Abstract
Pain recognition in cats is difficult and requires a multidisciplinary approach for diagnosis. A total of 103 client-owned cats were enrolled in this prospective, blinded clinical trial. Cats were invited to the clinic, or presented for annual rechecks/vaccinations, or gastrointestinal, dental or locomotor problems. The cats were of different breeds; both shorthaired and longhaired cats were included. Those cats that tolerated it were palpated and all cats were examined with the non-invasive method of thermographic imaging. Owners filled out a questionnaire about their cat’s behaviour and estimated whether the cat was in any pain. The agreement between a questionnaire and thermographic imaging or palpation was low. Also, the agreement between the owner’s estimation of pain and thermographic imaging or palpation was low. The agreement between palpation and thermographic imaging was moderate, suggesting that thermographic imaging is a potential tool in clinical practice for detecting and screening cats that are, potentially, in pain.
Introduction
Pain in cats often goes undetected as they have very subtle behavioural expression of pain.1,2 In cats, lameness and orthopaedic examination may be a challenge in the clinical setting owing to the clinician’s lack of experience and poor patient compliance. In this species, palpation and manual manipulation are often the only methods to detect pain in clinical practice. 3 Sometimes minor changes in behaviour can be the only indication of severe pain. 1 Cats suffer from the same painful diseases and conditions as dogs, humans and other mammals. As untreated pain can lead to more problems (eg, chronic pain and behavioural alterations),4,5 proper pain medication should be given to every cat in pain. 6 Owing to cats’ behaviour in hiding pain, veterinarians need new methods for assessment and recognition of pain in this species. A questionnaire to assess degenerative joint disease-associated pain in cats is, potentially, one such method. 7 A validated questionnaire for arthritic pain perception exists for dogs, 8 but one does not yet exist for cats. Aggressive behaviour is also often a problem with cats when they are in pain. Owing to this cats usually need to be sedated for examination, which may lead to something being overlooked as radiological examinations do not always correlate with pain in osteoarthritis.9,10 Furthermore, as feline patients are often elderly, sedation also has its challenges. 11
Thermographic imaging (also known as infrared thermography and infrared imaging) is a potential method for recognising injured or potentially painful areas in cats, as a change in superficial temperature may be an indicator of several illnesses and pain.12,13 It has been used in dogs,14–16 horses17–19 and farm animals 20 for various purposes, such as visualising orthopaedic problems. In humans it has been employed to detect, for example, breast cancer, 21 changes in vascular tissue 22 and digital osteoarthritis. 23 To our knowledge, thermographic imaging has not been used previously to study cats. Thermographic imaging is a non-invasive, quick and safe method of detecting changes in superficial temperature in animals17–20 and humans.21,22 An inflammation in subcutaneous and deeper tissues can be reflected as superficial tissue temperature changes23,24 of ≥1°C. 18 Modern infrared cameras have been claimed to be more than 10 times more sensitive in detecting temperature changes 18 than the human hand and fingers that can detect a ≥2°C difference in temperature on a patient’s skin. 25
Our primary objective was to compare the results of a palpation to thermographic images and pain questionnaire results in order to determine whether the indication of possible pain discovered in a palpation and a pain questionnaire correlates with the results of thermographic imaging. The secondary objective was to set reference limits for pain questionnaire scores to distinguish cats with no pain, with some clinical signs of pain, and definite pain. We also investigated the repeatability of a pain questionnaire, which was modified and translated from English to Finnish, by repeating the pain questionnaire and to show that the variance caused by this random component would be small enough to be considered as negligible.
Materials and methods
Cats
The study took place in the Veterinary Teaching Hospital at the University of Helsinki and a privately-owned cat clinic (CatVet) in Helsinki. One hundred and four client-owned cats were enrolled in the study. The cats studied at the Veterinary Teaching Hospital were invited to the hospital for the study, but the cats studied at the private cat clinic were regular patients attending the clinic for an annual clinical examination and vaccination, mild gastrointestinal problems, dental problems or for a suspected locomotor problem. Severely ill patients with advanced systemic disease (such as end-stage renal failure or cancer) were excluded from the study and one longhaired cat was excluded as the owner had not filled-out the entire questionnaire. All remaining cats (n = 103) were included. The hair coat was intact in the areas of interest of this study. Thermographic images of the cats were taken before palpation. The owners filled out the questionnaire twice (30 mins apart), before and after thermographic imaging in order to test the repeatability of the questionnaire. The owners were asked to sign written consent for participating before enrollment in the study. This study was approved by the Ethical Committee of the Viikki Campus at the University of Helsinki.
