Abstract
The continuous glucose monitoring system allows generation of detailed glucose curves via measurement of glucose concentration in interstitial fluid. The conventional site for sensor placement in diabetic cats is the subcutaneous tissue of the lateral chest wall. The aim of this study was to investigate the feasibility and accuracy of sensors placed in the lateral chest wall and in two alternative sites — the dorsal neck and lateral knee fold — of diabetic cats. Initialisation was successful in 15/20 lateral chest wall sensors, 9/10 neck sensors and 3/10 knee fold sensors. Compared with the reference portable blood glucose meter, 0.8% of measurements from lateral chest wall sensors, 0.7% from knee fold sensors and 0% from neck sensors would have resulted in erroneous treatment. This preliminary study suggests that dorsal neck placement may be superior to lateral chest wall and lateral knee fold; however, further investigation with a larger number of cases would be required to confirm this finding.
Introduction
A continuous glucose monitoring system (CGMS) has recently been introduced into veterinary medicine.1–4 This system measures glucose concentrations in the subcutaneous interstitial fluid every 5 mins for up to 72 h via a glucose oxidase-containing sensor. The technique provides a potentially less stressful alternative to blood glucose measurements and generates detailed glucose curves. A good correlation between interstitial fluid and blood glucose concentrations was shown with the first generation of CGMSs in cats and dogs.1–4 However, these older systems necessitate attachment of the monitor to the animal and manual downloading of the recorded data to a computer for analysis. The Guardian Real-Time (Medtronic) is a new generation CGMS that enables onscreen date recording over a 72-h period. This system has recently been validated for use in cats and provides clinically accurate and reproducible measurements in the euglycaemic and hyperglycaemic ranges, but slightly less accurate results in the hypoglycaemic range. 5
The manufacturer of the Guardian Real-Time CGMS recommends placing the sensor in an area with sufficient subcutaneous tissue. In humans, the most suitable sensor site is the abdominal para-umbilical region. However, a study based on glucose monitoring with microdialysis showed that readings of abdominal sensors were 20% lower than reference blood glucose concentrations or readings from sensors placed in the forearm. 6 In another study, clinical accuracy of measurements made using glucose oxidase-containing sensors or the microdialysis technique in the subcutaneous tissue of the shoulder was higher than in the upper leg. 7 Thus, the recorded values may differ depending on the site of sensor placement. There are no specific recommendations for sensor placement in cats. In most studies, the thoracic region has been used.3,8,9 We hypothesise that the position of the sensor interferes with its function in cats. The purpose of this study was to determine which sensor site in the subcutaneous tissue of diabetic cats is more practical and provides the most accurate results. For comparison the dorsal neck, the knee fold region and the lateral chest wall were evaluated.
Materials and methods
Animals
The study was approved by the Cantonal Veterinary Office of Zurich and conducted in accordance with guidelines established by the Animal Welfare Act of Switzerland (permission number 83/2008). Informed consent to participate in the study was provided by the owners.
Eighteen client-owned diabetic cats were used in the study and were hospitalised in the Clinic for Small Animal Internal Medicine in Zurich. Sixteen had been recently diagnosed with diabetes mellitus, one had been treated with porcine lente insulin (Caninsulin; Intervet) for 1 year and another for 3 years before recruitment. The median age of the cats was 11.0 years (range 7–21), median body weight was 5.0 kg (range 2.5–9.6) and median body condition score (Purina Pet Care Center) was 5.5 (range 2–9). Ten cats (55%) were neutered males and eight (44%) were spayed females. There were 17 (94%) domestic shorthair and one Birman cat.
