Abstract
This study was conducted to describe and validate a dorsal ultrasound-guided approach to block the femoral nerve (FN) in cats by means of anatomical and computed tomography (CT) studies. The anatomical study was carried out in four fresh feline cadavers to determine the anatomic landmarks to approach this nerve. Then, an ultrasonographic study of the FN was performed in another eight cadavers using a 13 MHz linear transducer. The accuracy of the neurolocation by ultrasonography (US) was determined in four cadavers by the injection of 1 ml blue ink around the FN. The staining of the nerve was evaluated in anatomical studies. The feasibility of this technique was also evaluated by CT after injecting 1 ml of an iodinated contrast medium (150 mgl/ml) around the FN in the other four cadavers. The landmarks to approach the FN were the cranial border of the iliac crest and the dorsal processes of L6 and L7. The FN was visualised as a round hypoechogenic structure surrounded by a hyperechogenic rim located within the iliopsoas muscle on transverse scans. The anatomical and CT studies confirmed the accuracy of the US location of the FN. The dorsal ultrasound-guided approach may allow feasible and accurate access to the FN in cats and it could be useful in producing successful blockade.
Introduction
Regional techniques to control surgical pain from procedures carried out on the pelvic limb have traditionally been limited to the neuraxial administration (epidural or spinal routes) of local anaesthetics (LA) in small animals. 1 The blockade of peripheral nerves in humans provides effective analgesia with potentially fewer side effects than neuraxial anaesthesia.2–5 Electrolocation of peripheral nerves has been one of the most commonly employed techniques to achieve a peripheral nerve block (PNB), although ultrasound (US)-guided techniques are now the most advocated for this purpose in humans. 6 The advantages of US-guided techniques to achieve a PNB have been described adequately in previous reports.2,3,7,8 In recent years, a great interest in implementing US-guided techniques to block the main nerve supply of the pelvic limb in dogs has been observed.9–13
The sciatic (ScN) and the femoral (FN) nerves are the main nerves supplying sensory function to the pelvic limb; thus, techniques producing a combined block of those nerves have been described as a valid alternative to epidural anaesthesia in dogs.12,14 The perioperative analgesia provided by a combined ScN and FN block for surgical procedures involving the stifle and structures distal to it has been reported as adequate in the dog. 15 Recently, the ultrasonographic appearance of the ScN, 16 as well as an US-guided approach to block this nerve, 17 have been reported in the cat. However, to our knowledge there are no similar descriptions regarding the FN in the cat. The description of a valid US-guided technique to block this nerve could be useful in producing a combined ScN and FN block in cats, similar to that reported in dogs. However, single FN block is recommended as an analgesic adjunct for a variety of surgical procedures, such as fracture of the neck and shaft of the femur, hip replacement, total knee arthroplasty, cranial cruciate ligament repair, and skin graft and muscle biopsy from the cranial aspect of the thigh. 18 Furthermore, US-guided techniques may reduce the dose of LA required to achieve a clinically effective nerve block.2,3 This advantage could be of importance in the cat, particularly when a combined PNB of the ScN and FN is intended, as this species has an increased risk of toxicity to LA.19,20
In previous studies, the accuracy of the neurolocation provided by electrolocation and US-guided techniques has been evaluated by observing the distribution of a staining solution along the target nerves.11,12,17,21–23 Computed tomography (CT) provides excellent anatomical visualisation and has been used to assess the efficacy of US needle placement 24 and to study the distribution of an injectate in PNBs. 25
The objective of this study was to develop a technique for an US-guided dorsal approach for the feline FN. Therefore, we identified the ultrasonographic landmarks for locating the FN and evaluated the feasibility and accuracy of this technique by means of anatomic and CT studies carried out in feline cadavers.
Materials and methods
Animals
The project was approved by the local animal care and ethics committee. The study was designed to be carried out in two phases. In the first phase, four fresh adult cat cadavers (two males and two females) with a mean weight of 3.7 kg (range 3.5–3.9 kg) were used to conduct an anatomical study. In the second phase, eight fresh adult cat cadavers (six males and two females) with a mean weight of 3.5 kg (range 2.4–4.1 kg) were used to conduct an imaging study. All the cats were obtained from the Local Zoonoses and Public Health Service and were euthanased humanely for reasons unrelated to pelvic limb lameness.