Palpation
Palpation of the spine, joints and muscles was performed by one experienced veterinarian who was blinded to the history of the cat. The results of the palpation, including even minor reactions to the palpation, wasted muscles and trigger points in the muscles, were scored by the examiner. The initial scoring was from 1 to 5, with 1 being normal. The scores were merged (1 = 1, 2–3 = 2, 4–5 = 3) for statistical analysis, producing a scale from 1 to 3, with 1 being normal.
Thermographic imaging
A thermal camera with a resolution of 320 × 240 pixels (T425, FLIR Systems, Sweden) was used for the imaging and the Color Palette high rainbow was chosen for displaying the images. The emissivity of one (e = 1) was used for the imaging. The freeware FLIR QuickReport 2.1. (FLIR, 2011) was applied to interpret the thermographic images. 26
Thermographic images were taken of each cat from the dorsal view, as well as from left and right lateral views, and from the cranial and caudal view, in addition to paw prints if possible (Figure 1). Those cats that were shy or frightened were allowed to stay in their carrier boxes with the lid off. In these cases, the minimum of the dorsal view and left and right lateral views were thermographed. Otherwise, cats were allowed to walk freely in an ambient room temperature of 21°C (69.8°F) for 15 mins. Owners were not allowed to touch the cats for 30 mins before or during the examination.
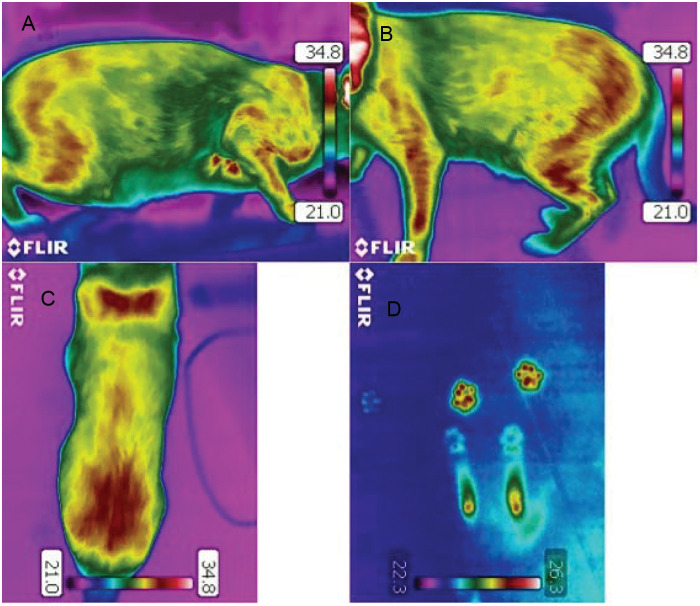
Figure 1.
Thermographic images of a clinically healthy cat (A) from the right lateral side of the cat, (B) from the left lateral side of the cat, (C) from the back (the most caudal parts are seen at the bottom of the picture) and (D) paw prints from the floor where the cat had remained stationary (front paw prints and hock area clearly visible)
One veterinarian trained in thermographic imaging evaluated the images. The veterinarian performing the evaluation was not aware of the cats’ history or signalment during the evaluation process. Notable temperature differences (≥1°C) 18 between the sides (left and right: lateral view and dorsal view, etc) of the cats were considered significant. Clinically abnormal cold or hot areas over the back within the same individual were also noted. The findings were scored from 1 to 3: 1 was considered to be normal with no findings; 2 was a mildly abnormal temperature finding (≥0.5°C difference but <1°C); and 3 was a temperature difference of ≥1°C. An example of the thermographic images with abnormal findings is presented in Figure 2.