Continuous glucose monitoring system
The Guardian Real-Time system for continuous glucose monitoring consists of a disposable glucose sensor, a transmitter and a recording monitor. The sensor is a flexible glucose electrode coated with the enzyme glucose oxidase. Glucose in the interstitial fluid undergoes the following electrochemical reaction:
glucose + oxygen → gluconic acid + H2O2
The produced peroxide dissociates to 2H+, oxygen and 2e-. This reaction generates a small electric current which is proportional to glucose concentration in the sensor environment and is referred to as the input signal for glucose (ISIG). The rechargeable transmitter connects directly to the glucose sensor and wirelessly sends ISIG data to the monitor, which can be up to 1.8 m away. The ISIG is subsequently converted to the prevailing glucose concentration (mmol/l) and can be read on the monitor in addition to the glucose value. The function of the monitor is to acquire, display and store signals from the subcutaneous glucose sensor. The glucose sensor signal is acquired every 10 s. The monitor stores the data and displays an average of the acquired signals every 5 mins in real-time for up to 24 h. The Guardian Real-Time monitor is not equipped with a signal indicator. But the monitor includes a programmable ‘weak signal’ alert that notifies when one or more expected transmissions are not received, as expected by the receiving device. The sensor can be left in place for up to 72 h. Glucose values can be downloaded onto a computer for analysis. The monitor has the capability to record glucose concentrations for up to 1 month before downloading.
Experimental design
For comparison of different sensor sites, two glucose sensors were placed one in the subcutaneous tissue of the lateral chest wall (standard sensor site) and the other in the subcutaneous tissue of the knee fold or dorsal neck area (alternative sensor sites). Sensors were placed in the lateral chest wall and dorsal neck region of 10 cats and in the lateral chest wall and knee fold of 10 other cats; in two cats both alternative sites were evaluated consecutively. After clipping and disinfecting the insertion site, the glucose sensor was placed in the subcutaneous tissue and secured to the skin with cyanoacrylate adhesive (Cyanolit universal classic; 3M Consumer Health Care). The transmitter was connected to the sensor and secured in place using a 2 × 5 cm piece of tape. The first CGMS calibration was carried out according to the manufacturer’s recommendation after a 2-h period of initialisation, during which time glucose measurements were not made. Thereafter, the CGMS was calibrated after 6 h and then every 10 h. Calibrations were achieved by measuring the glucose concentration of capillary blood from the inner pinna with the AlphaTrak (Abbott Animal Health) portable blood glucose meter (PBGM), which was used as a reference.10,11 Based on previous articles published by our group12–14 capillary glucose monitoring performed with the portable glucose meter is not stressful. Sampling of capillary blood using the Microlet Vaculance device (Bayer) was tolerated very well in all cats. The lancing device (Microlet Vaculance) enables easy and fast capillary blood sampling.11,14–16 All veterinarians taking care of the cats during the study were well trained in sampling capillary blood. Owing to intrinsic technical limits of the Guardian Real-Time system only glucose concentrations between 2.2 mmol/l and 22.2 mmol/l can be used for calibration. The CGMS, as it is so far marketed, is able to display glucose concentrations between 2.2 mmol/l and 22.2 mmol/l. However, concentrations beyond this range are recorded correctly by the CGMS, but need to be downloaded to be analysed. During our study, however, calibration was postponed until glucose concentration was within the working range. If calibration failed, 15 mins after entering the glucose value the CGMS monitor displayed ‘calibration error’. Based on the CGMS instructions provided by the manufacturer, sources of error can be one of the following: an incorrect blood glucose measurement entered from the meter into the monitor; blood glucose rising or falling rapidly; more time is needed for the sensor to stabilise after being inserted; or the sensor is no longer reading glucose correctly. Unfortunately, the CGMS displays ‘calibration error’, but does not indicate the cause of failure. The CGMS manufacturer recommends waiting for 10–15 mins after a ‘calibration error’ is displayed and then restarting with a new calibration. If glucose values are increasing or decreasing very fast, the manufacturer suggests waiting longer (ie, 15–20 mins) or until glucose values are stable. In the present study the suggested recommendations were followed. When calibration failed, recalibration was attempted after 10 mins and thereafter every 30 mins. Sensors were evaluated for up to 72 h.
Analysis
To identify the most practical and reliable site for placing the CGMS sensor in cats, the following items were analysed.
Sensor loosening
Detachment of the sensor from the skin on its own or by the cat was recorded for each site.
First sensor calibrations
The proportion of successful first calibrations was calculated for each site. First calibrations were considered successful when the CGMS was able to display glucose levels recorded by the sensor 2 h after the initialisation period. Owing to technical limits only calibrations with capillary blood glucose measurements between 2.2 mmol/l and 22.2 mmol/l were used.