Phase I: Anatomical study
Gross dissection of the FN
The left and right FN of two cadavers were dissected to investigate the anatomical characteristics of the FN and of its related structures. The crest of the ilium and the dorsal process of the fourth lumbar vertebra (L4) were the landmarks employed to dissect the FN. The skin of the lumbar area and the lateral aspect of the abdomen were reflected. Then, the external abdominal oblique, internal abdominal oblique, transverse, epaxial, psoas major, iliacus and quadratus lumborum muscles were detached from their insertion, and the FN identified. The FN was dissected from its origin to the point where it gives rise to the saphenous nerve.
Vascular injection and section
Red coloured latex was introduced through the thoracic aorta in two fresh feline cadavers. The cats were then frozen at -20°C for 24 h and then moved to -80°C for another 24 h. Later, transverse cryosections of the area of interest were made from the second lumbar vertebrae (L2) to the sacrum using a high-speed band saw at a thickness of 3 mm. The anatomic landmarks employed, and other anatomic structures related to the FN, were determined in this study. Photographs of each slide were taken to correlate the anatomical structures to the corresponding US images.
Phase II: Imaging study
Ultrasound nerve study and US-guided approach to the FN
Ultrasonographic scans were performed on the left and right FN of four fresh feline cadavers. The scans were carried out immediately after euthanasia. The cadavers were placed in lateral recumbency with the side of interest facing upwards. The hair from the lumbar area was clipped, the skin cleaned and coupling gel applied. A 4–13 MHz linear transducer (MyLab 70; Esaote) was used. A depth setting of 3 cm and two focal points at 0.5 and 1.5 cm were selected to perform the scans and guide the injections. The transducer was placed perpendicular to the spine, slightly ventral to the transverse process and cranial to crest of the ilium. The orientation marker of the probe was positioned dorsally. Then, the transducer was directed cranially from this point, trying to trace the projection of the FN on the lumbar area. Transverse images of the FN were obtained with the probe placed in the described position. Longitudinal images of this nerve were also obtained by rotating the transducer 90° from this point and placing the orientation marker of the probe towards the cranial aspect.
Once the FN was identified, 1 ml blue ink was injected under US guidance using an atraumatic PNB needle (Stimuplex; B-Braun) inserted close to this nerve to demonstrate the accuracy of the US nerve location. The injection was made between the sixth lumbar vertebrae (L6) and seventh lumbar vertebrae (L7) transverse processes, using a transverse cross-sectional view and inserting the needle by the use of an in-plane technique. If bone was felt the needle was re-directed slightly to pass between the transverse processes. Two of the cadavers were dissected immediately and the FN evaluated. The presence of blue ink staining the targeted nerve confirmed the accuracy of the US nerve location. The other two cadavers were frozen to obtain transverse cryosections, as described for phase I. Photographs of each slide were taken and the pattern of distribution of the ink studied.
CT study
Four fresh feline cadavers were employed in this part of the study. These cadavers were employed to inject 1 ml of iodinated contrast medium (150 mg/ml) obtained from iohexol 300 mg/ml (Omnitrast 300; Bayer) and diluted in saline solution 50:50 close to the FN. The injections were performed using the US-guided technique described above. Then, CT (High speed dual; General Electric) scans were performed immediately. The cadavers were positioned in lateral recumbency with side of the injection upwards. Axial transverse CT scans at a thickness of 1 mm were obtained from L2 to S3 and bone, and standard algorithm were used. The CT examinations were reviewed in both bone and a soft tissue window. The CT examinations were evaluated for location of the needle and distribution of the contrast medium.
Results
Phase I: Anatomical study
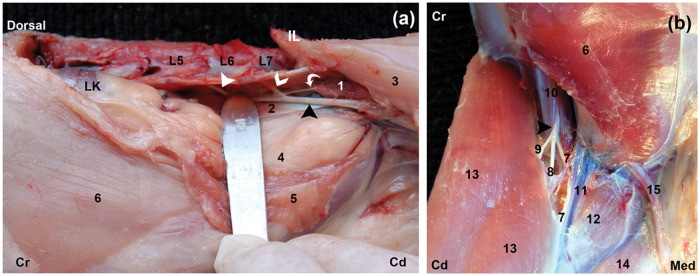
The FN was identified easily during the gross dissection in all cases. The FN was originated from the ventral branches of the fifth and sixth lumbar nerves, which emerged from the intervertebral foraminae of the vertebral bodies of L5 and L6. The major part of the FN was observed at the level of the L7 vertebral body within the substance of the iliopsoas muscle (IPM) (Figure 1a). It ran in a caudal direction to leave the abdomen through the muscular lacuna and divided after reaching the pelvic limb (Figure 1b).