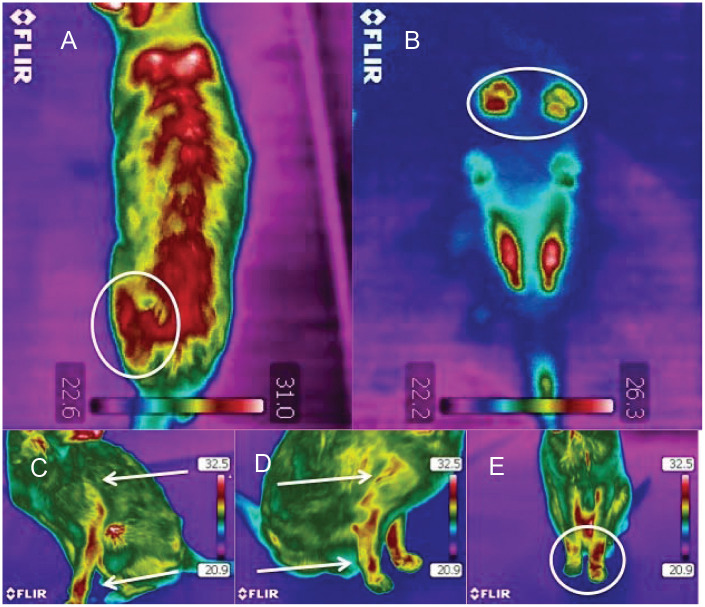
Figure 2.
Thermographic images of a cat with a suspected painful process: (A) uneven heat pattern in the lumbar area, with left hip area markedly warmer (circled); (B) uneven weight bearing in the front paws evident in the paw print thermographic image (circled); (C) and (D) slight differences in the heat patterns between the right and left front legs (arrows); (E) uneven heat pattern in the front legs (circled)
Questionnaire
Seventeen significant questions from the original pain questionnaire 7 were chosen and translated into Finnish from English. The chosen questions were piloted with cat owners (n = 9) and repeated (n = 5) after corrections. The questions were modified according to the translation and the feedback from the pilot (Table 1).
Table 1.
Modified questionnaire 7 translated from Finnish
| Questions | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| 1 | How does your cat walk? | Completely normally | Occasionally with difficulty | Sometimes with difficulty | Often with difficulty | With difficulty, limping |
| 2 | How does your cat run? | Completely normally | Occasionally with difficulty | Sometimes with difficulty | Often with difficulty | With difficulty, limping |
| 3 | How does your cat jump up? | Completely normally | Occasionally with difficulty | Sometimes with difficulty | Often with difficulty | With difficulty, doesn’t jump |
| 4 | How does your cat jump down? | Completely normally | Occasionally with difficulty | Sometimes with difficulty | Often with difficulty | With difficulty, doesn’t jump |
| 5 | How does your cat climb stairs | Completely normally | Occasionally with difficulty | Sometimes with difficulty | Often with difficulty | With difficulty, doesn’t climb |
| 6 | How does your cat descend stairs | Completely normally | Occasionally with difficulty | Sometimes with difficulty | Often with difficulty | With difficulty, doesn’t descend |
| 7 | How does your cat play with other pets? | Completely normally | Occasionally with difficulty | Sometimes with difficulty | Often with difficulty | With difficulty, doesn’t play |
| 8 | How does your cat rise from a resting position? | Completely normally | Occasionally with difficulty | Sometimes with difficulty | Often with difficulty | With major difficulty |
| 9 | How does your cat chase objects/toys/things? | Completely normally | Occasionally with difficulty | Sometimes with difficulty | Often with difficulty | With difficulty, doesn’t chase |
| 10 | How does your cat stretch? | Completely normally | Occasionally with difficulty | Sometimes with difficulty | Often with difficulty | With difficulty, doesn’t stretch |
| 11 | How does your cat eat (eating behaviour)? | Completely normally | Occasionally with difficulty | Sometimes with difficulty | Often with difficulty | With difficulty, poorly |
| 12 | Does your cat seek seclusion? | Normally | Occasionally | Quite often | Often | Constantly |
| 13 | How does your cat jump up high? | Completely normally | Occasionally with difficulty | Sometimes with difficulty | Often with difficulty | With difficulty, doesn’t jump |
| 14 | How does your cat jump down from high? | Completely normally | Occasionally with difficulty | Sometimes with difficulty | Often with difficulty | With difficulty, doesn’t jump |
| 15 | How does your cat sleep? | Completely normally | Occasionally more | Sometimes more | Often more | Mainly sleeps |
| 16 | How does your cat play with toys? | Completely normally | Occasionally less | Sometimes less | Doesn’t play often | Plays with difficulty, doesn’t play |
| 17 | What is your cat’s overall quality of life? | Normal/good | Quite good | Moderate | Worsened | Poor |