Sensor recording
For each sensor site the proportion of continuous recordings that lasted at least 48 h was calculated. Interruptions of recording shorter than 3 h were arbitrarily tolerated. In our experience interruptions not longer than 3 h over a period of 2 or more days, in a clinical setting, do not affect interpretation of the curves if the cat has stable glucose levels and shows no signs of hypoglycaemia (eg, restlessness, shivering).
Analytical and clinical accuracy
To evaluate accuracy of the CGMS, the glucose concentration of interstitial fluid at the different sensor sites was compared with capillary blood glucose concentrations measured with a PBGM every 4–6 h (reference method). Analytical accuracy was calculated by plotting differences between results of the CGMS and PBGM using Bland and Altmann plots. 17 Clinical accuracy was calculated using the Clarke error grid analysis. 18 The grid system assigns CGMS measured values (y-axis) versus actual glucose values (reference PBGM, x-axis) to five zones (A–E) and is based on the assumption that the clinical goal is to maintain blood glucose concentrations between 3.9 mmol/l and 10 mmol/l. Measurements in zones A and B are clinically accurate and lead to clinically correct treatment decisions. The CGMS readings in zone A deviate from the reference value by no more than 20% or both are <3.9 mmol/l. The CGMS readings in zone B represent benign errors and deviate from reference values by >20%; however, they do not lead to a change in treatment or treatment will not have any harmful effects. Values in zones C, D and E lead to treatment errors or failure to initiate treatment. Values in zone C lead to unnecessary correction or overcorrection of the acceptable glucose concentration, and cause the actual blood glucose concentration to fall below 3.9 mmol/l or to increase above 10 mmol/l. Zone D represents potentially dangerous errors of failing to detect and treat actual glucose values that are outside the target range because CGMS readings are within the target range. The CGMS readings in zone E are opposite to the actual glucose values and therapeutic actions would be opposite to those indicated.
Concordance between measurements of sensors and the reference method was determined by calculating the proportion of paired readings that were both in the normal (5–10 mmol/l), high (>10 mmol/l) and low (<5 mmol/l) glucose ranges. 9 However, because paired readings may be very close, yet assigned to different glycaemic ranges (eg, 4.9 versus 5.1 mmol/l), thus generating disagreement that does not have clinical relevance, we included an additional arbitrary criterion to evaluate concordance: paired samples considered not to be concordant required a difference of at least 10% in addition to being assigned to different glycaemic ranges.
Results
Feasibility of the three sensor sites
Placement of the sensor and transmitter, and visualisation of the data in real-time on the monitor were successful and straightforward in all cats. The sensor and transmitter were well tolerated by all cats and adverse skin reactions were not observed at any of the sensor sites.
Successful first calibrations 2 h after sensor placement were achieved in 15/20 (75%) sensors placed in the lateral chest wall, in 9/10 (90%) sensors placed in the neck region and in 3/10 (30%) sensors in the area of the knee fold.
Uninterrupted glucose concentration recordings over a 48-h period occurred in 17/20 (85%) of the sensors that were inserted in the lateral chest wall and in 7/10(70%) of the sensors that were inserted in the dorsal neck region and knee fold. Three of 20 (15%) lateral chest wall sensors, 3/10 (30%) dorsal neck sensors and 3/10 (30%) knee fold sensors provided uninterrupted recordings for periods shorter than 48 h. In all cases discontinuation of recordings occurred as a result of calibration errors. Two sensors (one in the lateral chest wall and one in the knee fold) never recorded glucose values because successful calibrations were not achieved. Macroscopically, the sensors were not deformed, broken or clogged by proteins or blood; no obvious sensor abnormalities were detected. One sensor in the dorsal neck region functioned for only 4 h. Two sensors each in the lateral chest wall, dorsal neck region and knee fold recorded glucose values for 15–38 h. The remaining 31 sensors provided glucose values for at least 48 h.