Figure 1.
Gross anatomy of the femoral nerve (FN). (a) Left lateral view of the FN; the skin, external and internal oblique, transverse, epaxial, psoas major and quadratus lumborum muscles were reflected and the origin of the FN visualised. The contributions of the fifth (full white arrowhead), sixth lumbar nerves (open white arrowhead) and the major part of the FN (black arrowhead) are observed. The obturator nerve (curved white arrow) is also visible at this level. (b) The FN (black arrowhead) is observed emerging briefly from the iliopsoas muscle (IPM) through the muscular lacuna dividing into three branches. Cr = cranial, Cd = caudal, Med = medial, IL = crest of ilium, L5 = fifth lumbar vertebrae, L6 = sixth lumbar vertebrae, L7 = seventh lumbar vertebrae, LK = left kidney, 1 = iliacus muscle, 2 = psoas minor tendon, 3 = sartorius, 4 = parietal peritoneum, 5 = internal abdominal oblique and transversal abdominal muscles, 6 = external abdominal oblique, 7 = saphenous nerve, 8 = muscular branch to the quadriceps femoris muscle, 9 = muscular branch to the sartorius, 10 = iliopsoas muscle, 11 = femoral artery and vein, 12 = pectineus muscle, 13 = sartorius muscle, 14 = gracilis muscle, 15 = spermatic cord
Phase II: Imaging study
Ultrasound nerve study and US-guided approach to the FN
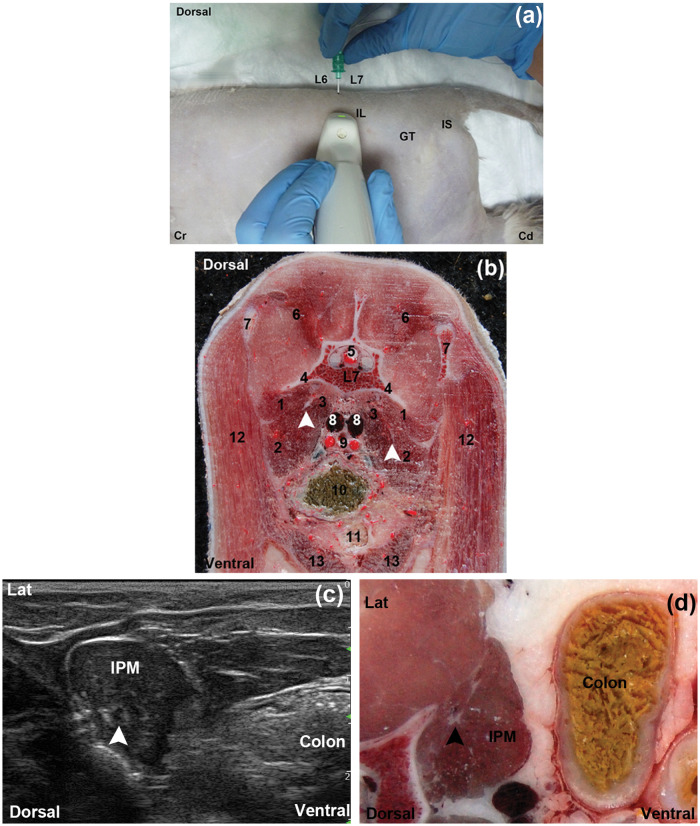
The FN was correctly identified by US in the tested dorsal approach. The crest of ilium and the dorsal processes of L6 and L7 were identified easily and considered as useful landmarks for this approach in all cases. The transducer was positioned perpendicular to the spine and cranial to the crest of ilium at the level of L6–L7 dorsal processes in the cadavers (Figure 2a, b). The IPM was observed as an ovoid to triangular hypoechoic structure with an internal pattern of scattering echoes in the transverse scans. The FN was visualised as a single, well-differentiated, rounded hypoechogenic structure surrounded by a marked hyperechogenic rim located within the IPM substance in the transverse scans (Figure 2c). In the longitudinal scans the FN appeared as a tubular hypoechogenic structure outlined by hyperechoic borders. There was a good correlation between the ultrasonographic images and the cryosections (Figure 2d).