In filling out the questionnaire of 17 questions owners had to choose one out of five alternative answers (scaled from 1 to 5 points) best describing their cat (Table 1). Points from the chosen 17 answers were then totaled, the minimum sum being 17 and the maximum 85. At the end of the questionnaire there was also a separate question about the owner’s opinion of whether their cat had any pain [none (1), occasionally (2), sometimes (3), often (4), constantly (5])]. The initial scoring for the question about pain was from 1 to 5, with 1 considered normal. The pain question scores were merged (1 = 1, 2–3 = 2, 4–5 = 3) for statistical analysis, the final scoring being from 1 to 3: 1 implied normal; 2 and 3 were considered moderate; and 4 and 5 were considered severe. The question concerning the assumed pain was analysed separately and compared to the questionnaire, as well as the thermographic and palpation findings.
Statistical analysis of thermographic images, palpation and questionnaire
The agreement between the owners’ evaluation of the cats’ pain, the palpation results and the thermographic image results were assessed pair-wise with the aid of Weighted Kappa coefficients using Cicchetti–Allison’s 27 weights. Weighted Kappa coefficients were selected instead of the unweighted option because unweighted kappa does not take into account the degree of disagreement between various methods and all levels of disagreement are treated equally as total disagreement. Kappa values of 0.01–0.20 were considered as slight agreement, 0.21–0.40 as fair agreement, 0.41–0.60 as moderate agreement, 0.61–0.80 as substantial agreement and 0.81–0.99 as almost perfect agreement. 28
The relationship between the pain questionnaire and the clinical examination and thermographic image results was assessed with one-way analysis of variance (ANOVA) models. Least square means from these two models were used in the determination of reference limits for pain questionnaire scores.
The quality of the determined reference limits was assessed in two different ways. First, sensitivity and specificity were calculated for dichotomised classification variables (pain/no pain). This analysis provided information on how well the classification based on the pain questionnaire identified painless cats and those in pain, assuming that the palpation/thermographic image results were correct. The other method applied in assessing the reference limits did not assume that the palpation and thermographic image results were correct and used the original three-category classification. The proportions of agreement (the ratios of consistency) and their 95% confidence intervals were calculated for each of the categories (no pain, probable pain, distinct pain).
The random variation caused by refilling the questionnaire was evaluated in two different ways, with a random effects model and an ANOVA model. The response in the models was the pain score. The effect of cat was used as the sole random/fixed effect (depending on the model). If a specific question was unanswered at the first or second time of taking the questionnaire, the specific question was also considered missing (for the specific cat) the other time in order to make the sums comparable. In the random effects model the residual variance component, and in the ANOVA model the within-group variation, describe the variation between questionnaire answers for a specific cat. Repeatability statistics between the two time points were calculated from the ANOVA model.
Statistical analyses were carried out using the SAS System for Windows, version 9.3 (SAS Institute).
Results
The cats represented 16 different breeds, were aged from 12 weeks to 20 years and weighed 0.9–7.5 kg (2.4–20.0 lb). Twenty-six of the cats were longhaired and 77 were shorthaired. All the cats that did not have short hair were considered to be longhaired cats. More details are shown in the demographic data presented in Table 2.
Table 2.