Sensors lost the proper placement under the skin in 1/20 (5%) sensors in the lateral chest wall, 2/10 (20%) sensors in the dorsal neck region and 2/10 (20%) sensors in the knee fold.
Analytical and clinical accuracy of the three sensor sites
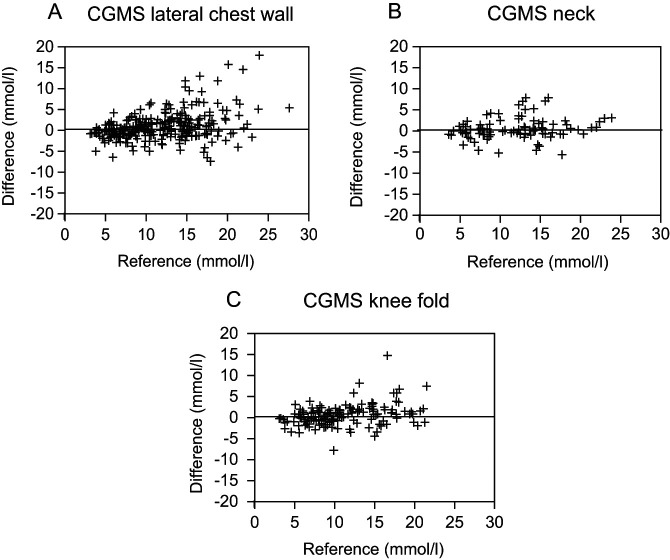
Four hundred and ninety-one paired samples were taken to compare glucose concentrations measured with the CGMS against the reference PBGM. Differences between glucose concentrations measured with the CGMS and PBGM are shown in Bland and Altmann plots (Figure 1). The mean ± two standard deviations (2SD) of the difference was 0.96 ± 6.76 mmol/l in the lateral chest wall, 0.60 ± 5.24 mmol/l in the dorsal neck and 0.62 ± 5.24 mmol/l in the knee fold region. The maximum deviation for sensors in the lateral chest wall was 18 mmol/l, in the dorsal neck region it was 7.9 mmol/l and in the knee fold area it was 14.8 mmol/l.
Figure 1.
Scatterplots of the differences between glucose concentrations obtained by use of the Guardian Real-Time continuous glucose monitoring system (CGMS) at different sensor sites [(A) lateral chest wall, (B) neck and (C) knee fold] versus blood glucose concentration obtained with the reference AlphaTrak portable blood glucose meter (PBGM) in cats
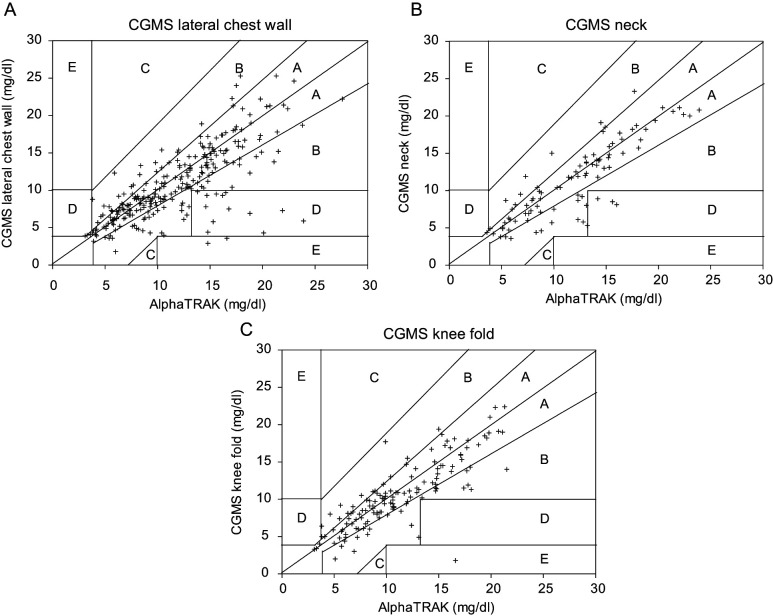
Results of the Clarke error grid analysis are shown in Figure 2. Overall, 472/491 (96.1%) paired glucose measurements were in zones A or B; this included 94.3% of glucose measurements from sensors in the lateral chest wall, 96.7% from the dorsal neck region and 99.3% from the knee fold. There were no measurements in zone C. Of the measurements from the lateral chest wall, 4.9% were in zone D and 0.8% in zone E, from the dorsal neck, 3.3% were in zone D and from the knee fold, 0.7% were in zone E.
