre-existing medical conditions, a potential inciting cause (if known), progression of clinical signs prior to presentation, presence of spinal hyperaesthesia at the time of presentation and clinical neurolocalisation. Based on the findings of neurological examinations performed at the time of presentation to the referral centre, cats were assigned a neurological grade of 1 (normal), 2 (ambulatory paresis), 3 (non-ambulatory paresis), 4 (plegic with intact nociception) or 5 (plegic with absent nociception). The findings of adjunctive clinical pathology investigations were recorded.
MR images were obtained using 1.5 Tesla MRI scanners (1.5 Tesla GE Signa echospeed and Phillips NT Intera). T2-weighted fast spin echo (FSE) images were acquired in sagittal and transverse planes in all cats. T1-weighted FSE, contrast-enhanced [Gadolinium: Omniscan (GE Healthcare), MultiHance (Bracco) or Gadovist (Bayer)] T1-weighted FSE and T2* gradient echo (GRE) images were obtained at the discretion of the radiologist. All images were reviewed by a board-certified radiologist.
In order to ascertain the imaging extent of the spinal cord lesions MR images were evaluated on a computer with proprietary software (ClearCanvas). The longitudinal (length of the lesion as a ratio to the length of C6 or L2 vertebral body) and transverse [percentage of the cross-sectional area (CSA) of the spinal cord] extents of each lesion were measured as described previously. 4 The mean, standard deviation and median measurements were determined for each of these values.
Follow-up information was obtained from the medical records, owners and referring veterinary surgeons (where owners could no longer be contacted) by means of a telephone questionnaire. A neurological re-evaluation was offered to owners of surviving cats.
The date of diagnosis was used as entry point in the survival analysis. The last reported date that the patient was seen alive was used as date of censor. Cats that were reported as dead owing to causes related directly to the ischaemic myelopathy were recorded as events.
Results
Nineteen cats met the inclusion criteria (Tables 1 and 2). The median age at the time of presentation was 10 years (range 6 months to 17 years; mean = 9 years and 7 months ± SD of 4 years and 7 months) with 10 cats aged between 9 and 13 years old. Eleven cats (58%) were neutered males, seven cats (37%) were spayed females and one cat (5%) was an entire female. Fourteen cats (73.7%) were domestic shorthair, with two (10.5%) domestic longhair, two (10.5%) Persian and one (5.3%) British Shorthair.
Table 1.
Signalment, severity of neurological dysfunction, lesion localisation and outcome of 19 cats with ischaemic myelopathy. Where a degree of lateralisation was noted, this has been recorded in brackets
| Case | Breed | Age (years/ months) | Sex | Potential cause identified | Neurological grade | Spinal pain on examination | Neuroanatomic localisation | MRI lesion location | Time to ambulation from onset (days) | Outcome (months since presentation) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DSH | 15 y 7 m | FS | Hypertension | 2 | No | C1–C5 | C3 | N/A | NQoL (6) |
| 2 | DSH | 11 y | MN | Suspected trauma | 2 | N/R | T3–L3 | T9–T10 | N/A | NQoL (29) |
| 3 | Persian | 10 y | MN | 2 | Lumbar | T3–L3 (left) | L2–L3 (left) | N/A | LTF after 34 months | |
| 4 | DLH | 13 y | MN | Hypertension | 3 | No | C1–C5 (left) | C3 (left) | 3 | CRTN (26) |
| 5 | DSH | 11 y 9 m | MN | Jumped/fell | 3 | No | T3–L3 | L2–L3 (left) | 11 | NQoL (67) |
| 6 | DSH | 6 m | FS | 3 | No | C6–T2 (left) | C6–C7 (left) | 3 | NQoL (55) | |
| 7 | DSH | 13 y | FS | Chronic renal insufficiency | 3 | Cervicothoracic | C6–T2 | C7–T1 | N/R | NQoL (51) |
| 8 | Persian | 12 y | MN | General anaesthetic | 3 | No | T3–L3 | T10–L1 (left) L3–L6 (left) | 19 | NQoL (33) |
| 9 | DSH | 6 y 10 m | MN | 3 | No | C1–C5 | C3 (right) | N/R | NQoL (37) | |
| 10 | DSH | 11 y 5 m | MN | 3 | No | C6–T2 (left) | C6–C7 (left) | 4 | NQoL (22) | |
| 11 | DSH | 12 y 11 m | FS | HCM, hypertension | 3 | No | C1–C5 (right) | C3 (right) | 4 | LTF after 39 months |
| 12 | DSH | 6 m | FE | Jumped/fell | 3 | No | C6–T2 (right) | C7–T1 (right) | N/R | LTF after 59 months |
| 13 | British SH | 9 y 7 m | FS | 3 | No | C6–T2 (left) | C6–T1 (left) | N/R | E 63 days after onset (renal infection) | |
| 14 | DSH | 11 y | MN | Trauma (stairs) | 3 | Cervical | C1–C5 | C3 | Euthanased non-ambulatory | E 7 days after onset (severe dyspnoea) |
| 15 | DSH | 17 y 11 m | FS | 3 | Generalised discomfort | C1–C5 (left) | C3 (left) | N/R | E 7 days after onset (FTI) | |
| 16 | DSH | 10 y | MN | Mild HCM | 4 | No | T3–L3 (left) | L2–L3 (left) | N/R | NQoL (82) |
| 17 | DSH | 9 y | FS | 4 | No | C1–T2 (left) | C5–C7 (left) | 3 | NQoL (124) | |
| 18 | DSH | 1 y | MN | Suspected trauma | 4 | No | L4–S1 (left) | L5–L7 (left) | 3 | LTF after 12 months |
| 19 | DLH | 6 y 3 m | MN | Chronic renal insufficiency | 4 | Lumbar | T3–S1 | L3–L4 | Euthanased non-ambulatory | E 24 days after onset (FTI) |
British SH = British Shorthair; CRTN = complete return to normal; DLH = domestic longhair; DSH = domestic shorthair; E = euthanased; FTI = failure to improve; FS = spayed female; HCM = hypertrophic cardiomyopathy; LTF = lost to follow-up; MN = neutered male; MRI = magnetic resonance imaging; N/A = not applicable; NQoL = normal quality of life; N/R = not recorded
Table 2.