Demographic data of the cats in the study
| Age in years (mean ± SD) |
Weight in kg (lb) (mean ± SD) |
Sex (n) |
|||||
|---|---|---|---|---|---|---|---|
| Breed | Cats (n) | Male | Female | Neutered male | Neutered Female | ||
| Abyssinian | 7 | 1.8 ± 1.3 | 2.9 ± 0.7 (7.8 ±1.9) | 2 | 2 | 3 | |
| Bengal | 4 | 7.0 ± 1.6 | 5.8 ± 1.2 (15 ± 3.2) | 3 | 1 | ||
| Birman | 1 | 21 | 3.0 (8.0) | 1 | |||
| Burmese | 3 | 8.3 ± 4.9 | 5.7 ± 1.7 (15 ± 4.6) | 1 | 1 | 1 | |
| Devon Rex | 2 | 2.3 ± 0.40 | 3.8 ± 0 (10 ± 0) | 2 | |||
| Domestic longhair | 6 | 9.5 ± 3.6 | 4.5 ± 0.73 (12 ± 2.0) | 1 | 3 | 2 | |
| Domestic shorthair | 44 | 7.7 ± 5.0 | 5.0 ± 1.3 (13 ± 3.5) | 2 | 1 | 25 | 16 |
| European shorthair | 2 | 11 ± 8.8 | 5.0 ± 0.20 (13 ± 0.50) | 1 | 1 | ||
| Korat | 4 | 2.4 ± 1.3 | 4.5 ± 0.60 (12 ± 1.6) | 4 | |||
| Maine Coon | 8 | 1.7 ± 1.7 | 5.2 ± 1.0 (14± 2.7) | 4 | 3 | 1 | |
| Norwegian Forest Cat | 3 | 10 ± 5.9 | 5.7 ± 1.2 (15 ± 3.2) | 1 | 2 | ||
| Oriental shorthair | 6 | 6.2 ± 5.8 | 3.1 ± 0.60 (8.3 ± 1.6) | 2 | 2 | 2 | |
| Persian | 1 | 6.0 | 3.3 (8.8) | 1 | |||
| Ragdoll | 7 | 4.7 ± 4.5 | 5.0 ± 1.2 (13 ± 3.2) | 1 | 2 | 4 | |
| Seychellois shorthair | 2 | 1.0 ± 0 | 3.9 ± 0.40 (10 ± 1.1) | 2 | |||
| Siamese | 3 | 10 ± 8.0 | 4.1 ± 1.1 (11 ± 2.9) | 2 | 1 | ||
| Total | 103 | 6.5 ± 5.2 | 4.7 ± 1.3 (13 ± 3.5) | 11 | 12 | 48 | 32 |
The palpation was executed only in those cats (n = 95) that tolerated handling in the study situation. Very frightened cats (n = 8) were not handled at all by the examiner. Of these, 47 (49%) showed signs of discomfort during the palpation, which was interpreted as possible pain. Out of the 47 cats that showed signs of discomfort during palpation, 39 had irregular heat patterns in their thermographic images. Irregular heat patterns (example seen in Figure 2) were found in the thermographic images of 56 palpated cats (59% of all cats). A total of 58 of the cats (56%) in the study (n = 103) had irregular heat patterns in their thermographic images.
Twenty-two cats were reported to have some pain sometimes (19%) or constantly (2%) by their owners. The rest of the cats were not considered to be in pain by the owner. Four cats had previously diagnosed osteoarthritis (4%) and two were suspected to have osteoarthritis (2%). None of the cats diagnosed with osteoarthritis were on non-steroidal anti-inflammatory drugs at the time of the study. One cat received acupuncture regularly, and five cats were on oral glucosamine sulphate with or without chondroitin sulphate at the time of the study. All the cats were able to move without significant difficulty or lameness. It was possible to take thermographic images of all the cats participating in this study without any resistance from the cats.
Comparison of palpation, thermographic images, questionnaire and owner’s estimate of pain
The agreement between palpation and thermographic imaging was found to be moderate (0.428) (Table 3). The agreement between the owner’s evaluation and thermographic image results or palpation results was found to be fair.
Table 3.