Figure 2.
Error grid analysis for the Guardian Real-Time continuous glucose monitoring system (CGMS) in cats. Results of the CGMS that fall in zone A deviate from the reference method value by no more than 20%, or the CGMS value and the reference method value are <3.9 mmol/l. Results of the CGMS that fall in zone B deviate from the reference method value by >20%, but reliance on results of the CGMS to make treatment decisions would not cause unacceptable errors in treatment. Values in zone C lead to unnecessary correction or overcorrection of glucose concentrations. Reliance on the CGMS value in zone D would result in a failure to detect glucose concentrations outside the reference interval. CGMS values in zone E would result in erroneous treatment with insulin. (A) The glucose sensors placed in the lateral chest wall yielded 94.3% in zones A or B, 4.9% in zone D and 0.8% in zone E. (B) Glucose sensors placed in the dorsal neck yielded 96.7% of measurements in zones A or B and 3.3% in zone D. (C) Glucose sensors at the knee fold yielded 99.3% of measurements in zones A or B and 0.7% in zone E
There was concordance between values generated by the CGMS and PBGM in 78.3% of measurements from sensors in the lateral chest wall, in 80.0% of measurements from sensors in the neck area and in 76.5% of measurements from sensors in the knee fold.
Discussion
This study investigated the feasibility and accuracy of CGMS sensors placed at different sites of the body in diabetic cats. Sensor placement in the subcutaneous tissue of the lateral chest wall, dorsal neck region and knee fold was quick and easy, and did not yield apparent discomfort. Sensors in all three sites were well tolerated and adverse skin reactions at the place of insertion were not observed after sensor removal. Overall, CGMS sensors placed in the dorsal neck region worked better than those placed in the lateral chest wall or knee fold. Following the initialisation period, successful first calibrations were achieved with 90% of sensors placed in the dorsal neck and 75% of sensors in the lateral chest wall, but with only 30% of sensors placed in the knee fold. The high proportion of unsuccessful first calibrations in the knee fold may have been attributable to poor capillary vascularisation in that region and thus insufficient contact between the sensor and interstitial fluid. Studies in humans and dogs suggest that the subcutaneous tissue in this area has less than optimal capillary blood supply.6,9 However, in cats the density of capillaries in the subcutaneous tissue of the knee fold has not been studied. In humans, blood flow in adipose tissue has been shown to differ among various regions of the body. 19
The sensors are expected to measure glucose concentrations without prolonged interruptions after the first calibration. The performance of sensors in the lateral chest wall, dorsal neck and knee fold with respect to uninterrupted function was similar; interruptions that lasted less than 3 h over a 48-h period occurred in 85%, 70% and 70% of the sensors, respectively. Of course, uninterrupted glucose concentrations recordings do not imply that recordings are necessarily accurate. However, it is important to know which of the different sensor sites yielded fewer problems after the first calibration. All interruptions were caused by calibration errors. Calibration errors were usually preceded by large discrepancies between the PBGM measurement used for calibration and the CGMS readings (eg, PBGM 15 mmol/l and CGMS 5 mmol/l), and the CGMS values were always lower than the PBGM reference values. A similar decrease in the ISIG before the occurrence of abnormal sensor function, which also led to calibration errors, has been documented in human diabetics. 20 The ISIG identifies the small electric current produced by the electrochemical reaction between glucose in the interstitial fluid and glucose oxidase on the sensor electrode. The ISIG is subsequently converted to glucose concentration (mmol/l). Thus, the direct relationship between the glucose concentration in the interstitial fluid and the ISIG means that as the glucose concentration increases or decreases in the interstitial fluid, so does the ISIG. However, a low ISIG can occur if the diffusion field nearby the sensor is disturbed. Other factors, like oxygen deficit, chemical interferences and enzyme inactivation can also impair the association between glucose concentration and sensor signal. 21 Further investigations are necessary to verify which of the above factors disturbs glucose sensor function in diabetic cats.