Median, mean and standard deviation values for the 19 cats with presumed ischaemic myelopathy. Note that the standard deviation is in reference to the mean
| Age | Time to presentation (days) | Neurological grade | Time to recover ambulation (days) where recorded/applicable | |
|---|---|---|---|---|
| Median | 10 y | <1 | 3 | 3.5 |
| Mean | 9 y 7 m | 1.7 | 3 | 6.3 |
| ± SD | 4 y 7 m | 2.3 | 0.6 | 5.4 |
Historical findings
Cats were presented within a median time of less than 1 day (with a range of <1–8 days) following peracute or acute onset of clinical signs of spinal dysfunction. Cats were typically found by their owners already displaying signs of spinal dysfunction. Five cats were witnessed or suspected (on the basis of location or presence of shredded claws) to have undergone a trauma immediately prior to onset of clinical signs and one cat displayed signs of myelopathy on recovery from a general anaesthetic.
Pre-referral treatment consisted of corticosteroids (three cats), antibiotics (four cats), opioid analgesia (two cats), non-steroidal anti-inflammatory (one cat), intravenous fluid therapy (four cats) and lactulose (one cat). Ten cats did not receive medication prior to referral.
For the five cats in which it was noted, only two (cases 5 and 11) were reported by their owners to have exhibited signs of pain at the onset of neurological dysfunction.
The pattern of progression of clinical signs prior to presentation was reported in 13 cats: seven were reported to have shown neurological deterioration during the 24 h from the time of onset (with time to presentation ranging from less than 1 to up to 6 days), four were unchanged (with time to presentation ranging from less than 1 and up to 8 days) and two were considered clinically improved (with time to presentation of less than 1–2 days).
Clinical and MRI findings
Clinical and MRI findings for each of the 19 cats are summarised in Table 1. Neurological grade ranged from two (ambulatory paretic) to four (plegic with intact nociception) with both a median and mean score of 3 (non-ambulatory paresis; ± SD of 0.6). Of the 18 cats for which the presence or absence of hyperaesthesia was recorded at the time of presentation, four displayed focal spinal pain. One further cat displayed generalised discomfort on physical examination; however, its fractious nature reportedly made differentiation of true discomfort and a behavioural component impossible. Both cats that were reported by their owners to have shown pain at the time of onset were considered non-painful by the time they were presented to the referral centre at 4 days and less than 1 day.
MRI lesion localisation was to the spinal cord segments of C1–C5 (six cats), C6–T2 (six cats), T3–L3 (five cats) and L4–S3 (three cats) with one cat affected by two separate lesions. In all but the one cat with two lesions clinical and MRI neurolocalisations of affected spinal cord segments corresponded. In all 11 cases in which a clinical lateralisation was present, this corresponded to MRI lesion lateralisation. In three cats a lesion lateralisation was evident on MRI, but was not clinically overt (Table 1).
One cat (case 8) had two separate lesions identified on MRI. For analysis of the lesion extent both lesions were considered as separate and measured appropriately. For one cat (case 1) the extent of the lesion could not be measured accurately as the MRI study did not include transverse slices over the full length of the lesion or the C6 vertebral body. This case was, therefore, excluded from MRI measurements.
All 20 intramedullary lesions were hyperintense to normal grey matter on T2-weighted imaging (Figure 1). Seventeen (85%) were isointense to normal grey matter on T1-weighted imaging and the remainder were hypointense (15%). Four (22%) lesions (cases 7, 10, 12 and 13) displayed a degree of enhancement following intravenous administration of gadolinium; these cats underwent MRI 10, 6, 4 and within 1 day of the onset of clinical signs, respectively. GRE sequences were performed on four lesions – all appeared isointense to normal grey matter.