Figure 2.
(a) Anatomical landmarks for the dorsal approach to the FN. (b) Cross-sectional anatomical image of the dorsal approach at the seventh lumbar vertebrae level (caudal view). The left and right femoral nerves (FN) (arrows) are visualisation embedded on the sublumbar muscles. (c) Transverse ultrasonographic image of the FN (arrowhead) at the dorsal approach. (d) Corresponding close cross-sectional anatomic image. Lat = lateral, Cr = cranial, Cd = caudal, IL = crest of ilium, GT = greater trochanter, IS = ischial tuber, IPM = iliopsoas muscle, L6 = sixth lumbar dorsal process, L7 = seventh lumbar dorsal process, 1 = quadratus lumborum muscle, 2 = psoas major muscle, 3 = iliacus muscle, 4 = seventh lumbar vertebrae transverse process, 5 = spinal cord, 6 = epaxial muscles, 7 = wing of ilium, 8 = iliac veins, 9 = external and internal iliac arteries, 10 = colon, 11 = urinary bladder, 12 = sartorius muscle, 13 = rectus abdomini
The injection site was located approximately 1 cm lateral to the midline of the spine. The needle was inserted using an in-plane technique and directed close to the target nerve. The ink was observed staining successfully the fifth and sixth lumbar nerves, as well as the FN. The distribution of the injectate from the injection site was observed to occur simultaneously in cranial and caudal directions staining the muscles surrounding the target nerve (quadratus lumborum, psoas major, psoas minor and iliacus) (Figure 3a). The anatomical cryosections showed that the spreading of injectate occurred in the interfascial space, within the IPM, as well as between the iliopsoas and the quadratus lumborum muscles in the directions described above. There was no evidence of dye distribution within the vertebral canal (Figure 3b).
Figure 3.

Accuracy of the FN location and blockade. (a) Gross dissection, left lateral view showing the FN (black arrowhead) stained. (b) Transverse cryosection; notice the ink (*) at the iliopsoas muscle. Cr = cranial, Cd = caudal, L6 = sixth lumbar vertebrae, L7 = seventh lumbar vertebrae, IL = wing of ilium
CT study
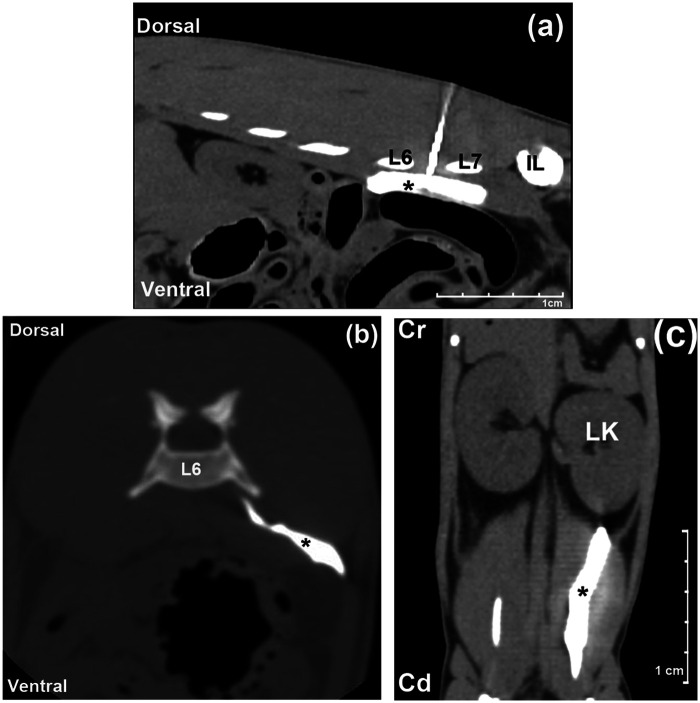
The CT scans demonstrated an accurate location of the needle between the transverse processes of L6 and L7, and within the IPM in all cases (Figure 4a). A spreading of contrast medium within the IPM and around the sublumbar musculature, with cranial and caudal distributions, was observed from the midpoint of the vertebral body of L5 to the inguinal region. A small amount of contrast medium was observed outside the IPM distributed into the retroperitoneal space in three cases (3/4) (Figure 4b). No evidence of contrast medium within the vertebral canal was noticed in any cases. The mean length of contrast distribution within the IPM was 4.6 cm (range 3–6.4 cm) (Figure 4c).