The sensor lost the proper placement in only five cats. This occurred in one sensor in the lateral chest wall, two sensors in the dorsal neck and two sensors in the knee fold, suggesting that the thoracic region is a more secure location for sensor placement. Hind limb movement resulting in detachment of the sensor from the skin may explain why loosening occurred more frequently in the knee fold region. Likewise, frequent and vigorous lateral and vertical movements of the head may gradually break the attachment between the sensor and the skin in the neck area.
Similar to the results of our previous study, 5 the glucose concentration measured by the sensors was generally lower than the reference value, regardless of sensor location. This discrepancy notwithstanding, error grid analysis revealed a satisfactory clinical accuracy, with 96.1% of glucose values being in zone A and B. A small proportion of measurements from sensors in the lateral chest wall (0.8%) and knee fold (0.7%) were in zone E. In these cases, the CGMS yielded glucose values in the hypoglycaemic range, whereas the actual capillary glucose concentration measured with the PBGM was in the hyperglycaemic range, which would confuse treatment of hyperglycaemia with treatment of hypoglycaemia. The reason for this underestimation of the glucose concentration is not clear, but may be due to insufficient sensor perfusion leading to drop in the ISIG. Sensors in the dorsal neck yielded no readings in zone E and thus none of those measurements would have confused treatment. Based on these results, sensors placed in the dorsal neck appear to be clinically more accurate and reliable. Furthermore, concordance between values generated by the PBGM and sensors in the dorsal neck was better than for the sensors in the other two locations, although the difference was minimal. Differences between CGMS and PBGM measurements tended to increase at higher glucose concentrations, especially for the lateral chest and knee sites. This finding is in agreement with previous observations in cats. 5 Analytical accuracy is lower in the hyperglycaemic range, although the clinical relevance is minimal. 5
There are some limitations to this study that need to be discussed. First, differences between the three sensor sites were not assessed with statistical methods in order to objectively verify whether one was superior to the others. Analysis was not performed because sample size was relatively small, in particular for knee and neck regions, and because, for correct interpretation of the results, sensors should have been placed at the three sensor sites simultaneously. For animal welfare reasons this was not considered acceptable by the Veterinary Office that supervised the study. Second, interruptions of recording shorter than 3 h were tolerated arbitrarily. Although in our experience interruptions shorter than 3 h (over a period of 2 or more days) do not affect interpretation of the curves if the cat has stable glucose levels and shows no signs of hypoglycaemia; however, no specific investigation has been performed to assess whether this is true in a clinical setting. Finally, it is worth mentioning that results of the CGMS were compared with those of the reference PBGM. Although the PBGM has been previously validated, 10 small differences with the true gold standard (ie, the hexokinase method) may have, at least in part, biased the results of the present study.
Conclusions
Sensor placement is feasible in any of the three sites used in this study and did not cause any adverse reactions in cats. This preliminary study suggests that dorsal neck placement may be superior to lateral chest wall and lateral knee fold; however, further investigation with a larger number of cases would be required to confirm this finding.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors do not have any potential conflicts of interest to declare.