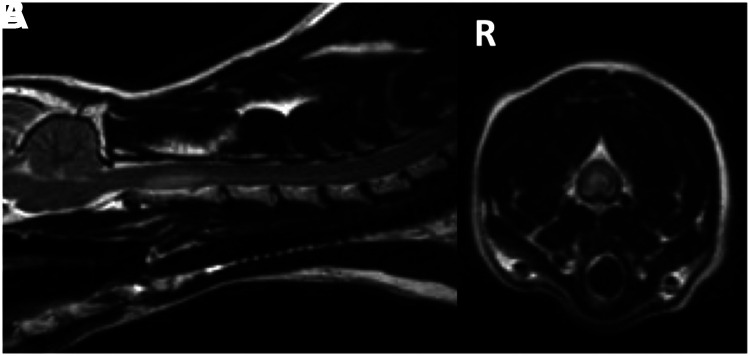
Figure 1.
Midline sagittal (A) and transverse (B) T2-weighted images of case 11 depicting the hyperintense intramedullary lesion affecting the spinal cord dorsal to the C2 vertebral body with right (R)-sided lateralisation evident in the transverse image
Eleven intramedullary lesions were located within the C1–T2 spinal cord segments, and their longitudinal and transverse extents have been summarised in Table 3. The eight T3–S1 lesions are summarised in Table 4. Measurements of statistical significance with respect to neurological grade or outcome were not applied to these data sets given the low number of cases in each group.
Table 3.
Measured extent of intramedullary lesions in the C1–T2 spinal cord segments. Note that the standard deviation is in reference to the mean
| Case | Neurological grade | L:C6 | % CSA | Outcome | |
|---|---|---|---|---|---|
| 1 | 2 | 1.5:1 | 51.6 | NQoL | |
| 4 | 3 | 2.0:1 | 41.6 | CRTN | |
| 6 | 3 | 2.5:1 | 33.2 | NQoL | |
| 7 | 3 | 2.4:1 | 34.2 | NQoL | |
| 9 | 3 | 1.6:1 | 44.7 | NQoL | |
| 10 | 3 | 2.5:1 | 66.9 | NQoL | |
| 11 | 3 | 2.1:1 | 39.0 | LTF after 39 months | |
| 12 | 3 | 1.8:1 | 65.9 | LTF after 59 months | |
| 13 | 3 | 3.1:1 | 59.5 | E 63 days after onset | |
| 14 | 3 | 2.6:1 | 49.1 | E 7 days after onset | |
| 15 | 3 | 1.7:1 | 54.5 | E 7 days after onset | |
| 17 | 4 | 3.1:1 | 60.8 | NQoL | |
| Median | 2.3:1 | 50.4 | |||
| Mean | 2.2:1 | 50.1 | |||
| ± SD | 0.5:1 | 11.3 |
%CSA = percentage of the cross-sectional area of the spinal cord affected by the lesion as measured on transverse MR images; CRTN = complete return to normal; E = euthanased; L:C6 = ratio of the length of the lesion to the length of the C6 vertebral body as measured on sagittal MR images; LTF = lost to follow-up; NQoL = normal quality of life
Table 4.
Measured extent of intramedullary lesions in the T3–S1 spinal cord segments. Note that the standard deviation is in reference to the mean
| Case | Neurological grade | L:L2 | %CSA | Outcome | |
|---|---|---|---|---|---|
| 2 | 2 | 2.3:1 | 54.3 | NQoL | |
| 3 | 2 | 1.6:1 | 27.1 | LTF after 34 months | |
| 5 | 3 | 1.2:1 | 60 | NQoL | |
| 8a | 3 | 2.3:1 | 60.6 | NQoL | |
| 8b | 3 | 2.3:1 | 39 | NQoL | |
| 16 | 4 | 2.3:1 | 57.9 | NQoL | |
| 18 | 4 | 1.3:1 | 31.4 | LTF after 12 months | |
| 19 | 4 | 3.6:1 | 54.3 | E 24 days after onset | |
| Median | 2.3:1 | 54.3 | |||
| Mean | 2.1:1 | 48.1 | |||
| ± SD | 0.7:1 | 12.6 |
%CSA = percentage of the cross-sectional area of the spinal cord affected by the lesion as measured on transverse MR images; E = euthanased; L:L2 = ratio of the length of the lesion to the length of the L2 vertebral body as measured on sagittal MR images; LTF = lost to follow-up; NQoL = normal quality of life
One cat (case 18) displayed evidence of soft tissue trauma on MRI (increased T2-weighted signal within the paralumbar musculature, with extensive change the length from L3 to L7 vertebral bodies on the left and, to a lesser severity, the lengths of L5 and L6 on the right). Pleural and abdominal effusions were also evident in this cat.
Ancillary diagnostic investigations performed in the 19 cats are summarised in Tables 5 and 6.