Figure 4.
Pattern of the distribution of the injectate in the computed tomography study. (a) Sagittal view; the needle is observed between L6 and L7 transverse processes, and the contrast media (*) is observed spreading at the iliopsoas muscle. (b) Transverse view; notice the contrast media (*) at the iliopsoas and spreading outside on the retroperitoneal space. (c) Dorsal view, showing the length spreading of the contrast media (*) at the sublumbar musculature. Cr = cranial, Cd = caudal, IL = wing of ilium, LK = left kidney
Discussion
The aim of this study was to describe the US appearance of the FN and develop an US-guided approach for the FN block in cats, studying its feasibility and accuracy by means of anatomical and CT studies. To our knowledge, this is the first study to investigate the use of US to locate and block the FN in the cat.
The anatomical study was useful for establishing an adequate acoustic window to block the FN. The anatomical features of the FN and its related structures within the IPM were similar to previous descriptions. 26 In humans and in dogs, the FN has been described as a hyperechogenic triangular structure when it is observed by US at the level of the femoral triangle.11,27 In our study, the FN within the IPM was observed by US as a hypoechogenic oval structure surrounded by a hyperechogenic rim. These differences may be explained by the effect of the tissues covering the FN at different anatomical regions. The FN is covered by multiple facial planes and fat tissue at the level of the femoral triangle, while it is only surrounded by muscle mass within the IPM. 12 Moreover, the visualisation of the FN may be superior using a dorsal approach than by other approaches because the hyperechogenic border of the nerve is better contrasted as it is surrounded by a hypoechoic muscle tissue.
The anatomical landmarks established to localise the FN were the iliac crest and the dorsal processes of L6 and L7. The L7 dorsal process is distinguished easily by palpation because it is shorter than the L6 dorsal process. These anatomical landmarks can be difficult to use in dogs with spinal abnormalities and in obese patients. 28 The presence of anatomical variations of the lumbar and sacral segments is relatively uncommon in the cat. 29
The localisation of the FN by US-guided dorsal approach was successful in all cases. This nerve was visualised easily because the hyperechogenic border of the nerve was well contrasted against the hypoechoic muscle tissue, which was surrounding it. 12 Another advantage of this approach is that it was possible to observe the needle and the nerve in the same plane. The visualisation of the shaft of the needle is been considered necessary to accomplish a safer approach to a peripheral nerve. 30 Moreover, the observation of the target nerve in a cross-sectional view allows a detailed observation of the distribution of the anaesthetic solution around the nerve. This fact is of importance in blocking a peripheral nerve using a small volume of LA,6,31 which could be useful in cats because of their low capacity for hepatic glucoronidation.19,20 One disadvantage of the studied approach could be that the needle has to cross between the transverse processes of L6 and L7 to reach the FN, with the potential risk of reaching bone. 12 In our study, when it occurred, the needle was redirected slightly and the target nerve was always reached successfully.