Accepted: 10 September 2012
References
- 1. Ristic JM, Herrtage ME, Walti-Lauger SM, et al. Evaluation of a continuous glucose monitoring system in cats with diabetes mellitus. J Feline Med Surg 2005; 7:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wiedmeyer CE, Johnson PJ, Cohn LA, Meadows RL. Evaluation of a continuous glucose monitoring system for use in dogs, cats, and horses. J Am Vet Med Assoc 2003; 223: 987–992. [DOI] [PubMed] [Google Scholar]
- 3. Wiedmeyer CE, DeClue AE. Continuous glucose monitoring in dogs and cats. J Vet Intern Med 2008; 22: 2–8. [DOI] [PubMed] [Google Scholar]
- 4. Davison LJ, Slater LA, Herrtage ME, et al. Evaluation of continuous glucose monitoring system in diabetic dogs. J Small Anim Pract 2003; 44: 435–442. [DOI] [PubMed] [Google Scholar]
- 5. Moretti S, Tschuor F, Osto M, et al. Evaluation of a novel real-time continuous glucose-monitoring system for use in cats. J Vet Intern Med 2010; 24: 120–126. [DOI] [PubMed] [Google Scholar]
- 6. von Dobeln A, Adamson U, Lins PE. Nocturnal differences in subcutaneous tissue glucose between forearm and abdominal sites during continuous glucose monitoring in normal subjects. Diabetes Metab 2005; 31: 347–352. [DOI] [PubMed] [Google Scholar]
- 7. Vriesendorp TM, Devries JH, Holleman F, et al. The use of two continuous glucose sensors during and after surgery. Diabetes Technol Ther 2005; 7: 315–322. [DOI] [PubMed] [Google Scholar]
- 8. Reineke EL, Fletcher DJ, King LG, et al. Accuracy of continuous glucose monitoring system in dogs and cats with diabetic ketoacidosis. J Vet Emerg Crit Care 2010; 20: 303–312. [DOI] [PubMed] [Google Scholar]
- 9. Bilicki KL, Schermerhorn T, Klocke EE, et al. Evaluation of a real-time, continuous monitor of glucose concentration in healthy dogs during anesthesia. Am J Vet Res 2010; 71: 11–16. [DOI] [PubMed] [Google Scholar]
- 10. Zini E, Moretti S, Tschuor F, Reusch CE. Evaluation of a new portable glucose meter designed for the use in cats. Schweiz Arch Tierheilkd 2009; 151: 448–451. [DOI] [PubMed] [Google Scholar]
- 11. Thompson MD, Taylor SM, Adams VJ, et al. Comparison of glucose concentrations in blood samples obtained with a marginal ear vein nick technique versus from a peripheral vein in healthy cats and cats with diabetes mellitus. J Am Vet Med Assoc 2002; 221: 389–392. [DOI] [PubMed] [Google Scholar]
- 12. Kley S, Casella M, Reusch CE. Evaluation of long-term home monitoring of blood glucose concentrations in cats with diabetes mellitus: 26 cases (1999–2002). J Am Vet Med Assoc 2004; 225: 261–266. [DOI] [PubMed] [Google Scholar]
- 13. Sieber-Ruckstuhl NS, Casella M, Reusch CE. Home monitoring of blood glucose concentrations by owners of diabetic dogs and cats. Schweiz Arch Tierheilkd 2003; 145: 537–543. [DOI] [PubMed] [Google Scholar]
- 14. Reusch CE, Kley S, Casella M. Home monitoring of the diabetic cat. J Feline Med Surg 2006; 8: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wess G, Reusch CE. Capillary blood sampling from the ear of dogs and cats use of portable meters to measure glucose concentration. J Small Anim Pract 2000; 41: 60–66. [DOI] [PubMed] [Google Scholar]
- 16. Casella M, Wess G, Reusch CE. Measurement of capillary blood glucose concentrations by pet owners: a new tool in the management of diabetes mellitus. J Am Anim Hosp Assoc 2002; 38: 239–245. [DOI] [PubMed] [Google Scholar]
- 17. Bland JM, Altmann DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–310. [PubMed] [Google Scholar]
- 18. Clarke WL, Cox D, Gonder-Frederick LA, et al. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care 1987; 10: 622–628. [DOI] [PubMed] [Google Scholar]
- 19. Munck O, Andersen AM, Binder C. Clearance of 4-Iodoantipyrine-125-I after subcutaneous injection in various regions. Scand J Clin Lab Invest 1967; 99 (Suppl): 39–45. [PubMed] [Google Scholar]
- 20. Jadviscokova T, Fajkusova Z, Pallayova M, et al. Occurrence of adverse events due to continuous glucose monitoring. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2007; 151: 263–266. [DOI] [PubMed] [Google Scholar]
- 21. Gough DA, Armour JC. Development of the implantable glucose sensor: What are the prospects and why is it taking so long? Diabetes 1995; 44: 1005–1009. [DOI] [PubMed] [Google Scholar]