Table 5.
Ancillary diagnostic investigations performed in 19 cats with ischaemic myelopathy
| Diagnostic test | Number of cats |
|---|---|
| Biochemistry profile | 17 |
| Haematology profile | 16 |
| CSF analysis | 16 |
| FIV/FeLV (serology and/or CSF PCR) | 8 |
| Spinal radiographs | 7 |
| Abdominal ultrasound | 7 |
| Urinalysis | 6 |
| Toxoplasma species (serology and/or CSF PCR) | 6 |
| Feline coronavirus (serology and/or CSF PCR) | 6 |
| Thoracic radiographs | 6 |
| Cardiac ultrasound | 5 |
| Blood pressure measurement | 5 |
| Neospora species (serology and/or CSF PCR) | 3 |
| T4 measurement | 3 |
| Abdominal radiographs | 3 |
| Myelography | 2 |
| Electromyography | 2 |
CSF = cerebrospinal fluid; FeLV = feline leukaemia virus; FIV = feline immunodeficiency virus; PCR = polymerase chain reaction
Table 6.
Abnormal findings detected on ancillary diagnostic investigations in each of the 19 cats with ischaemic myelopathy
| Case | Abnormalities on ancillary diagnostic tests |
|---|---|
| 1 | Hypertension (190 mmHg) |
| 2 | No abnormalities detected |
| 3 | Suspected multiple renal infarcts on MRI |
| 4 | Mild hypercholesterolaemia, moderately elevated creatine kinase, hypertension (182 mmHg), elevated cCSF TP (0.38 g/l) |
| 5 | Proteinuria and elevated leukocytes in urine (no bacterial growth), elevated lCSF TP (0.63 g/l) |
| 6 | Mild neutrophilic pleocytosis (NCC = 55/µl), elevated cCSF TP (1.13 g/l) |
| 7 | Mild segmented neutrophilia |
| 8 | No abnormalities detected |
| 9 | No abnormalities detected |
| 10 | CSF neutrophilic pleocytosis (NCC = 12/µl) and elevated lCSF TP (0.56 g/l) |
| 11 | Hypertrophic cardiomyopathy, hypertension (209 mmHg) |
| 12 | Elevated lCSF TP (0.65 g/l) |
| 13 | No abnormalities detected |
| 14 | Elevated lCSF TP (1.03 g/l) |
| 15 | Anaemia, mild hypercholesterolaemia, elevated lCSF TP (2.1 g/l) |
| 16 | Mild hypertrophic cardiomyopathy, urinary infection (Escherichia coli) |
| 17 | CSF neutrophilic pleocytosis (NCC = 23/µl) and elevated lCSF TP (0.64 g/l) |
| 18 | No abnormalities detected |
| 19 | Anaemia, renal insufficiency, moderately elevated creatine kinase, haematuria and proteinuria (no bacterial growth) |
CSF = cerebrospinal fluid; cCSF = cisternal CSF sample; lCSF = lumbar CSF sample; NCC = nucleated cell count; TP = total protein
Treatment and outcome
Treatment for all cats consisted of rest, supportive care and physiotherapy. Three cats (cases 6, 7 and 14) received antibiotic therapy (13–20 mg/kg clindamycin q12h) while awaiting serum titres for protozoal organisms. One cat (case 16) received cephalexin (15 mg/kg q12h) for treatment of a urinary tract infection. Three cats initially received corticosteroid treatment [prednisolone (cases 8 and 10) at 0.5–1.0 mg/kg/day, dexamethasone (case 14) at 0.2 mg/kg once daily until euthanasia 3 days after admission]. One cat (case 18) received meloxicam for treatment of soft tissue trauma. Two cats required pharmacological assistance for bladder management [diazepam (case 16) and prazosin (case 2), respectively]. Two cats (cases 1 and 11) were treated with amlodipine (1.25 mg orally once daily) for hypertension.
The mean and median follow-up for this cohort of cats was, respectively, 36 and 33 months with a range of 1 week to 124 months. The mean survival time was 88 months [95 confidence interval (CI) 76–121 months), while the median was not reached as 15 cats were censored. The statistical analysis was limited to the Kaplan–Meier survival plot (Figure 2) because most cats were censored precluding analysis of specific prognostic factors.
Figure 2.