The puncture site was located 1 cm lateral to the midline between L6 and L7. This insertion site was chosen because in the anatomic study the major part of the FN was visualised at the level to the L7 vertebral body. It is recommended that the FN is blocked before it is divided in its different branches, as the distal components of the FN, including its sensory (saphenous nerve) and motor branches will be blocked producing a clinically more effective blockade. 32 In the dog, when performing a blind technique using a similar dorsal approach to the one employed in our study, the puncture site was between L5 and L6. 21 This is because in dogs the FN receives branches from the fourth, fifth and sixth lumbar nerves, and a more cranial approach is required. 33 In the dog, variable success rates of 68–100% have been reported when nerve electrolocation was employed to block the FN through dorsal 21 or femoral triangle10,32 approaches. This may be explained by the lower success rate of blind techniques to locate accurately the target nerves. 21 These blind techniques can also be associated with inadvertent vascular or nerve puncture. 32 When the FN block was performed using an US-guided technique, a 50% failure rate was reported using a femoral triangle approach. 11 Contrarily, the same authors reported a success rate of 100% when a suprainguinal approach to this nerve was employed. 12 The low success rate of the FN block for the femoral triangle approach has been related to difficulties in visualising the FN by US owing to the great amount of connective tissue around the FN in this area and the reduced length of the FN and its branches at this level. 11 Furthermore, there is an increased risk of accidental puncture of the femoral artery and of inadvertent intravascular injection of LA when this approach is used. 32 The suprainguinal approach locates the FN within the IPM before it bifurcates, which improves the visualisation of the FN, explaining the higher success rate reported for this approach. 12 Similarly, the dorsal approach to the feline FN employed in our study locates the main trunk of the FN within the IPM and, therefore, a high success rate should be expected.
This cadaveric study only allows us to speculate on the clinical efficacy of the FN block by the observation of the extent to which the methylene blue dye was distributed along the target nerve. Results from this study showed that the US-guided dorsal approach to the FN in cats produced an adequate staining of the FN in the anatomic study, as well as a good distribution of the contrast medium along the area of interest in the CT study. The length of the nerve in contact with the LA is a major factor in determining the success of a PNB. An in vitro study suggested that a staining of ≥2 cm along a peripheral nerve should be considered sufficient to produce a clinically effective nerve blockade. 34 In the present study, in which 1 ml of injectate was administered close to the FN, the length of nerve stained was within a range of 3–6.4 cm. The distribution of the injectate was observed to occur in the fascial plane within the IPM and around the sublumbar musculature, with a cranial and a caudal spreading. This spreading pattern of the injectate may produce its diffusion towards the three main nerve components of the lumbar plexus either within the IPM or within the fascial planes of the local musculature.21,35 However, further research is required to confirm this hypothesis. In the CT study, most of the contrast medium remained within the IPM, but some extension of contrast media outside the IPM toward retroperitoneal space occurred in three out of four cases. Traces of dye outside the IPM have been also reported in dogs after a dorsal approach. 21 Further imaging studies carried out with live animals are required to provide more information about this potential complication and to demonstrate clinically the success of the FN block technique studied in this research. Epidural spread of LA following a lumbar plexus block through a dorsal approach is a reported complication with an incidence of 9% in human patients 36 and 8.7% in the dog. 21 This complication might lead to the blockade of the contralateral limb 37 and may also cause a sympathetic blockade of the affected dermatomes. 21 In our study carried out on cats cadavers, evidence of epidural or bilateral paravertebral spreading of contrast medium or ink was not seen in any case. The factors that contribute to the epidural spreading of LA after a lumbar plexus block using a dorsal approach are still not well defined. However, this could be a multifactorial complication related to factors such as the position of the needle before the injection, unintended injection close to the intervertebral foramen, administration of larger volumes of LA and the presence of spinal deformity. 38
Conclusions
The described technique of a dorsal US-guided approach may allow feasible and accurate access to the FN to produce successful blockade in cats. It produced adequate staining of the FN in the anatomical study and a good spreading pattern of contrast medium in the CT scans. Further research is required to evaluate the clinical efficacy of this nerve block in live cats.
Footnotes
Funding: This research was supported by CONACYT Consejo Nacional de Ciencia y Tecnología, México.