Weighted kappa statistics and 95% confidence intervals (CI) comparing owner’s evaluation of the pain, palpation results and thermographic imaging scores
| Comparison | Weighted kappa | Lower 95% CI | Upper 95% CI |
|---|---|---|---|
| Pain (owner’s evaluation) vs mean of palpation and thermographic image | 0.287 | 0.182 | 0.392 |
| Pain (owner’s evaluation) vs palpation | 0.323 | 0.205 | 0.441 |
| Pain (owner’s evaluation) vs thermographic image | 0.268 | 0.148 | 0.388 |
| Thermal image vs palpation | 0.428 | 0.285 | 0.571 |
Based on the fitted ANOVA models, two different cut-off values for pain questionnaire scores to classify cats as being in pain or painless were selected (Table 4). Furthermore, values for classifying the pain into three categories in the questionnaire were determined. Two different limits were assessed; these cut-off values are presented in Table 5. Using the first limits of Table 4, we achieved excellent specificity results (Table 6), but the downside was quite poor sensitivity.
Table 4.
Cut-off values for classifying cats as being in pain or painless based on the pain questionnaire
| No pain | Pain | |
|---|---|---|
| First limit | <22 points | ≥22 points |
| Second limit | ≤19 points | >19 points |
Table 5.
Cut-off values for classifying cats into three pain categories based on the pain questionnaire
| No pain | Probable pain | Distinct pain | |
|---|---|---|---|
| First limit | <22 points | ≥22; ≤28 points | >28 points |
| Second limit | ≤19 points | >19; ≤28 points | >28 points |
Table 6.
Sensitivity and specificity of the pain questionnaire compared to palpation results and thermographic image score
| Compared measure | Limits | Sensitivity | Specificity |
|---|---|---|---|
| Mean of palpation and thermographic image | 1 | 50.00 | 97.30 |
| Palpation result | 1 | 61.70 | 89.58 |
| Thermographic image | 1 | 48.28 | 86.67 |
| Mean of palpation and thermographic image | 2 | 60.61 | 81.08 |
| Palpation result | 2 | 72.34 | 75.00 |
| Thermographic image | 2 | 60.34 | 73.33 |
Generally speaking, the proportions of agreement between the pain questionnaire and thermographic images, or between the pain questionnaire and palpation results, were quite low, especially for the two categories (probable pain and distinct pain) indicating pain. Separating cats with less pain from those with more pain seemed to be more challenging with the pain questionnaire. Based on the results, the pain questionnaire would work better in classifying the cats into only two categories: no pain versus pain.
Repeatability of questionnaire
The repeatability was 0.97, indicating that the variation between repeats represented a very small part of the total variation. The results of the random effects model gave the same signal as the repeatability statistics. The estimate of the variance component was 3.24, which was very small compared with the between-cats variation (107.64).
Discussion
Our results suggest that owners not trained in evaluating their cats’ pain miss the signs of possible pain more often than a veterinarian performing palpation and using thermographic imaging. Thermographic imaging is a sensitive method for detecting changes in superficial temperature.12,13 Such a change can be the result of an inflammatory process in an osteoarthritic joint, 23 but it can also be caused by a change in weight bearing. An indication of changes in weight bearing, as seen in thermographic images, can be the result of any kind of painful process, but it can also be the result of the cat lying in the transport box with the other flank down before the thermographic imaging. As this is possible where cats are concerned, the cats in the present study were allowed to roam the room freely, when possible, for a minimum of 15 mins and the owners were not allowed to touch to cats 30 mins before thermographic imaging to avoid false-positive findings. The treatment regimes (acupuncture, glucosamine sulphate with or without chondroitin sulphate) some of the cats were on during the study may also have had a small effect on the results.
Our results suggest that the questionnaire could be used only to detect some cats with severe pain. Using the higher cut-off value of 22 points, we could be very confident that the cat was in pain. However, some cats that had some pain would have been diagnosed as having no pain. Using the second limits of Table 4, we were able to improve the sensitivity of the test, but lost a degree of specificity compared with the first limits (Table 6).