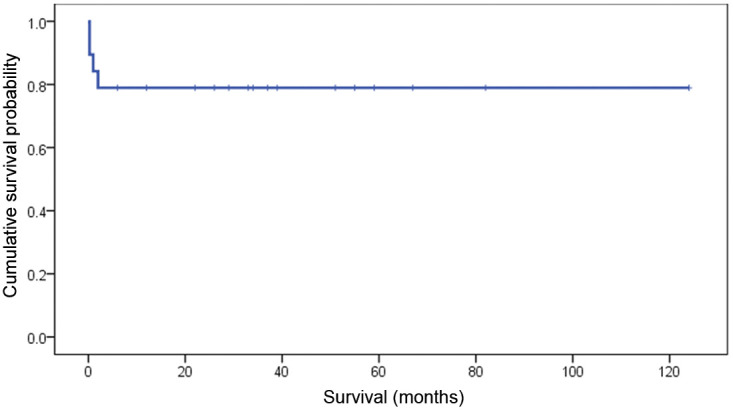
Kaplan–Meier survival plot depicting survival outcome of the 19 cats
Four cats were considered to have died as a direct consequence of ischaemic myelopathy. Two were euthanased during hospitalisation: the first was euthanased owing to failure to regain any voluntary limb movement after 3 weeks of physiotherapy and the second developed acute onset severe pulmonary oedema 6 days after the onset of neurological signs. Histopathological confirmation of the presumptive diagnosis of ischaemic myelopathy was achieved in the latter case. The cause of the pulmonary oedema was not evident, although cardiac abnormalities (cardiomyocyte degeneration and mild left ventricle hypertrophy) were present and occasional axons within the brainstem were reported to be swollen. Two cats were euthanased within 2 months of discharge owing to perceived poor quality of life and renal infection. Case 13 was reported to be ambulatory with assistance at the time of discharge and bladder management had not been required during hospitalisation. It was therefore unknown whether the renal infection was related to the presumed IM.
For the 17 cats that survived until discharge, the time to ambulation was available in 8/14 initially non-ambulatory cats with a median of 3.5 days (range 3–19 days) from onset of clinical signs. Time to discharge for the 17 cats was a median of 5 days (range 2–18 days) with five (29%) cats being hospitalised for more than 10 days. At the time of discharge, six cats were non-ambulatory, although two of these cats were reported to display good limb movement when supported. Four cats were re-examined at the referral institutions 1–8 months following discharge. Three of these cats demonstrated a residual decrease in postural reactions and strength at that time.
With the exception of two cats euthanased within 2 months of discharge, long-term follow-up data (ranging from 6 months to 10 years and 4 months, median 3 years and 1 month) was available in all of the 17 cats discharged. In four cases the owners were not contactable. Based on the records of the referring veterinary surgeons, before being lost to follow-up, these cats were known to be alive between 1 and 5 years following their presentation to the referral veterinary centres. No mention of neurological status was made in the final file entries of any of these cats, suggesting that neurological impairment was not a concern of their owners.
For the 13 cats in which the owners could be contacted, follow-up ranged from 6 months to 10 years and 4 months (median 2 years 9 months) following onset of clinical signs. Eleven of these 13 cats (84.6%) had returned to the same lifestyle they had enjoyed prior to developing IM. Of these, one was reported to make a full recovery and was considered completely normal by its owner. The residual neurological deficits of the remaining 10 cats were reported to be mild ataxia, paresis and stiffness of the affected limbs. Owners reported a time to maximal improvement of 2 days (one cat), 2–3 weeks (four cats), 1–3 months (four cats) and 1 year (two cats). Clinical re-evaluation was declined by the owners in all cases owing to logistical reasons and/or lack of a perceived need given their pet’s quality of life.
Of the surviving cats with owner-derived follow-up, only two owners reported difficulties in continuing their cat’s treatment at home, with one cat poorly tolerant to physiotherapy and one cat considered too active despite attempts to rest him. Two cats (cases 4 and 17) were reported to later develop self-resolving signs of vestibulocerebellar dysfunction, but were not referred for investigation. One cat (case 5) was euthanased owing to development of progressive incontinence (which was not investigated) just prior to euthanasia, 2.5 years following discharge. All other cats were alive at the time of owner contact.
Discussion
Based on the majority of previously published data the prognosis for feline IM appears to be poor. However, of the cats reported here, 15/19 (79%) survived more than 6 months after discharge, suggesting the prognosis may, in fact, be considered favourable.
The median age of the cats presented (10 years) was consistent with that obtained by combining results of previously published cases of feline IM (10 years).3,7,9,10,14–18 Also comparable to previously published reports was a greater number of male cats, with a ratio of 1.4 male:1 female in our study and 1.25:13,7,9,10,14,16–18 after combination of data from previous reports of feline IM. Although the cause of a gender bias has not been hypothesised, the over-representation of males reported in this study is also consistent with the findings of previous studies in dogs, with reports of male to female ratios of up to 4.5:1.4,5,13,19
Potential inciting or predisposing causes for development of spinal infarction were identified in 12 cats, with physical exertion, trauma, general anaesthesia and pre-existing medical conditions (renal disease, hyperthyroidism, hypertension and hypertrophic cardiomyopathy) identified. In confirmed, or presumed, canine FCEM, preceding trauma or exercise has been reported in 43–79% of cases.13,20 It has been hypothesised that increased intra-discal pressure could cause penetration of the nucleus pulposus into the spinal cord vasculature, resulting in embolisation and ischaemic necrosis of dependent spinal cord parenchyma. In this study, five cats had known or presumed trauma immediately prior to the onset of clinical signs. A canine study evaluating causes of cerebrovascular infarction identified chronic hypertension and renal disease 21 as common concurrent medical conditions. One cat (case 4), identified as hypertensive, later developed self-resolving signs of vestibulocerebellar dysfunction. Although this was not investigated, the possibility of a second ischaemic incident must be considered in this case. While heart disease as a concurrent disorder in dogs affected by ischaemic cerebrovascular accidents is considered rare, it has been reported.21, 22 Slight, and presumed clinically-insignificant, aortic valve thickening has been reported previously to have caused turbulence in blood flow in a cat diagnosed with presumed IM. 7 Although a cause–effect relationship between the above medical disorders and IM cannot be proven, concurrent conditions predisposing to infarction should be investigated in cats with IM.