The authors do not have any potential conflicts of interest to declare
Accepted: 20 August 2012
References
- 1. Valverde A. Epidural analgesia and anesthesia in dogs and cats. Vet Clin North Am Small Anim Pract 2008; 38: 1205–1230. [DOI] [PubMed] [Google Scholar]
- 2. Casati A, Baciarello M, Di Cianni S, et al. Effects of ultrasound guidance on the minimum effective anaesthetic volume required to block the femoral nerve. Br J Anaesth 2007; 98: 823–827. [DOI] [PubMed] [Google Scholar]
- 3. Oberndorfer U, Marhofer P, Bosenberg A, et al. Ultrasonographic guidance for sciatic and femoral nerve blocks in children. Br J Anaesth 2007; 98: 797–801. [DOI] [PubMed] [Google Scholar]
- 4. Singelyn FJ, Ferrant T, Malisse MF, et al. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous femoral nerve sheath block on rehabilitation after unilateral total-hip arthroplasty. Reg Anesth Pain Med 2005; 30: 452–457. [DOI] [PubMed] [Google Scholar]
- 5. Davies AF, Segar EP, Murdoch J, et al. Epidural infusion or combined femoral and sciatic nerve blocks as perioperative analgesia for knee arthroplasty. Br J Anaesth 2004; 93: 368–374. [DOI] [PubMed] [Google Scholar]
- 6. Marhofer P, Greher M, Kapral S. Ultrasound guidance in regional anaesthesia. Br J Anaesth 2005; 94: 7–17. [DOI] [PubMed] [Google Scholar]
- 7. Enneking FK, Chan V, Greger J, et al. Lower-extremity peripheral nerve blockade: Essentials of our current understanding. Reg Anesth Pain Med 2005; 30: 4–35. [DOI] [PubMed] [Google Scholar]
- 8. Gray AT, Collins AB, Schafhalter-Zoppoth I. An introduction to femoral nerve and associated lumbar plexus nerve blocks under ultrasonic guidance. Tech Reg Anesth Pain Manag 2004; 8: 155–163. [Google Scholar]
- 9. Costa-Farre C, Blanch XS, Cruz JI, et al. Ultrasound guidance for the performance of sciatic and saphenous nerve blocks in dogs. Vet J 2011; 187: 221–224. [DOI] [PubMed] [Google Scholar]
- 10. Campoy L, Bezuidenhout AJ, Gleed RD, et al. Ultrasound-guided approach for axillary brachial plexus, femoral nerve, and sciatic nerve blocks in dogs. Vet Anaesth Analg 2010; 37: 144–153. [DOI] [PubMed] [Google Scholar]
- 11. Echeverry DF, Gil F, Laredo F, et al. Ultrasound-guided block of the sciatic and femoral nerves in dogs: a descriptive study. Vet J 2010; 186: 210–215. [DOI] [PubMed] [Google Scholar]
- 12. Echeverry DF, Laredo FG, Gil F, et al. Ventral ultrasound-guided suprainguinal approach to block the femoral nerve in the dog. Vet J 2012; 192: 333–337. [DOI] [PubMed] [Google Scholar]
- 13. Shilo Y, Pascoe PJ, Cissell D, et al. Ultrasound-guided nerve blocks of the pelvic limb in dogs. Vet Anaesth Analg 2010; 37: 460–470. [DOI] [PubMed] [Google Scholar]
- 14. Campoy L, Martin-Flores M, Ludders JW, et al. Comparison of bupivacaine femoral and sciatic nerve block versus bupivacaine and morphine epidural for stifle surgery in dogs. Vet Anaesth Analg 2012; 39: 91–98. [DOI] [PubMed] [Google Scholar]
- 15. Campoy L, Martin-Flores M, Ludders JW, et al. Procedural sedation combined with locoregional anesthesia for orthopedic surgery of the pelvic limb in 10 dogs: case series. Vet Anaesth Analg 2012; 39: 436–440. [DOI] [PubMed] [Google Scholar]
- 16. Haro P, Gil F, Laredo F, et al. Ultrasonographic study of the feline sciatic nerve. J Feline Med Surg 2011; 13: 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haro P, Laredo F, Gil F, et al. Ultrasound-guided block of the feline sciatic nerve. J Feline Med Surg 2012; 14: 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Szucs S, Morau D, Iohom G. Femoral nerve blockade. Med Ultrason 2010; 12: 139–144. [PubMed] [Google Scholar]
- 19. Court MH, Grenblatt DJ. Molecular basis for deficient acetaminophen glucuronidation in cats. An interspecies comparison of enzyme kinetics in liver cromosomes. Biochem Pharmacol 1997; 53: 1041–1047. [DOI] [PubMed] [Google Scholar]