Although there are many pain scoring systems to choose from,29–31 no definitive method for diagnosing mild pain in cats exists. In our study the patient selection was limited to normal cats that were assumed healthy (<5 years) and those that were suspected or known to have osteoarthritis or another painful orthopedic problem. Other diagnostic modalities were not used in this study as our aim was to study the thermographic method with cats in basic veterinary clinic conditions that may not include a radiological examination or computed tomography devices; furthermore, not all the painful cases of osteoarthritis, for example, can be detected in radiological examinations.9,10
Thermographic imaging has not been used previously to study cats. The reason for this could be that cats are difficult to restrain without touching them and too small for using harnesses or collars without affecting the thermographic images. However, thermographic imaging has been used successfully with dogs14–16 and horses,17–19 and we did not see any reason not to extrapolate the reported information to our study. In the present study, we found the technical problems concerning the imaging of movable targets (cats) to be relatively minor, hence the method could be used on cats. Moreover, the cats seemed quite relaxed, which is why the owners also responded enthusiastically to the study.
Conclusions
Our results indicate that when combined with palpation, thermographic imaging might be a useful tool in differentiating painful cats from non-painful cats. In our results, palpation findings associated relatively well with thermographic images. This is another reason why thermographic imaging seems to be a good method to be used on tense cats. Further studies are required to determine the final scope and potential of the method of thermographic imaging.
Acknowledgments
FLIR Sweden and Infradex Oy Finland (Seppo Vihinen) made this study possible by providing the camera. Special thanks are owed to the personnel and patients of CatVet Kissaklinikka Oy for cooperation in this study.
Footnotes
Funding: The authors thank Suomen Kissaliitto ry (Finnish Cat Association) for taking an interest in this study.
The authors do not have any potential conflicts of interest to declare.
Accepted: 17 September 2012
References
- 1. Hellyer PW, Gaynor JS. Acute postsurgical pain in dogs and cats. Compend Contin Educ Pract Vet.1998; 2: 140–153. [Google Scholar]
- 2. Beale BS. Orthopedic problems in geriatric dogs and cats. Vet Clin North Am Small Anim Pract 2005; 35: 655–674. [DOI] [PubMed] [Google Scholar]
- 3. Kerwin S. Orthopedic examination in the cat: clinical tips for ruling in/out common musculoskeletal disease. J Feline Med Surg 2012; 1: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taddio A, Katz J, Ilersich AL, Gideon Koren. Effect of neonatal circumcision on pain response during subsequent routine vaccination. Lancet 1997; 349: 599–603. [DOI] [PubMed] [Google Scholar]
- 5. Väisänen MA-M, Tuomikoski SK, Vainio OM. Behavioral alterations and severity of pain in cats recovering at home following elective ovariohysterectomy or castration. J Am Vet Med Assoc 2007; 231: 236–242. [DOI] [PubMed] [Google Scholar]
- 6. Slingsby LS, Waterman-Pearson AE. Postoperative analgesia in the cat after ovariohysterectomy by use of carprofen, ketoprofen, meloxicam or tolfenamic acid. J Small Anim Pract 2000; 41: 447–450. [DOI] [PubMed] [Google Scholar]
- 7. Zamprogno H, Hansen BD, Bondell HD, Sumrell AT, Simpson W, Robertson ID, et al. Item generation and design testing of a questionnaire to assess degenerative joint disease-associated pain in cats. Am J Vet Res 2010; 71: 1417–1424. [DOI] [PubMed] [Google Scholar]
- 8. Hielm-Björkman AK, Rita H, Tulamo R-M. Psychometric testing of the Helsinki chronic pain index by completion of a questionnaire in Finnish by owners of dogs with chronic signs of pain caused by osteoarthritis. Am J Vet Res 2009; 6: 727–734. [DOI] [PubMed] [Google Scholar]