When a neurological grade was assigned to the cats in the feline literature3,7,9,10,14–18 (with a more severe grade assigned when ambulation status was unclear), this was similar to those of the cats included in our study, ranging from 2 to 4, with a median of 3. In previously reported feline cases, involvement of the C6–T2 spinal cord segments was most commonly reported (42% of cases published) and T3–L3 spinal cord segment involvement was the least commonly reported (11% of cases publish-ed).3,7,9,10,14–18 In our case series the most common lesion localisations (based on MRI) were to the C1–C5 and C6–T2 spinal cord segments, each accounting for 30% of the localisations, while T3–L3 spinal cord segment lesions were present in 25% of the cats. For all six cats with a C1–C5 spinal cord segment neurolocalisation the lesion solely involved the C3 spinal cord segment. Interestingly, in dogs, IM lesions affecting the C1–C5 spinal segments are less frequently reported, with frequencies of only 0–9.5% in three large case studies.4,13,20 Furthermore, while in our feline population the L4–S3 spinal cord segments were the least commonly affected (15%), this location has been reported more frequently both in previously reported cats (21% of previously reported cases3,7,9,10,14–18) and dogs (43–47% of the large case studies4,13,20). As many previously published reports provide post-mortem confirmation of the diagnosis of IM, it is possible that a poorer outcome has been perceived previously for cases affected by lower motor neuron signs, resulting in this location bias.
Clinical and MRI localisations were in agreement in 18/19 (95%) cases and 19/20 (95%) lesions, with the one discrepancy resulting from the presence of two lesions in the same patient. Lesion lateralisation was identified clinically in 11/19 (58%) cats and on MRI in 15/20 (75%) lesions. Of the MRI-identified lateralisations, 12/15 (80%) of lesions exhibited a degree of lateralisation towards the left. A trend towards left-sided lateralisation of clinical signs or MRI findings has been reported previously in dogs, 4 although a potential cause for this has not been identified. Three cats (16%) displayed lesion lateralisation on MR images without clinical lateralisation. In each case the lesions affected the spinal cord bilaterally with the degree of lateralisation on MRI considered to be mild.
As characteristic MRI findings were necessary for inclusion in this study, it is unsurprising that the MRI features of our cats were consistent with those previously described for IM. However, compared with the findings of a large study of presumed IM in dogs, 4 a higher proportion of lesions in our cats were hypo-intense on T1-weighted imaging (15% compared with 5% of dogs), displayed a degree of contrast enhancement (22% compared to 12.5%) and were isointense on GRE images (100% compared to 63%). Contrast enhancement of presumed IM lesions in dogs has been reported to typically occur 5–7 days following the onset of clinical signs, although 12.5% of dogs imaged within 24 h of onset also displayed contrast enhancement. 4 Similarly, contrast enhancement was noted in 3/4 cats imaged more than 4 days following onset of neurological signs in our study. Although 21% of cases in this canine study did not exhibit an overt lesion on MRI, evaluation of a similar finding was not possible in our study as cases with a normal MRI did not meet our inclusion criteria.
In our case series, 17/19 cats (89%) survived until discharge, although two cats were subsequently euthanased. All 15 surviving cats were known to have lived for at least a further 6 months with one cat (case 17) reported by her owner to still be active at 20 years of age, 10 years and 4 months following her episode of presumed IM. For the 13 cases for which owner-derived follow-up was available, 11 (84.6%) returned to a normal lifestyle, with their owners reporting no significant long-term effects to their quality of life. The median time to recover ambulation was shorter in the cats of our study at 3.5 days (range 3–19 days) compared with a median of 11.5 days (range 2–27 days) in the feline literature9,18 and a similar figure in canine studies.12,13
Euthanasia of case 15 was performed shortly following discharge (7 days after onset of clinical signs). It is possible that sufficient improvement may have been noted in this cat had further recovery time been permitted. This highlights the impact of difficulties perceived by owners of non-ambulatory patients on their survival. Even with the inclusion of both cats euthanased following discharge, the total mortality rate (21%) in our study is half that reported previously in the combined feline veterinary literature. As neurological grades were similar between our cats and those of the previously published literature, the greater survival rate in our study may be a result of the lower number of cats with pelvic limb lower motor neuron (L4–S3 spinal cord segments) signs and/or a longer recovery time being permitted by the owners of our cats. In the published literature, euthanased cats only survived 1–12 days following presentation.3,9,10,14–16 Given that the time to recover ambulation has been reported to be up to 27 days, 18 it is possible that some of these cats may have made a recovery with time.