- 20. Robertson SA, Taylor PM. Pain management in cats – past, present and future. Part 2. Treatment of pain – clinical pharmacology. J Feline Med Surg 2004; 6: 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Campoy L, Martin-Flores M, Looney AL, et al. Distribution of a lidocaine-methylene blue solution staining in brachial plexus, lumbar plexus and sciatic nerve blocks in the dog. Vet Anaesth Analg 2008; 35: 348–354. [DOI] [PubMed] [Google Scholar]
- 22. Rasmussen LM, Lipowitz AJ, Graham LF. Development and verification of saphenous, tibial and common peroneal nerve block techniques for analgesia below the thigh in the nonchondrodystrophoid dog. Vet Anaesth Analg 2006; 33: 36–48. [DOI] [PubMed] [Google Scholar]
- 23. Rigaud M, Filip P, Lirk P, et al. Guidance of block needle insertion by electrical nerve stimulation. Anesthesiol 2008; 109: 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kirchmair L, Entner T, Kapral S, et al. Ultrasound guidance for the psoas compartment block: An imaging study. Anesth Analg 2002; 94: 706–710. [DOI] [PubMed] [Google Scholar]
- 25. Bagshaw HS, Larenza MP, Seiler GS. A technique for ultrasound-guided paravertebral brachial plexus injections in dogs. Vet Radiol Ultrasound 2009; 50: 649–654. [DOI] [PubMed] [Google Scholar]
- 26. Crouch JE. Text-atlas of cat anatomy. Philadelphia: Lea & Febiger, 1969, pp 244–245. [Google Scholar]
- 27. Gruber H, Peer S, Kovacs P, et al. The ultrasonographic appearance of the femoral nerve and cases of iatrogenic impairment. J Ultrasound Med 2003; 22: 163–172. [DOI] [PubMed] [Google Scholar]
- 28. Mihelić D, Zobundzija M, Brkić A, et al. Anatomical possibilities of access to and blockade of n. femoralis in the dog. Veterinární Medicína 1995; 40: 283–287. [PubMed] [Google Scholar]
- 29. Newitt A, German AJ, Barr FJ. Congenital abnormalities of the feline vertebral column. Vet Radiol Ultrasound 2008; 49: 35–41. [DOI] [PubMed] [Google Scholar]
- 30. Marhofer P, Harrop-Griffiths W, Kettner SC, et al. Fifteen years of ultrasound guidance in regional anaesthesia: Part 1. Br J Anaesth 2010; 104: 538–546. [DOI] [PubMed] [Google Scholar]
- 31. Kumar PA, Gentry WB, Arora H. Ultrasound guidance in regional anaesthesia. J Anaesthesiol Clin Pharmacol 2007; 23: 121–128. [Google Scholar]
- 32. Mahler SP, Adogwa AO. Anatomical and experimental studies of brachial plexus, sciatic, and femoral nerve-location using peripheral nerve stimulation in the dog. Vet Anaesth Analg 2008; 35: 80–89. [DOI] [PubMed] [Google Scholar]
- 33. Kitchell, Evans H. The spinal nerves. In: Evans H. (ed). Evans Miller’s anatomy of the dog. 3rd ed. Philadelphia: WB Saunders, 1993, pp 829–893. [Google Scholar]
- 34. Raymond SA, Steffensen SC, Gugino LD, et al. The role of length of nerve exposed to local anesthetics in impulse blocking action. Anesth Analg 1989; 68: 563–570. [PubMed] [Google Scholar]
- 35. Mannion S, Barrett J, Kelly D, et al. A description of the spread of injectate after psoas compartment block using magnetic resonance imaging. Reg Anesth Pain Med 2005; 30: 567–571. [DOI] [PubMed] [Google Scholar]
- 36. Farny J, Girard M, Drolet P. Posterior approach to the lumbar plexus combined with a sciatic nerve block using lidocaine. Can J Anaesth 1994; 41: 486–491. [DOI] [PubMed] [Google Scholar]
- 37. Campoy L. Fundamentals of regional anesthesia using nerve stimulation in the dog. In: Gleed RD, Ludders JW. (eds). Recent advances in veterinary anesthesia and analgesia: Companion Animals. Ithaca, NY: International Veterinary Information Service, 2008, http://www.ivis.org/advances/Anesthesia_Gleed/campoy/chapter.asp?LA=1 (accessed 10 February 2011). [Google Scholar]
- 38. De Biasi P, Lupescu R, Burgun G, et al. Continuous lumbar plexus block: Use of radiography to determine catheter tip location. Reg Anesth Pain Med 2003; 28: 135–139. [DOI] [PubMed] [Google Scholar]