- 9. Clarce SP, Bennet D. Feline osteoarthritis: a prospective study of 28 cases. J Small Anim Pract 2006; 8: 439–445. [DOI] [PubMed] [Google Scholar]
- 10. Lascelles BD, Dong YH, Marcellin-Little DJ, Thomson A, Wheeler S, Correa M. Relationship of orthopedic examination, goniometric measurements, and radiographic signs of degenerative joint disease in cats. Vet Res 2012; 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bednarski R, Grimm K, Harvey R, Lukasik VM, Penn WS, Sargent B, Spelts K. AAHA anesthesia guidelines for dogs and cats. J Am Anim Hosp Assoc 2011; 6: 377–685. [DOI] [PubMed] [Google Scholar]
- 12. Head JF, Elliott RL. Infrared imaging: making progress in fulfilling its medical promise. IEEE Eng Med Biol 2002; 21: 80–85. [DOI] [PubMed] [Google Scholar]
- 13. Jiang LJ, Ng EYK, Yeo ACB, Wu S, Pan F, Yau WY, et al. A perspective on medical infrared imaging. J Med Eng Technol 2005; 29: 257–267. [DOI] [PubMed] [Google Scholar]
- 14. Infernuso T, Loughin CA, Marino DJ, Umbaugh SE, Solt PS. Thermal imaging of normal and cranial cruciate ligament-deficient stifles in dogs. Vet Surg 2010; 39: 410–417. [DOI] [PubMed] [Google Scholar]
- 15. Loughin CA, Marino DJ. Evaluation of thermographic imaging of the limbs of healthy dogs. Am J Vet Res 2007; 67: 1064–1069. [DOI] [PubMed] [Google Scholar]
- 16. Marino DJ, Loughin CA. Diagnostic imaging of the canine stifle: a review. Vet Surg 2010; 39: 284–295. [DOI] [PubMed] [Google Scholar]
- 17. Schweinitz von DG. Thermographic diagnostics in equine back pain. Vet Clin N Am-Equine 1999; 15: 161–177. [DOI] [PubMed] [Google Scholar]
- 18. Turner TA. Diagnostic thermography. Vet Clin N Am-Equine 2001; 17: 95–113. [DOI] [PubMed] [Google Scholar]
- 19. Levet T, Mertens A, Devisscher L, Duchateau L, Bogaert L, Vlaminck L. Distal limb cast sores in horses: risk factors and early detection using thermography. Equine Vet J 2009; 41: 18–23. [DOI] [PubMed] [Google Scholar]
- 20. Kízková I, Kunc P. Applications of infrared thermography in animal production. J Faculty Agriculture 2007; 22: 329–336. [Google Scholar]
- 21. Ng EYK, Kee EC. Advanced integrated technique in breast cancer thermography. J Med Eng Technol 2008; 32: 103–114. [DOI] [PubMed] [Google Scholar]
- 22. Tan J-H, Ng EYK, Acharya UR, Chee C. Infrared thermography on ocular surface temperature: a review. Infrared Phys Technol 2009; 52: 97–108. [Google Scholar]
- 23. Varjú G, Pieper CF, Renner JB, Kraus VB. Assessment of hand osteoarthritis: correlation between thermographic and radiographic methods. Rheumatology 2004; 43: 915–919. [DOI] [PubMed] [Google Scholar]
- 24. Vianna DML, Carrive P. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur J Neurosci 2005; 21: 2505–2512. [DOI] [PubMed] [Google Scholar]
- 25. Holmes LC, Gaughan EM, Gorondy DA, Hogge S, Spire MF. The effect of perineural anesthesia on infrared thermographic images of the forelimb digits of normal horses. Can Vet J 2003; 44: 392–396. [PMC free article] [PubMed] [Google Scholar]
- 26.FLIR Systems, Inc. http://www.flir.com/cs/emea/en/view/?id=42406 (1999–2012, accessed 18 April 2012).
- 27. Cicchetti DV, Allison T. A new procedure for assessing reliability of scoring EEG sleep recordings. Am J EEG Technol 1971; 11: 101–119. [Google Scholar]
- 28. Viera A, Garrett J. Understanding interobserver agreement: The Kappa statistic. Fam Med. 2005; 37: 360–363. [PubMed] [Google Scholar]
- 29. Robertson SA. Managing pain in feline patients. Vet Clin Small Anim 2008; 38: 1267–1290. [DOI] [PubMed] [Google Scholar]
- 30. Cambridge AJ, Tobias KM, Newberry RC, Sarkar DK. Subjective and objective measurements of postoperative pain in cats. J Am Vet Med Assoc 2000; 217: 685–690. [DOI] [PubMed] [Google Scholar]
- 31. Dixon MJ, Robertson SA, Taylor PM. A thermal threshold testing device for evaluation of analgesics in cats. Res Vet Sci 2002; 72: 205–210. [DOI] [PubMed] [Google Scholar]