Loss of nociception has been reported previously to be a negative prognostic factor for recovery from IM in dogs.11,13,20 In our study, nociception was retained in all cats, precluding this factor from evaluation. Although an influence on prognosis has been reported in dogs, the number of cats within each neurological grade 4 or with lower motor neuron signs11,20 was too low to evaluate statistical associations between presenting clinical signs and outcome in our study. However, only 1/4 cats with a neurological grade of 4 (the most severe grade observed in this study) was euthanased owing to failure to improve. While another of these cats was lost to follow-up after 12 months the remaining two cats were reported to return to a normal quality of life with follow-up periods of 82 and 124 months. In the reported literature 3/5 cats with grade 4 clinical signs7,9,10,15 also survived to regain ambulation. Combining data from our study and the previously published literature, 6/9 (67%) cats that were clinically plegic with intact nociception at presentation recovered ambulation.
The availability of MRI as a diagnostic tool for our cases provided improved detail regarding the underlying cause of the spinal dysfunction compared with imaging tools such as myelography. This enabled clinicians to advise owners of treatment options and prognosis accordingly, and may have contributed to the owners’ willingness to persist in conservative management. Another factor that may bias our study towards a more optimistic prognosis is the fact that six of nine previous publications have been case reports that relied on a histopathological diagnosis. Only one other study (including six cats with presumed IM undergoing 0.3T MRI within 14 days of onset) has reported a favourable prognosis. None of these cats were reported to be plegic. 18
Although statistical significance could not be reached owing to the small case numbers, in each of the C1–T2 and T3–S1 lesion location groups the patients with the greatest ratio of lesion length to the length of C6 or L2 vertebral bodies were euthanased within 63 days of onset of clinical signs, whereas the patients with the greatest percentage CSA values returned to a normal quality of life. This finding was unexpected as, in a similar investigation in a large study of dogs, the percentage of the CSA of the lesion was associated significantly with outcome, with a value ≥67% being 100% predictive of an unsuccessful outcome.4,12 However, it must be noted that no lesions in our study were ≥67%, preventing direct comparison of dogs and cats. Further work is in progress to investigate the prognostic value of MRI on feline IM based on a larger population set.
With the exception of one case, in our study the diagnosis of IM was made on suggestive clinical and diagnostic findings, rather than histopathological confirmation. It was therefore not possible to provide a conclusive diagnosis in 18/19 cats. Furthermore, owing to the retrospective nature of this study, investigation into potential underlying causes of IM was not standardised. The small number of cases available also precluded the identification of statistically significant conclusions regarding potential prognostic indicators. Follow-up information was obtained from owners, rather than by re-examination by a veterinary neurologist. Potentially, this may have resulted in failure to detect some persistent neurological dysfunction, preventing objective conclusions regarding the degree of clinical improvement. However, as, ultimately, survival of a veterinary patient is dictated by their owner’s interpretation of quality of life, ease of management after discharge and recovery time, owner-derived assessment was considered to be valuable in determining survival outcomes in our cats.
Conclusions
Although uncommonly reported in veterinary literature, a presumptive diagnosis of IM in cats has, historically, resulted in a perceived poor prognosis, with almost half of the cases reported resulting in euthanasia. However, this study highlights that, even when plegia is present, the majority of cats with suspected IM can return to an owner-decreed normal lifestyle with supportive care and time. As IM may result from causes other than fibrocartilaginous embolisation, investigation into potential underlying causes of embolism or thrombosis should be undertaken to identify possible predisposing factors for infarction.
Acknowledgments
The authors would like to thank the AHT and RVC for assessing the manuscript according to their good research practice (RVC authorisation number: VCS_00363).
Footnotes
Funding: This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
The authors do not have any potential conflicts of interest to declare.
Accepted: 17 September 2012
References
- 1. De Risio L, Platt SR. Fibrocartilaginous embolic myelopathy in small animals. Vet Clin North Am Small Anim Pract 2010; 40: 859–869. [DOI] [PubMed] [Google Scholar]
- 2. Garosi LS. Cerebrovascular disease in dogs and cats. Vet Clin North Am Small Anim Pract 2010; 40: 65–79. [DOI] [PubMed] [Google Scholar]
- 3. Abramson CJ, Platt SR, Stedman NL. Tetraparesis in a cat with fibrocartilaginous emboli. J Am Anim Hosp Assoc 2002; 38: 153–156. [DOI] [PubMed] [Google Scholar]
- 4. De Risio L, Adams V, Dennis R, McConnell F, Platt S. Magnetic resonance imaging findings and clinical associations in 52 dogs with suspected ischemic myelopathy. J Vet Intern Med 2007; 21: 1290–1298. [DOI] [PubMed] [Google Scholar]
- 5. Abramson CJ, Garosi L, Platt SR, Dennis R, McConnell JF. Magnetic resonance imaging appearance of suspected ischemic myelopathy in dogs. Vet Radiol Ultrasound 2005; 46: 225–229. [DOI] [PubMed] [Google Scholar]
- 6. Duprez TP, Danvoye L, Hernalsteen D, Cosnard G, Sindic CJ, Godfraind C. Fibrocartilaginous embolization to the spinal cord: serial MR imaging monitoring and pathologic study. AJNR Am J Neuroradiol 2005; 26: 496–501. [PMC free article] [PubMed] [Google Scholar]
- 7. MacKay AD, Rusbridge C, Sparkes AH, Platt SR. MRI characteristics of suspected acute spinal cord infarction in two cats, and a review of the literature. J Feline Med Surg 2005; 7: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mateen FJ, Monrad PA, Hunderfund AN, Robertson CE, Sorenson EJ. Clinically suspected fibrocartilaginous embolism: clinical characteristics, treatments, and outcomes. Eur J Neurol 2011; 18: 218–225. [DOI] [PubMed] [Google Scholar]
- 9. Mikszewski JS, Van Winkle TJ, Troxel MT. Fibrocartilaginous embolic myelopathy in five cats. J Am Anim Hosp Assoc 2006; 42: 226–233. [DOI] [PubMed] [Google Scholar]
- 10. Scott HW, O’Leary MT. Fibro-cartilaginous embolism in a cat. J Small Anim Pract 1996; 37: 228–231. [DOI] [PubMed] [Google Scholar]
- 11. Gilmore DR, deLahunta A. Necrotizing myelopathy secondary to presumed or confirmed fibrocartilaginous embolism in 24 dogs. J Am Anim Hosp Assoc 1987; 23: 373–376. [Google Scholar]
- 12. De Risio L, Adams V, Dennis R, McConnell FJ, Platt SR. Association of clinical and magnetic resonance imaging findings with outcome in dogs suspected to have ischemic myelopathy: 50 cases (2000–2006). J Am Vet Med Assoc 2008; 233: 129–135. [DOI] [PubMed] [Google Scholar]
- 13. Gandini G, Cizinauskas S, Lang J, Fatzer R, Jaggy A. Fibrocartilaginous embolism in 75 dogs: clinical findings and factors influencing the recovery rate. J Small Anim Pract 2003; 44: 76–80. [DOI] [PubMed] [Google Scholar]
- 14. Zaki FA, Prata RG, Werner LL. Necrotizing myelopathy in a cat. J Am Vet Med Assoc 1976; 169: 228–229. [PubMed] [Google Scholar]
- 15. Bichsel J, Vandevelde M, Lang J. Spinal cord infarction following fibrocartilaginous embolism in the dog and cat. Schweiz Arch Tierheilkd 1984; 126: 387–397 . [PubMed] [Google Scholar]
- 16. Turner PV, Percy DH, Allyson K. Fibrocartilaginous embolic myelopathy in a cat. Can Vet J 1995; 36: 712–713. [PMC free article] [PubMed] [Google Scholar]
- 17. Coradini M, Johnstone I, Filippich LJ, Armit S. Suspected fibrocartilaginous embolism in a cat. Aust Vet J 2005; 83: 550–551. [DOI] [PubMed] [Google Scholar]
- 18. Nakamoto Y, Ozawa T, Mashita T, Mitsuda M, Katakabe K, Nakaichi M. Clinical outcomes of suspected ischemic myelopathy in cats. J Vet Med Sci 2010; 72: 1657–1660. [DOI] [PubMed] [Google Scholar]
- 19. Hawthorne JC, Wallace LJ, Fenner WR, Waters DJ. Fibrocartilaginous embolic myelopathy in miniature schnauzers. J Am Anim Hosp Assoc 2001; 37: 374–383. [DOI] [PubMed] [Google Scholar]
- 20. Cauzinille L, Kornegay JN. Fibrocartilaginous embolism of the spinal cord in dogs: review of 36 histologically confirmed cases and retrospective study of 26 suspected cases. J Vet Intern Med 1996; 10: 241–245. [DOI] [PubMed] [Google Scholar]
- 21. Garosi L, McConnell JE, Platt SR, Barone G, Baron JC, de Lahunta A, Schatzberg SJ. Results of diagnostic investigations and long-term outcome of 33 dogs with brain infarction (2000–2004). J Vet Intern Med 2005; 19: 725–731. [DOI] [PubMed] [Google Scholar]
- 22. Paul AE, Lenard Z, Mansfield CS. Computed tomography diagnosis of eight dogs with brain infarction. Aust Vet J 88: 374–380. [DOI] [PubMed] [Google Scholar]